Precision medicine for respiratory
diseases
Respiratory illnesses pose a significant cause of
illness and mortality on a global scale. Despite the substantial
expenses linked to the accurate diagnosis and treatment of these
conditions, a number of patients suffering from chronic respiratory
diseases or malignancies cannot be effectively treated using
current therapies. There are often delays in diagnoses, treatments
are frequently subpar, and in several cases, patients do not
receive adequate care due to treatment unavailability or poor
prognoses. Additionally, some patients encounter adverse reactions
or limitations in the usefulness of available treatments due to
side-effects (1).
In recent years, there has been a marked improvement
in the understanding of the mechanisms through which respiratory
diseases develop, allowing for tailored medical treatments based on
individual patient traits. Understanding the genetic roots,
molecular pathways and environmental influences driving disease
development has opened avenues for preventive strategies and has
enhanced diagnostic and treatment strategies personalized for each
patient (2).
Precision medicine, broadly defined as strategies
considering individual differences in preventing and treating
ailments, aims to categorize individuals into groups with varying
susceptibilities, pathogeneses, manifestations and responses to
therapies for specific diseases. This approach seeks to enhance
patient care by combining advancements in medical research and
computer science, as highlighted by the substantial allocation of
funds to the Precision Medicine Initiative (3,4).
Patient stratification based on molecular and
phenotypic distinctions is not a new concept in medicine. Over
time, disease classifications have evolved with advances in
understanding disease origins, manifestations and prognoses
(5).
Cutting-edge technologies, such as whole-genome
sequencing, transcriptome profiling, proteomics and imaging
techniques are transforming the classification of disease subtypes.
Progress in comprehending regulatory networks and essential
metabolic pathways allows for adjusting immune responses and
crafting specific treatments, with some already in active clinical
deployment. The goal of therapeutic drug monitoring is to offer
customized therapies, aiming to reduce adverse effects, while
ongoing efforts focus on pinpointing biomarkers for monitoring
treatment responses and refining the duration of therapies. Yet,
the challenge remains in managing the vast amount of complex data,
requiring integrated knowledge systems and standardized data
analysis in precision medicine (6).
In due course, precision medicine is expected to
lead to diagnostic tools that identify individuals who will benefit
most from specific therapies, while avoiding unnecessary treatments
and expenses for those who will not benefit. Sharma et al
(7) reported the application of
precision medicine in the treatment of lung cancer, an area where
tailoring patient care is at the forefront within the field of
respiratory medicine. They discussed significant discoveries that
have advanced the current knowledge of the molecular pathways
underlying the onset and spread of lung cancer, as well as the
development of immune checkpoint inhibitor therapy and targeted
therapies, which have significantly improved the prognosis of
patients with particular subtypes of the disease (7).
Salzer et al (8) provided a thorough overview of the use
of precision medicine in the treatment of patients with long-term
respiratory infectious diseases, such as tuberculosis,
nontuberculous mycobacterial pulmonary disorders and chronic
pulmonary aspergillosis. They presented an overview of the use of
cutting-edge therapeutic applications and diagnostics in the
future, including host-directed therapies that will significantly
improve patient prognoses and enable them to be cured of these
diseases. They also compared the current state of care to expert
management standards (8).
Heaney and McGarvey (9) evaluated the conventional method of
treating respiratory conditions (asthma and chronic obstructive
pulmonary disease) in stages. The impact of comorbidities on the
expression of airway disease was critically examined, along with
new developments in the fields of airway inflammometry, biomarkers,
and companion diagnostics that characterize endotypes of severe
asthma and chronic obstructive pulmonary disease and stratify the
response to biologics and small-molecule inhibitors, behavioral
psychology, and the introduction of quantitative tools to measure
inhaler adherence using digital technologies (9).
An impartial characterization of phenotypes or
endotypes (which are phenotypes classified by their mechanisms) is
a crucial initial stage in advancing precision medicine for asthma.
