Introduction
Decompressive craniectomy (DC) is considered a
cornerstone in managing refractory intracranial hypertension
(1,2). Proposals suggest that the use of DC can
significantly improve the increased intracranial pressure (ICP)
values by facilitating the expansion of the edematous cerebral
hemispheres (1,3). Furthermore, some researchers have
argued that DC disrupts the vicious cycle of intracranial
hypertension by reducing cerebral perfusion pressure (1,4).
In late 1890, Kocher introduced the concept of
opening-box decompression, which involves removing a variable
amount of calvaria (1). In 1901,
Kocher proposed opening the skull to relieve increased intracranial
pressure, and Cushing (5) performed
a subtemporal DC to treat moribund edema due to an intracranial
neoplastic disorder. Miyazaki (6)
initially described the concept of large cranial and dural
decompression, along with the removal of any underlying
space-occupying lesions in 1971, while Kjellberg and Prieto
(7) refined this surgical technique
in 1971.
For decades, DC was known as an occasionally
lifesaving procedure, associated, however, with numerous serious
complications (8,9). Therefore, the majority of neurosurgeons
were not very eager to incorporate DC into the trauma neurosurgical
armamentarium. Characteristically, in 1968, Clark (10), stated that the only reason for
reporting his experience of performing DCs in patients with of
severe traumatic brain injury (STBI) was to warn other
neurosurgeons to avoid performing similar surgery. Advancements
being made in neuroimaging, prehospital care, and neurointensive
care and rehabilitation services have led to a reconsideration of
DC as the final treatment option for refractory intracranial
hypertension (1,11,12).
In 1999, Guerra et al (13) presented a satisfactory outcome in 56%
of cases with STB treated with DC, leading to the re-introduction
of DC. Since then, the literature has thoroughly discussed the use
of DC in STBI cases, leading to numerous retrospective series and
randomized controlled trial (RCTs), with controversial results
(14,15). Researchers have evaluated this
promising new intervention in various neurosurgical emergencies,
including stroke, malignant middle cerebral artery (MCA) infarcts
(MMCA), acute subarachnoid hemorrhage (SAH), tumor cases, large
intracerebral hemorrhage (ICH), cerebral vein thrombosis (CVT) and
severe intracranial infections (1).
In the literature, there are an ample amount of encouraging studies
with extended indications (1,3,4).
According to Professor Servadei, DC was considered a
panacea (a cure for everything) for various pathological entities
(11). However, DC is an aggressive
amputative procedure that is associated with high mortality rates,
higher morbidity rates and various types of complications (seizure,
subdural hygroma, hydrocephalus, local infection, bone graft
resorption and refractory cerebral edema following cranioplasty)
(16-18).
Conversely, scholars have extensively discussed and assessed the
complications associated with DC (19,20). The
proportion of cases that may have functional outcomes is relatively
small, and that will be feasible after a long-term hospital stay
and long-term, high-quality rehabilitation services (21,22).
Every active neurosurgeon is familiar with the
procedure in everyday practice; however, the ideal candidate who
would benefit from this aggressive amputation procedure is still
under investigation and surrounded by controversy. Therefore, it
remains debatable whether DC is a panacea or whether it is merely
an avenue for other possibilities.
The present study is based on the authors working
experience with DCs. This was a single-center retrospective
case-series study on 321 consecutive patients who underwent DC. The
aim of the present study was to present the authors' experience in
dealing with DCs in a single-center retrospective case-series study
that included a number of different pathologies, each with a
different pathophysiology, clinical course and management, apart
from DC and rehabilitation. In addition, under the prisma of new
and old controversies, the present study aimed to address which
specific patient may benefit from this amputating procedure, as it
is considered that the abundant use of DC is not optimal.
Materials and methods
Study design and population
The present study was a single-center retrospective
study on DC cases. The study included patients that underwent DC in
different neurosurgical entities at the authors' local institution
(University Hospital of Larissa, Larissa, Greece) between January,
2010 and December, 2020. In total, 321 patients that underwent DC
were analyzed in the present study. Data on the age of the
patients, sex, a history of anticoagulant use, diabetes and
hypertension, site and size of DC external ventricular drain (EVD)
placement, post-surgical cases with an ICP ≥20 mmHg, hospital stay
and intensive care unit (ICU) stay, Glasgow outcome scale (GOS) and
mortality were collected. Patients with an age >75 years were
not included (as the comorbidities following craniectomy are
increased). The sex as inclusion criteria was not affected our
study. All patients were divided into four groups as follows: Group
A included patients who suffered from a space-occupying MCA
ischemic event; group B included patients who developed ICH; group
C included patients admitted for TBI; and group D included patients
with other neurosurgical entities that underwent DC, such as SAH,
tumors, brain abscess and cerebral ventricular sinus thrombosis
(CVST) events. These groups were recognized based on the following
demographic, clinical and radiographic data that were reclaimed
from the medical archives when available: Age, sex, a history of
anticoagulant use, diabetes and hypertension, site and size of DC,
EVD placement, post-surgical cases with an ICP ≥20 mmHg, hospital
stay and ICU stay, GOS and mortality (Table I). The exclusion criteria were
patients that succumbed the first 24 h and cases that were
transferred to the ICU in other facilities and thus, lost from the
follow-up. All participants had a follow-up period of 1 to 12 years
from the day of discharge from the hospital. The primary outcome
was defined as a GOS score >2, and the secondary outcomes were
the following: Post-surgical cases with an ICP ≥20 mmHg, hospital
stay, ICU stay and mortality.
 | Table IBaseline demographic characteristics
of the patients. |
Table I
Baseline demographic characteristics
of the patients.
| Parameter | All patients, n=321
(100%) | Group A
(space-occupying MCA event), n=52 (16.1%) | Group B (ICH), n=51
(15.8%) | Group C (TBI),
n=164 (51.0%) | Group D (other),
n=54 (16.8%) | P-value |
|---|
| Age, mean ± SD
(years) | 53.0±19 | 63.0±13 | 62.3±12 | 42.7±19 | 65.7±10 | 0.001b |
| Sex (male), n
(%) | 235 (73.2) | 33 (10.2) | 34 (10.5) | 135 (42.0) | 33 (10.2) | 0.002b |
| Anticoagulant, n
(%) | 74 (23.0) | 13 (4.0) | 15 (4.6) | 34 (10.5) | 12 (3.7) | 0.616 |
| Diabetes, n
(%) | 67 (20.8) | 8 (2.4) | 15 (4.6) | 29 (9.0) | 15 (4.9) | 0.123 |
| Hypertension, n
(%) | 99 (30.8) | 11 (3.4) | 20 (6.2) | 48 (14.9) | 20 (6.2) | 0.163 |
| Site of
craniectomy, n (%) | | | | | | |
|
Right | 121 (37.6) | 18 (5.6) | 18 (5.6) | 63 (19.6) | 22 (6.8) | 0.568 |
|
Left | 173 (53.8) | 30 (9.3) | 32 (9.9) | 84 (26.1) | 27 (8.4) | |
|
Bilateral | 27 (8.4) | 4 (1.2) | 1 (0.3) | 17 (5.2) | 5 (1.5) | |
| Size of
craniectomy, n (%) | | | | | | |
|
Extended
(≥120 cm2) | 231 (71.9) | 33 (10.2) | 33 (10.2) | 129 (40.1) | 36 (11.2) | 0.055 |
|
<120
cm2 | 90 (28.0) | 19 (5.9) | 18 (5.6) | 35 (10.9) | 18 (5.6) | |
| EVD, n (%) | 87 (27.0) | 12 (3.7) | 20 (6.2) | 35 (10.9) | 20 (6.2) | 0.022a |
| Post-surgical ICP,
n (%) | | | | | | |
|
≥20
mmHg | 105 (32.7) | 11 (3.4) | 22 (6.8) | 51 (15.8) | 21 (6.5) | 0.078 |
|
<20
mmHg | 216 (67.2) | 41 (12.7) | 29 (9.0) | 113 (35.2) | 33 (10.2) | |
| Hospital stay, mean
± SD (days) | 42.4±22 | 40.3±22 | 35.1±15 | 43.8±24 | 46.9±24 | 0.062 |
| ICU stay, mean ± SD
(days) | 24.8±16 | 24.9±17 | 21.2±13 | 25.2±16 | 26.8 ±16 | 0.390 |
| GOS, mean ± SD | 2.8±1 | 3.4±1 | 2.5±1 | 2.8±1 | 2.7±1 | 0.003b |
| Mortality, n
(%) | 72 (22.4) | 8 (2.4) | 17 (5.2) | 33 (10.2) | 14 (4.3) | 0.119 |
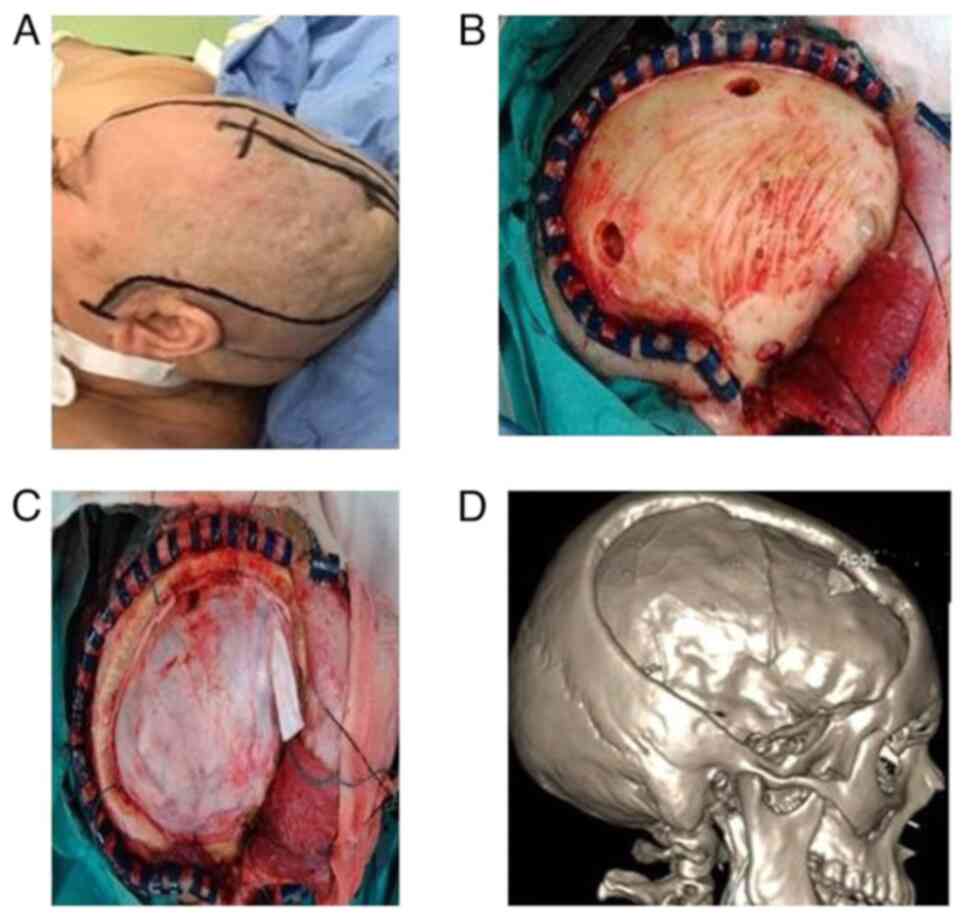
Surgical technique
The literature reports various types of
decompression. DC is unanimously characterized as the typical
fronto-temporo-parietal craniectomy/hemicraniectomy that consists
of theoretically extensive bone resection, exposing practically the
whole underlying cerebral hemisphere (23,24).
The key elements of the procedure included C-spine
precautions during positioning. The extended reverse question mark
skin incision begins at 1 cm in front of the tragus, extends above
and behind the ipsilateral ear, then curves forward 2 cm laterally
from the midline, ending just behind the hairline. The
decompression extends from the floor of the middle cranial fossa to
the zygomatic arch, preserving the superficial temporal artery and
the branches of the facial nerve.
The underlying brain edema causes the wound to close
in anatomic layers, avoiding tension in the skin margins, and
reapproximating the temporalis muscle with a few sutures.
Statistical analysis
Statistical analyses was performed using the
Statistical Package for the Social Sciences (SPSS 11; SPSS, Inc.).
Fisher's exact test was used to compare the categorial variables
between the groups, and the Mann-Whitney U test for the comparison
of continuous data. Data between multiple groups was analyzed using
the Kruskal-Wallis test and Steel-Dwass test. To determine the
independent contribution of risk factors (explanatory variables)
that were statistically significant in the univariable analysis,
such as group A (MCA), size of craniectomy-extended (≥120
cm2, EVD, post-surgical ICP ≥20 mmHg, hospital stay and
mortality, to the development of the GOS (response variable),
multivariable analysis was performed. Thus, the risk of an outcome
(GOS) may be modified by other risk variables or by their
interactions, and these effects can be assessed by multivariable
analysis. Linear regression was used with continuous outcomes,
while logistic regression was used with binary outcomes.