While there have been definitions and treatment targets established
for allergic or eosinophilic asthma categorized as T2-high asthma,
identifying non-T2 phenotypes remains a primary focus. The
effectiveness of precision medicine also hinges on identifying
biomarkers that can distinguish between these phenotypes, aiding in
the identification of patients suitable for specific targeted
therapies. Hence, precision medicine establishes connections
between phenotypes (endotypes) and targeted treatments, aiming for
improved outcomes (10).
Recently, Karampitsakos et al (11) summarized the current knowledge of
precision medicine in idiopathic pulmonary fibrosis (IPF) and
highlighted barriers to translating these research findings into
clinical practice. Over the past decade, there has been a surge in
scientific advancements, resulting in the identification of
multiple biomarkers and two anti-fibrotic compounds that can
attenuate the progression of IPF. The current challenge lies in
translating these biomarkers into cost-effective tools that offer
valuable guidance for clinicians. An essential biomarker should
significantly influence decisions of clinicians, whether by aiding
in early diagnosis, providing insight into disease activity, or
determining the need for treatment adjustments. Achieving this
could involve the use of individual biomarkers, comprehensive
indexes, or polygenic risk scores (11).
Identifying biomarkers that align with disease
activity could play a pivotal role in determining when intervention
is necessary. Particularly for patients with interstitial lung
abnormalities (ILA) and mild functional impairment, where the
initiation of antifibrotic treatment is occasionally delayed, these
biomarkers could prove invaluable. Additionally, biomarkers capable
of identifying specific endotypes are crucial (12-14).
Instead of using the term ‘idiopathic’, categorizing pulmonary
fibrosis based on high-risk genomic pulmonary fibrosis,
MUC5B-pulmonary fibrosis, or telomeropathy-pulmonary fibrosis may
offer a more effective approach. Utilizing precision medicine and
endotyping could pave the way for pharmacogenetic approaches and
may help guide treatment decisions. Tailoring treatments to
specific endotypes based on distinct biomarker expression levels
could enhance the effectiveness of future therapies, while
minimizing adverse events. Examples include ongoing clinical, such
as the TELO-SCOPE Study (NCT04638517) and the study with the title
‘Low-Dose Danazol for the Treatment of Telomere Related Diseases’
(NCT03312400) testing the synthetic androgen, danazol, for patients
with short telomeres, based on previous findings suggesting the
potential of androgens to restore telomerase activity in IPF, as
well as the PRECISIONS trial for NAC involving TOLLIP gene variants
(15,16).
Transitioning from a standardized approach to a
patient-centered one is crucial. Trials evaluating therapeutic
biomarkers alongside the weight-based dosing of antifibrotics or
investigating reduced doses in patients with ILA could offer
benefits, while minimizing adverse events that often lead to
treatment cessation. Encouraging studies focused on managing
symptoms in a personalized manner are essential. For instance, the
extended-release form of nalbuphine, acting as a dual κ opioid
receptor agonist/μ opioid receptor antagonist, exhibits promise in
alleviating chronic cough in patients with IPF (17-19).
While not all respiratory disease cases can be
cured, precision medicine continues to enhance patient prognoses.
It emphasizes personalized care, yet a holistic approach centered
on the patient beyond scientific advancements ensures a truly
individualized and humane medical practice. The concept of
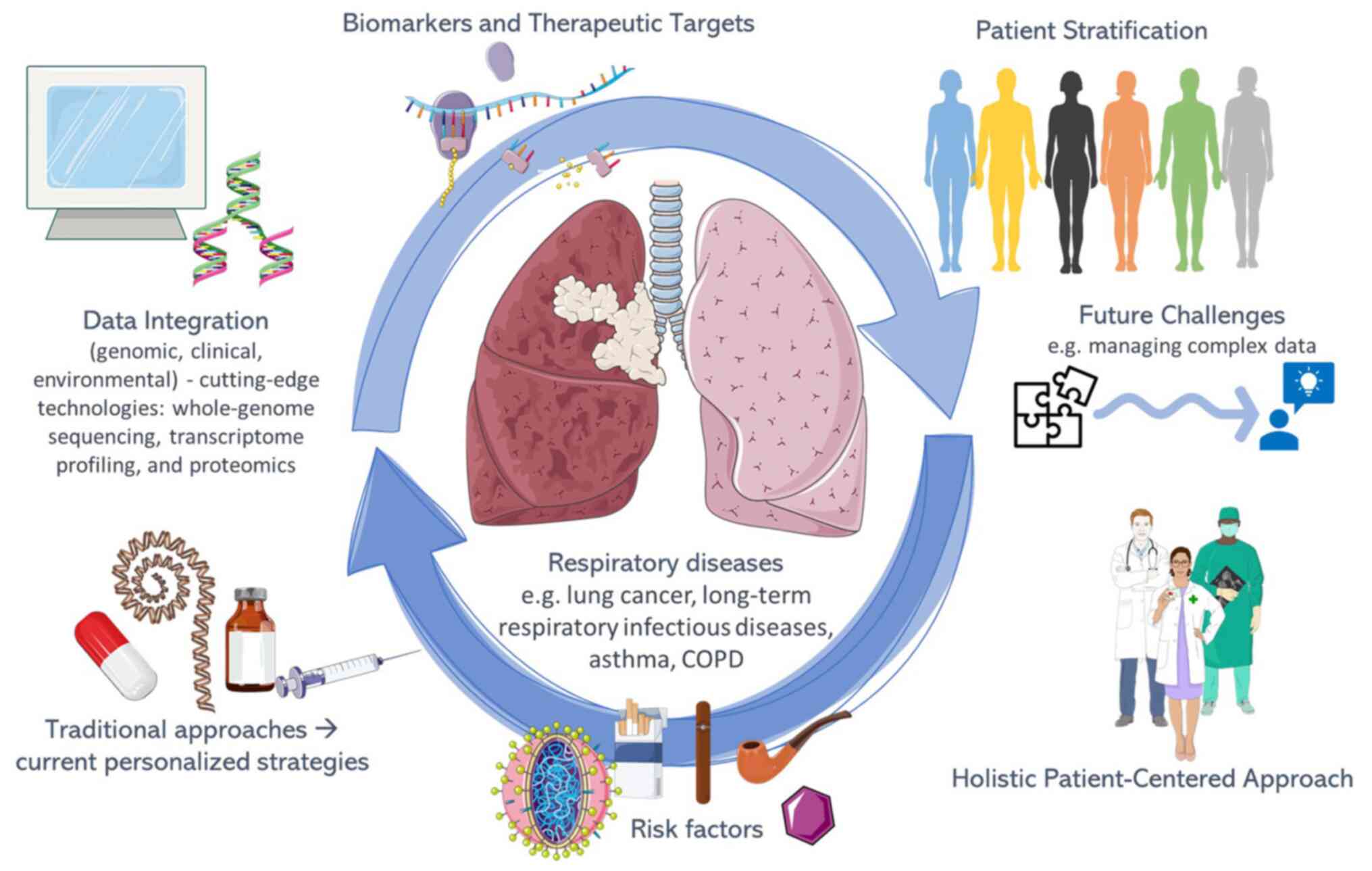
precision medicine in the field of respiratory diseases is
illustrated in Fig. 1.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
VEG, and DAS conceptualized the study. IGL, VEG, NT,
PS and DAS designed and wrote the manuscript. All authors have read
and approved the final manuscript. Data authentication is not
applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Use of artificial intelligence tools
During the preparation of this work, AI tools were
used to improve the readability and language of the manuscript or
to generate images, and subsequently, the authors revised and
edited the content produced by the AI tools as necessary, taking
full responsibility for the ultimate content of the present
manuscript.
References
|
1
|
GBD Chronic Respiratory Disease
Collaborators. Prevalence and attributable health burden of chronic
respiratory diseases, 1990-2017: a systematic analysis for the
Global Burden of Disease Study 2017. Lancet Respir Med. 8:585–596.
2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Obeidat M and Hall IP: Genetics of complex
respiratory diseases: implications for pathophysiology and
pharmacology studies. Br J Pharmacol. 163:96–105. 2011.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Jameson JL and Longo DL: Precision
medicine - personalized, problematic, and promising. N Engl J Med.