Proportional hazards (Cox) regression analysis was used when the
outcome was the elapsed time to an event. A P-value <0.05 was
considered to indicate a statistically significant difference. The
overall survival time was estimated using Kaplan-Meier analysis and
the log-rank test was used to compare the survival curves between
groups (group A, 52 patients; group B, 51 patients; group C, 164
patients; group D, 54 patients) for a 1-year observation
period.
Results
The present study enrolled a total of 348 patients
who underwent DC (the DC procedure is illustrated in Fig. 1). A total of 27 patients succumbed
within the first 24 h or were transferred to the ICU at other
facilities and were thus lost from the follow-up. The remaining 321
patients were included in the present study: Group A included 52
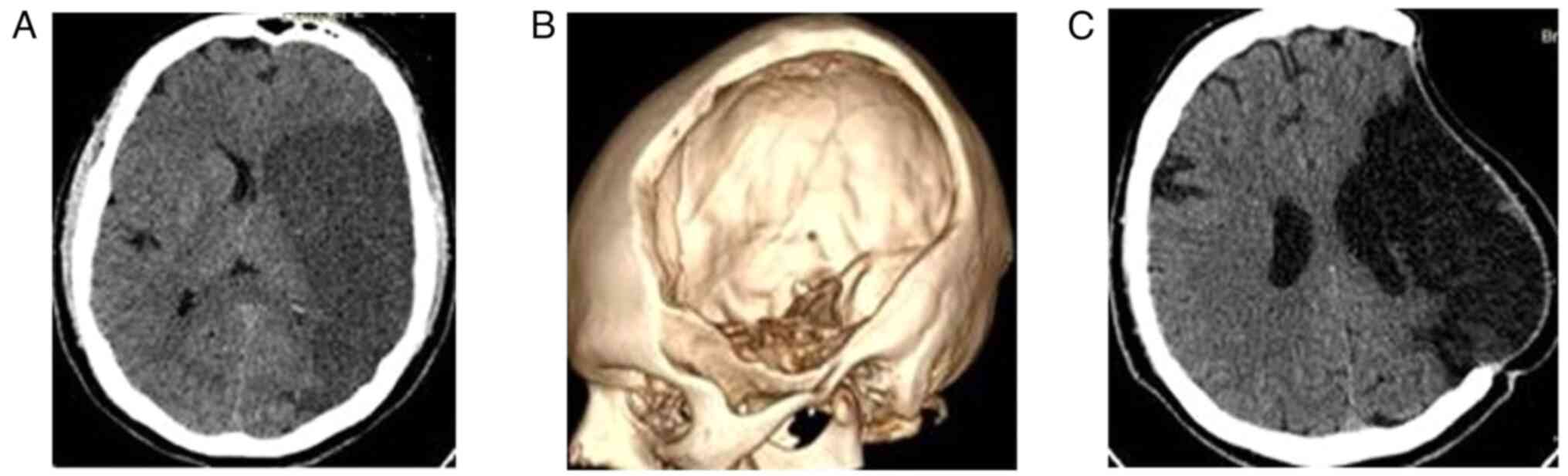
out of the 321 (16.1%) patients (Fig.
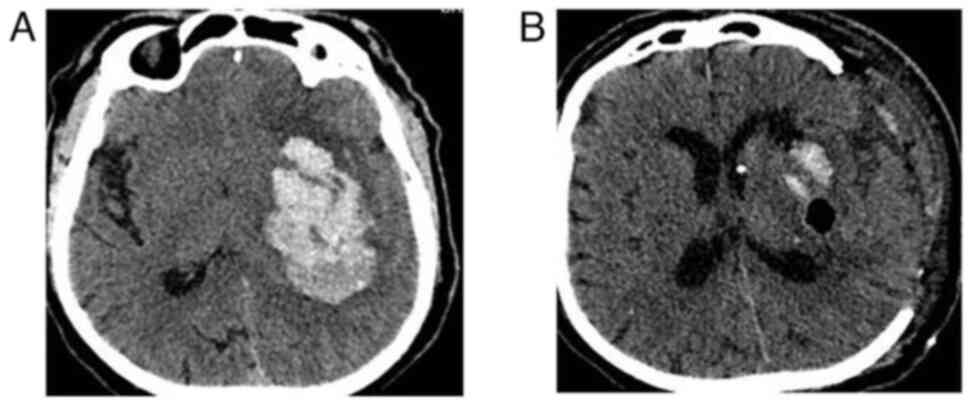
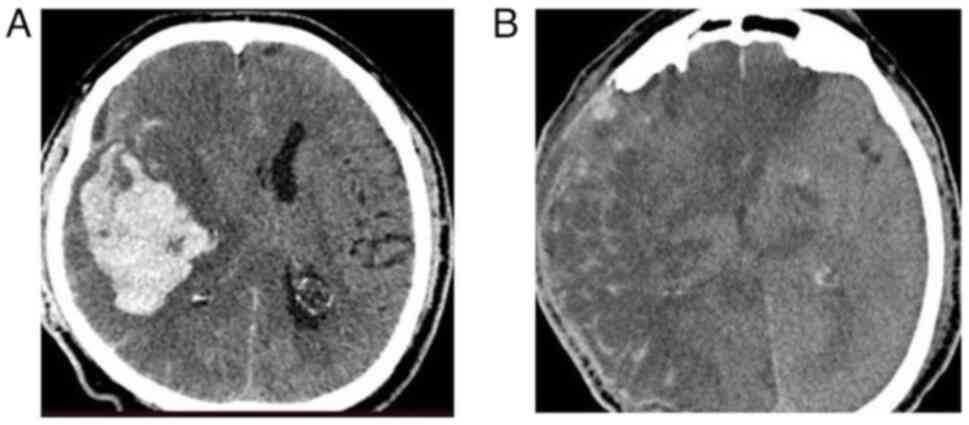
2); group B included 51 (15.8%) patients (Fig. 3); group C included 164 (51.0%)
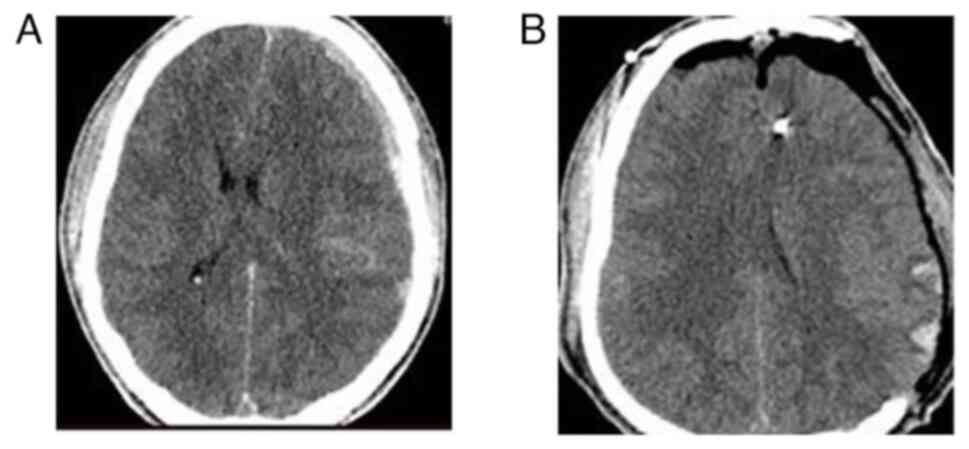
patients (Fig. 4); and group D
included 54 (16.8%) patients (Fig.