372:2229–2234. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Collins FS and Varmus H: A new initiative
on precision medicine. N Engl J Med. 372:793–795. 2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Haendel MA, Chute CG and Robinson PN:
Classification, Ontology, and Precision Medicine. N Engl J Med.
379:1452–1462. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Wörheide MA, Krumsiek J, Kastenmüller G
and Arnold M: Multi-omics integration in biomedical research - A
metabolomics-centric review. Anal Chim Acta. 1141:144–162.
2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Sharma J, Shum E, Chau V, Paucar D, Cheng
H and Halmos B: The Evolving Role of Biomarkers in Personalized
Lung Cancer Therapy. Respiration. 93:1–14. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Salzer HJ, Wassilew N, Köhler N, Olaru ID,
Günther G, Herzmann C, Kalsdorf B, Sanchez-Carballo P, Terhalle E,
Rolling T, et al: Personalized Medicine for Chronic Respiratory
Infectious Diseases: Tuberculosis, Nontuberculous Mycobacterial
Pulmonary Diseases, and Chronic Pulmonary Aspergillosis.
Respiration. 92:199–214. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Heaney LG and McGarvey LP: Personalised
Medicine for Asthma and Chronic Obstructive Pulmonary Disease.
Respiration. 93:153–161. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Chung KF: Precision medicine in asthma:
linking phenotypes to targeted treatments. Curr Opin Pulm Med.
24:4–10. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Karampitsakos T, Juan-Guardela BM,
Tzouvelekis A and Herazo-Maya JD: Precision medicine advances in
idiopathic pulmonary fibrosis. EBioMedicine.
95(104766)2023.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Juan-Guardela BM and Herazo-Maya JD:
Immunity, Ciliated Epithelium, and Mortality: Are We Ready to
Identify Idiopathic Pulmonary Fibrosis Endotypes With Prognostic
Significance? Chest. 161:1440–1441. 2022.PubMed/NCBI View Article : Google Scholar
|
|
13
|
De Sadeleer LJ, Verleden SE, Schupp JC,
McDonough JE, Goos T, Yserbyt J, Bargagli E, Rottoli P, Kaminski N,
Prasse A and Wuyts WA: BAL Transcriptomes Characterize Idiopathic
Pulmonary Fibrosis Endotypes With Prognostic Impact. Chest.
161:1576–1588. 2022.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Vassilakis DA, Sourvinos G, Spandidos DA,
Siafakas NM and Bouros D: Frequent genetic alterations at the
microsatellite level in cytologic sputum samples of patients with
idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 162 (3 Pt
1):1115–1119. 2000.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Townsley DM, Dumitriu B, Liu D, Biancotto
A, Weinstein B, Chen C, Hardy N, Mihalek AD, Lingala S, Kim YJ, et
al: Danazol Treatment for Telomere Diseases. N Engl J Med.
374:1922–1931. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Podolanczuk AJ, Kim JS, Cooper CB, Lasky
JA, Murray S, Oldham JM, Raghu G, Flaherty KR, Spino C, Noth I and
Martinez FJ: PRECISIONS Study Team. Design and rationale for the
prospective treatment efficacy in IPF using genotype for NAC
selection (PRECISIONS) clinical trial. BMC Pulm Med.
22(475)2022.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Antoniou KM, Tsitoura E, Vasarmidi E,
Symvoulakis EK, Aidinis V, Tzilas V, Tzouvelekis A and Bouros D:
Precision medicine in idiopathic pulmonary fibrosis therapy: From
translational research to patient-centered care. Curr Opin
Pharmacol. 57:71–80. 2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Thannickal VJ and Antony VB: Is
personalized medicine a realistic goal in idiopathic pulmonary
fibrosis? Expert Rev Respir Med. 12:441–443. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Maher TM, Avram C, Bortey E, Hart SP,
Hirani N, Molyneux PL, Porter JC, Smith JA and Sciascia T:
Nalbuphine tablets for cough in patients with idiopathic pulmonary
fibrosis. NEJM Evid. 2(EVIDoa2300083)2023.PubMed/NCBI View Article : Google Scholar
|