5). Of the 321 patients, 235 (73.2%) were males, and the median
age was 53.7 years. The patients taking anticoagulant medication
were 74 of 321 (23%); those with diabetes were 67 of 321 (20.8%);
those with hypertension were 99 of 321 (30.8%); those who underwent
a right craniectomy were 121 of 321 (37.6%); those who underwent a
left craniectomy were 173 of 321 (53.8%); those who underwent a
bilateral craniectomy were 27 (8.4%) and those with EVD placement
were 87 of 321 (27%). The baseline characteristics of the patients
included in the present study are listed in Table I.
Univariate analysis revealed that there was a
statistically significant difference in the patients in group A, in
the size of craniectomy, EVD placement, cases with a post-surgical
ICP ≥20 mmHg, hospital stay and mortality between the participants
who had a GOS score >2 and those who had a GOS score ≤2
(P<0.05, Table II).
 | Table IIUnivariate analysis for GOS. |
Table II
Univariate analysis for GOS.
| Parameters | GOS score >2,
n=211 | GOS score ≤2,
n=110 | P-value |
|---|
| Groups, n (%) | | | |
|
Group A
(space-occupying MCA event) | 41 (12.7) | 10 (3.1) | 0.016a |
|
Group B
(ICH) | 33 (10.2) | 18 (5.6) | 0.866 |
|
Group C
(TBI) | 100 (31.1) | 64 (19.9) | 0.066 |
|
Group D
(Other) | 36 (11.2) | 18 (5.6) | 0.874 |
| Age, mean ± SD
(years) | 53.7±19 | 51.7±19 | 0.396 |
| Sex (male), n
(%) | 150 (46.7) | 85 (26.4) | 0.235 |
| Anticoagulant, n
(%) | 46 (14.3) | 28 (8.7) | 0.461 |
| Diabetes, n
(%) | 48 (14.9) | 19 (5.9) | 0.252 |
| Hypertension, n
(%) | 68 (21.1) | 31 (9.6) | 0.456 |
| Site of
craniectomy, n (%) | | | |
|
Right | 78 (24.2) | 40 (12.4) | 0.782 |
|
Left | 111 (34.5) | 62 (19.3) | |
|
Bilateral | 19 (5.9) | 8 (2.4) | |
| Size of
craniectomy, n (%) | | | |
|
Extended
(≥120 cm2) | 162 (50.4) | 69 (21.4) | 0.008b |
|
<120
cm2 | 49 (15.2) | 41 (12.7) | |
| EVD, n (%) | 48 (14.9) | 39 (12.1) | 0.015a |
| Post-surgical
ICP | | | |
|
≥20
mmHg | 61 (19.0) | 44 (13.7) | 0.044a |
|
<20
mmHg | 150 (46.7) | 66 (20.5) | |
| Hospital stay, mean
± SD (days) | 41.3±23 | 44.4±22 | 0.039a |
| ICU stay, mean ± SD
(days) | 24.0±15 | 26.3±16 | 0.161 |
| Mortality, n
(%) | 3 (0.9) | 69 (21.4) | 0.001b |
The multivariate analysis (Table III) revealed that the group A (MCA)
parameter and mortality were independent factors associated with a
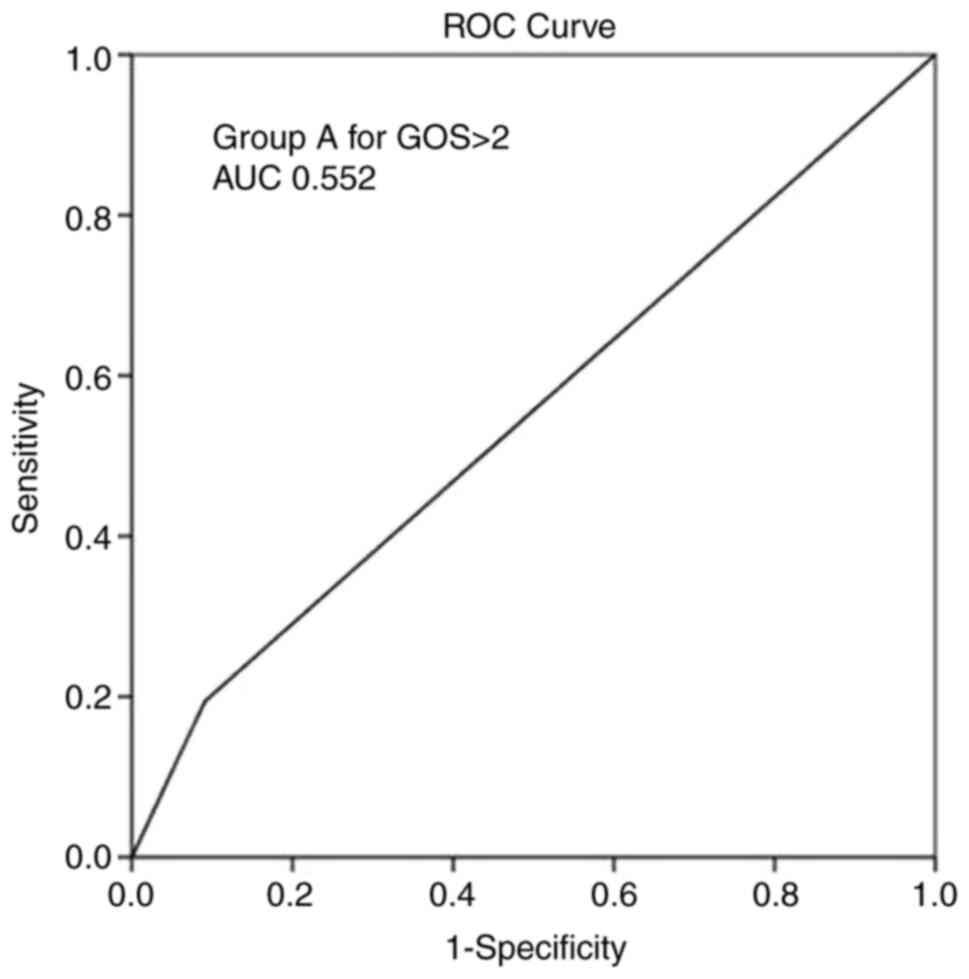
GOS score >2 during follow-up (P<0.05). Overall, ROC analysis
demonstrated that the group A parameter exhibited the optimal
performance to predict a GOS score >2, as evaluated by an area
under the curve standard error [AUC (SE)] of [0.552 (0.033)] and
(P=0.128) (Table IV and Fig. 6).
 | Table IIIMultivariate analysis for a GOS score
>2. |
Table III
Multivariate analysis for a GOS score
>2.
| | 95% CI for Exp
(B) |
|---|
| Parameters | P-value | Exp (B) | Lower | Upper |
|---|
| Groups-group A
(space-occupying MCA event) | 0.030a | 0.089 | 0.011 | 0.219 |
| Size of craniectomy
- extended (≥120 cm2) | 0.657 | -0.020 | -0.112 | 0.071 |
| EVD | 0.675 | 0.019 | -0.076 | 0.117 |
| Post-surgical
ICP-≥20 mmHg | 0.494 | 0.030 | -0.058 | 0.119 |
| Hospital stay | 0.195 | -0.052 | -0.003 | 0.001 |
| Mortality | 0.001b | -0.697 | -0.887 | -0.700 |
 | Table IVROC analysis for GOS. |
Table IV
ROC analysis for GOS.
| Parameters | Area | Std. error | CI (95%)
lower-upper | P-value |
|---|
| Groups-group A
(space-occupying MCA event) | 0.552 | 0.033 | 0.487-0.616 | 0.128 |
Kaplan-Meier (Fig. 7)
analysis estimated the survival rates of the groups of patients
that underwent DC (Table V). The
log-rank test revealed a statistically significant difference
(P=0.006), indicating the difference between the survival times
among the four groups.
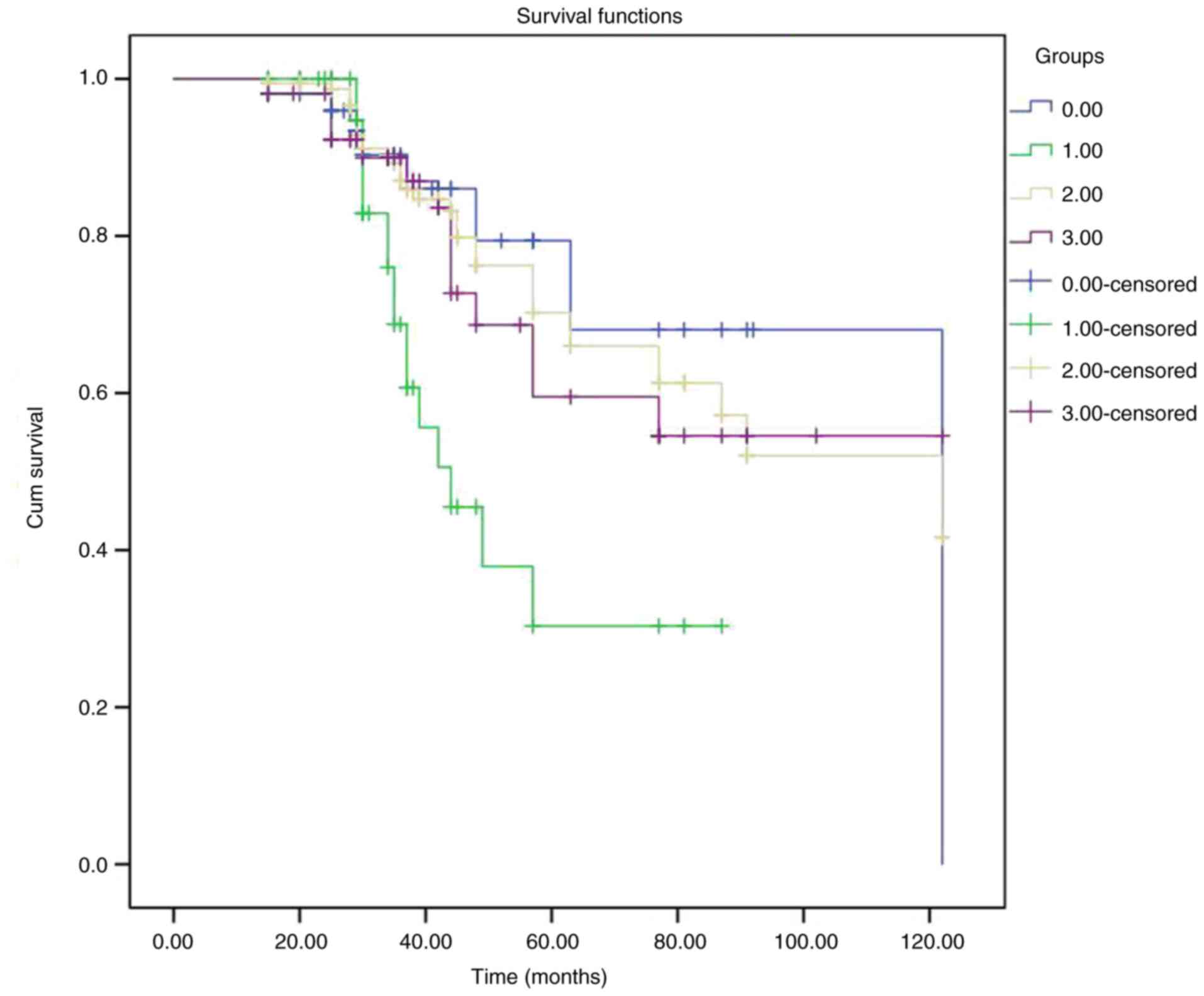 | Figure 7Graph illustrating representative
Kaplan-Meier survival curves of the patients who underwent
decompressive craniectomy and grouped according to different
neurosurgical entities. In the figure, the groups are indicated as
follows: Group A (DC, space-occupying middle cerebral artery
event), blue color; group B (DC, intracerebral hemorrhage), green
color; group C (DC, traumatic brain injury), yellow color; group D
(DC, other entities), purple color. DC, decompressive
craniectomy. |
 | Table VKaplan-Meier analysis for patient
survival. |
Table V
Kaplan-Meier analysis for patient
survival.
| A, Mortality and
groups |
|---|
| Parameters | Estimate
(days) | Std. error
(days) | CI (95%)
lower-upper |
|---|
| Group A
(space-occupying MCA event) | 97.41 | 9.4 | 78.85-115.98 |
| Group B (ICH) | 53.69 | 5.0 | 43.84-63.55 |
| Group C (TBI) | 90.12 | 4.6 | 80.92-99.32 |
| Group D
(other) | 87.28 | 7.2 | 73.00-101.56 |
| B, Test of equality
of survival distributions in the different groups |
| | Chi-squared test
value | P-value | |
| Log-rank
(Mantel-Cox) | 12.39 | 0.006a | |
Discussion
The results of the present study suggest that among
patients who underwent DC with different neurological entities,
those who experienced MCA events had more favorable outcomes with a
GOS score >2 and lower mortality rates. Thus, the role of DC in
daily clinical practice may be more efficient for these
patients.
The literature demonstrates highly variable DC
outcome rates in different entities (25). Tagliaferri et al (25) retrospectively analyzed 526
consecutive cases treated with DC, reporting poor outcomes in 77%
of the cases, which escalated to 93% in patients over 65. For
patients aged 18 to 65 years, they stated that the only
statistically significant parameters were age, the time of
decompression and tge size of the bone flap (25). Goedemans et al (26) presented 204 cases of DC, reporting a
range of functional outcomes from 91% in CVT cases to 0% in SAH
cases with ischemia; 26% of the cases with STBI and 39% of the
stroke cases survived independently. Kapapa et al (27) assessed 134 cases with various
entities treated with DC, and concluded that the outcomes after DC
do not differ significantly among patients with different
pathologies.
STBI
The literature extensively assesses the use of DC in
patients with STBI (27), using
RCTs, numerous retrospective series, consensus and guidelines
(12,24,28-31).
The results, conclusions and limitations of recently published RCTs
are thoroughly considered in the literature (12,24,28-31).
The RCTs for STBI have established that DC does indeed lower ICP
rates and occasionally, even mortality rates; however, the majority
of survivors fail to achieve optimal functional outcomes (28-31).
It is important to emphasize that DC does not reverse the effects
of the disease, and factors affecting the outcome include age,
other concomitant injuries, co-morbidities, chronic medication,
substance abuse and the genetic profile of patients. High-quality
ICU and rehabilitation services are detrimental in order to achieve
the desired outcomes.
There is an increase in the number of survivors in a
vegetative state. Thus, there is a belief that DC translates
mortality into survival with severe disability and dependency
(21,22,31-33).
Furthermore, in the literature, there is ample criticism about the
vast differences and equalities in the management and outcome of
cases with STBI between middle-income countries (MICs) and
low-middle-income countries (LMICs) (21,22,31-33).
Despite the fact that LMICs and health systems with limited
resources report 90% of trauma-related deaths, there is minimal or
no participation in RCTs, particularly in the ICU, and the
availability of rehabilitation is crucial (21,22,31-33).
DC continues to be proposed as a prophylactic measure for the
management of STBI, where sophisticated monitoring resources are
not feasible (28). Clinical
practice has undergone a reassessment due to the lack of solid
evidence and the heterogeneity of STBI worldwide. It is recommended
that therapy be individualized following a patient-centered
discussion about realistic outcome expectations (34).
MMCA
In the era of thrombolysis and thrombectomy, DC for
stroke has earned a distinguished place, particularly in cases of
MMCA. DC decreases ICP, improves perfusion and blood flow, and
reduces mortality rates. The RCTs of the past two decades have
revealed a significant benefit in functional outcomes in all
predefined subgroups (35). In the
majority of cases, that study reported good outcomes, with a GOS
score >2 in 78% of the cases (35). Older patients have a lower margin of
benefit (35). There is no evidence
that DC affects outcomes when delayed 48 to 92 h following the
onset of stroke, but surely before herniation (35). As for dominant infarcts, there is a
bias toward worse outcomes related to aphasia; the literature does
not support withholding DC based on laterality (36). The decision to perform DC should not
be based on ICP values, but if available, post-DC ICP monitoring
may be useful (36). Predictors of
malignant edema and optimizing provider settings are the key
elements of achieving the optimal outcome possible.
ICH
ICH constitutes a devastating disease with high
mortality and morbidity rates (37).
Yao et al (37), in their
systematic review and meta-analysis, stated that DC effectively
reduced mortality in cases with ICH, and that DC may improve
functional outcomes in certain populations and warrants further
verification. In the present study, the results are not so
disheartening; however, there is probably a bias, since older
individuals and patients with major co-morbidities were not
subjected to DC. Additionally, decompression was only applied when
the Glasgow coma scale (GCS) score decreases, and certainly not
before herniation.
Minimally invasive procedures may be feasible in
elective cases. DC with or without hematoma evacuation should be
considered as a life-saving approach when neurological
deterioration is evolving (38).
Other neurosurgical entities
There are only retrospective case series available
that evaluate other neurosurgical entities treated with DC,
including SAH, tumors, brain abscesses and CVST (39). However, the pertinent literature
reports a wide variation in clinical outcomes and ill-defined
indications for DC in patients (39). RCTs have not provided solid evidence
(28-31,35)
that DC can ameliorate functional outcomes in the other entities.
In the literature, there are highly variable rates of complication
mortality morbidity; there is a trend that cases with an SAH have
poorer prognoses (39). The results
of the present study are positive. Notably, there are case reports
assessing DC in entities, such as encephalopathies and diabetic
ketoacidosis (40,41).
Complications
DC has been associated with high mortality rates,
mainly due to the severity of the underlying trauma, as well as
numerous and occasionally severe complications (20). Researchers have extensively studied
and reported peri-operative, early post-operative and late
post-operative complications (19,20).
Researchers have identified several factors that
predispose to the development of DC-associated complications
(20). These include a low GCS score
upon admission, the age of the patient, co-morbidity, and the
systematic pre-operative anticoagulant administration (20).
Cranioplasty
A drawback of DC is that a second surgical
intervention is required to repair the bone defect (42). There are a number of colleagues who
consider that cranioplasty is the normal sequel of DC and that the
surgical procedure of CP is an extension of DC. Without any medical
contraindications, we should make every effort to perform CP after
DC.
Although CP is considered a straightforward
procedure, it is well documented that it is associated with
considerable complication rates that are widely variable in the
literature (42-44).
DC and survival for different
pathologies
When comparing DC for different pathologies, DC for
MCA stroke has a trend toward improved outcomes, probably due to
the high alert of physicians who address cases to neurosurgical
facilities on time and before herniation. The benefit of DC in
cases with malignant MCA stroke is well-established in the
literature, and studies have documented significant benefits in
functional outcomes (35,36). The different groups assessed are
highly variable In subsequent pathophysiology and further clinical
management, and the categorization in these groups may be
arbitrary. The present study reported disheartening results for the
ICH cases. ICH constitutes a devastating disease, and the reason to
perform DC is lethal intracranial hypertension; without DC the
outcome would be a GOS score of 1 or 2.
The present study has certain limitations which
should be mentioned. The present study harbors all the limitations
of a single-center retrospective case-series study. The
heterogeneity of the population, different pathologies with unique
pathophysiological sequelae, and diverse treatment modalities other
than DC cannot surely fit into one basket. In addition, the
different groups assessed are highly variable. In subsequent
pathophysiology and further clinical management, the categorization
in these groups may be arbitrary; the authors aimed to report their
experience in these complex cases and to reveal any trends that may
be clinically significant.
In the near future, novel evaluation methods need to
be endorsed, as optimizing medical and neurocritical care, optimal
provider settings and high-quality rehabilitation services are
detrimental to ameliorating the outcome. Determining functional
outcomes via the modified Rankin scale, GOS and the GOS extended
may not be capable of sufficiently describing all aspects of
patients, families, caregivers, surgeons and health system
expectations.
Current medicine regularly discusses the major issue
of quality of life. Furthermore, the socioeconomic effects create a
heavy burden that challenges even sophisticated and plentiful
health systems. Environments with limited capabilities drastically
exacerbate this issue. These vast distances and differences between
HMICs and LMICs constitute a highly controversial topic (21,22,32,33).
Subpopulations should also receive special attention due to
age-limit comorbidities (34).
Despite the clear postulation that DC cannot cure
unsalvageable patients while providing them with a reasonable
quality of life, overtreatment remains a contentious issue
(8,9).
In conclusion, DC is an aggressive amputation
procedure with numerous complications that effectively reduces ICP
values and mortality rates. However, the impact on functional
outcomes and the quality of life of survivors remains highly
controversial, and the current evidence has several limitations.
Furthermore, individuals demand high-quality mandatory
rehabilitation services and prolong recovery times. It is difficult
to withhold DC from a young patient who develops refractory
intracranial hypertension and has at least some chance of survival
with an accepted disability. One cannot overemphasize the
importance of individualized treatment and patient-centered
discussions. However, before performing one, the well-documented
risks and drastic complications of DC must be seriously considered.
In carefully selected cases, these risks may outweigh the expected
benefits. Finally, the excessive use of DC could be more
optimal.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CG and GF conceptualized the study. VEG, CG, AT, GC,
TS, AK, KP, GF, NT, PS and KNF analyzed the data, and wrote and
prepared the draft of the manuscript. VEG and GF provided critical
revisions. All authors contributed to manuscript revision and have
read and approved the final version of the manuscript. GF and CG
confirm the authenticity of all the raw data. All authors
contributed to manuscript revision and have read and approved the
final version of the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Review Board (IRB) of the University of Thessaly, Greece/The School
of Medicine/School of Health Sciences approved the study (approval
IRB no. 12783/20-05-2020). The present study was in line with the
Declaration of Helsinki (1995; as revised in Edinburgh 2000).
Written informed consent was obtained from all included patients or
their next-of-kin before the surgery.
Patient consent for publication
Written informed consent was obtained from all
included patients or their next-of-kin before surgery for the
publication of their data and any related images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kolias AG, Kirkpatrick PJ and Hutchinson
PJ: Decompressive craniectomy: Past, present and future. Nat Rev
Neurol. 9:405–415. 2013.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Fotakopoulos G, Tsianaka E, Vagkopoulos K
and Fountas KN: According to which factors in severe traumatic
brain injury craniectomy could be beneficial. Surg Neurol Int.
7(19)2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Fotakopoulos G, Tsianaka E, Siasios G,
Vagkopoulos K and Fountas K: Posttraumatic hydrocephalus after
decompressive craniectomy in 126 patients with severe traumatic
brain injury. J Neurol Surg A Cent Eur Neurosurg. 77:88–92.
2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Pachatouridis D, Alexiou GA, Zigouris A,
Michos E, Drosos D, Fotakopoulos G and Voulgaris S: Management of
hydrocephalus after decompressive craniectomy. Turk Neurosurg.
24:855–858. 2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Cushing H: The establishment of cerebral
hernia as a decompressive measure for inaccessible brain tumors;
with the description of intramuscular methods of making the bone
defect in temporal and occipital regions. In: Surgery, Gynecology
and Obstetrics. Vol I, No. 4. North Books: Used & Rare, St.
Louis, MO, pp297-314, 1905.
|
|
6
|
Miyazaki Y: Bifrontal external
decompression for traumatic brain edema. Jpn J Surg. 1:83–92.
1971.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kjellberg RN and Prieto A Jr: Bifrontal
decompressive craniotomy for massive cerebral edema. J Neurosurg.
34:488–493. 1971.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Cooper PR, Hagler Hs, Clark WK and Barnett
P: Enhancement of experimental cerebral edema after decompressive
craniectomy: Implications for the management of severe head
injuries. Neurosurg. 4:296–300. 1979.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Cooper PR, Rovit RL and Ransohoff J:
Hemicraniectomy in the treatment of acute subdural hematoma: A
re-appraisal. Surg Neurol. 5:25–28. 1976.PubMed/NCBI
|
|
10
|
Clark K, Nash TM and Hutchison GC: The
failure of circumferential craniotomyin acute traumatic cerebral
swelling. J Neurosurg. 29:367–371. 1968.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Rossini Z, Nicolosi F, Kolias AG,
Hutchinson PJ, De Sanctis P and Servadei F: The history of
decompressive craniectomy in traumatic brain injury. Front Neurol.
10(458)2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Sahuquillo J and Dennis JA: Decompressive
craniectomy for the treatment of high intracranial pressure in
closed traumatic brain injury. Cochrane Database Syst Rev.
12(CD003983)2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Guerra WK, Gaab MR, Dietz H, Mueller JU,
Piek J and Fritsch MJ: Surgical decompression for traumatic brain
swelling: Indications and results. J Neurosurg. 90:187–196.
1999.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Cooper DJ, Rosenfeld JV, Murray L, Arabi
YM, Davies AR, D'Urso P, Kossmann T, Ponsford J, Seppelt I, Reilly
P, et al: Decompressive craniectomy in diffuse traumatic brain
injury. N Engl J Med. 364:1493–1502. 2011.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Hutchinson PJ, Kolias AG, Timofeev IS,
Corteen EA, Czosnyka M, Timothy J, Anderson I, Bulters DO, Belli A,
Eynon CA, et al: Trial of decompressive craniectomy for traumatic
intracranial hypertension. N Engl J Med. 375:1119–1130.
2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Honeybul S, Ho KM, Lind CRP and Gillett
GR: The current role of decompressive craniectomy for severe
traumatic brain injury. J Clin Neurosci. 43:11–15. 2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Honeybul S and Ho KM: The current role of
decompressive craniectomy in the management of neurological
emergencies. Brain Inj. 27:979–991. 2013.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Kolias AG, Viaroli E, Rubiano AM, Adams H,
Khan T, Gupta D, Adeleye A, Iaccarino C, Servadei F, Devi BI and
Hutchinson PJ: The current status of decompressive craniectomy in
traumatic brain injury. Curr Trauma Rep. 4:326–332. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Stiver SI: Complications of decompressive
craniectomy for traumatic brain injury. Neurosurg Focus.
26(E7)2009.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Kurland DB, Khaladj-Ghom A, Stokum JA,
Carusillo B, Karimy JK, Gerzanich V, Sahuquillo J and Simard JM:
Complications associated with decompressive craniectomy: A
systematic review. Neurocrit Care. 23:292–304. 2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Tropeano MP, Spaggiari R, Ileyassoff H,
Park KB, Kolias AG, Hutchinson PJ and Servadei F: A comparison of
publication to TBI burden ratio of low- and middle-income countries
versus high-income countries: How can we improve worldwide care of
TBI? Neurosurg Focus. 47(E5)2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Mohan M, Layard Horsfall H, Solla DJF,
Robertson FC, Adeleye AO, Teklemariam TL, Khan MM, Servadei F, Khan
T, Karekezi C, et al: Decompressive craniotomy: An international
survey of practice. Acta Neurochir (Wien). 163:1415–1422.
2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Timofeev I, Santarius T, Kolias AG and
Hutchinson P: Decompressive craniectomy-operative technique and
perioperative care. J Adv Tech Stand Neurosurg. 38:115–136.
2012.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Hawryluk GWJ, Rubiano AM, Totten AM,
O'Reilly C, Ullman JS, Bratton SL, Chesnut R, Harris OA, Kissoon N,
Shutter L, et al: Guidelines for the management of severe traumatic
brain injury: 2020 update of the decompressive craniectomy
recommendations. Neurosurgery. 87:427–434. 2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Tagliaferri F, Zani G, Iaccarino C, Ferro
S, Ridolfi L, Basaglia N, Hutchinson P and Servadei F:
Decompressive craniectomies, facts and fiction: A retrospective
analysis of 526 cases. Acta Neurochir (Wien). 154:919–926.
2012.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Goedemans T, Verbaan D, Coert BA, Kerklaan
BJ, van den Berg R, Coutinho JM, van Middelaar T, Nederkoorn PJ,
Vandertop WP and van den Munckhof P: Neurologic outcome after
decompressive craniectomy: Predictors of outcome in different
pathologic conditions. World Neurosurg. 105:765–774.
2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Kapapa T, Brand C, Wirtz CR and Woischneck
D: Outcome after decompressive craniectomy in different
pathologies. World Neurosurg. 93:389–397. 2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Servadei F: Clinical value of
decompressive craniectomy. N Engl J Med. 364:1558–1559.
2011.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Hutchinson PJ, Kolias AG, Tajsic T,
Adeleye A, Aklilu AT, Apriawan T, Bajamal AH, Barthélemy EJ, Devi
BI, Bhat D, et al: Consensus statement from the international
consensus meeting on the role of decompressive craniectomy in the
management of traumatic brain injury: Consensus statement. Acta
Neurochir (Wien). 161:1261–1274. 2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Smith M, Servadei F and Hutchinson PJ:
What is new in decompressive craniectomy in neurological
emergencies: The good, the bad and the ugly. Intensive Care Med.
46:1023–1026. 2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Barthélemy EJ, Melis M, Gordon E, Ullman
JS and Germano IM: Decompressive craniectomy for severe traumatic
brain injury: A systematic review. World Neurosurg. 88:411–420.
2016.PubMed/NCBI View Article : Google Scholar
|
|
32
|
De Silva MJ, Roberts I, Perel P, Edwards
P, Kenward MG, Fernandes J, Shakur H and Patel V: CRASH Trial
Collaborators. Patient outcome after traumatic brain injury in
high-, middle- and low-income countries: Analysis of data on 8927
patients in 46 countries. Int J Epidemiol. 38:452–458.
2009.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Rubiano AM, Carney N, Khan AA and Ammirati
M: The role of decompressive craniectomy in the context of severe
traumatic brain injury: Summary of results and analysis of the
confidence level of conclusions from systematic reviews and
meta-analyses. Front Neurol. 10(1063)2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Honeybul S: Balancing the short-term
benefits and long-term outcomes of decompressive craniectomy for
severe traumatic brain injury. Expert Rev Neurother. 20:333–340.
2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Powers WJ, Rabinstein AA, Ackerson T,
Adeoye OM, Bambakidis NC, Becker K, Biller J, Brown M, Demaerschalk
BM, Hoh B, et al: Guidelines for the early management of patients
with acute ischemic stroke: 2019 update to the 2018 guidelines for
the early management of acute ischemic stroke: A guideline for
healthcare professionals from the american heart
association/American stroke association. Stroke. 50:e344–e418.
2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Räty S, Georgiopoulos G, Aarnio K,
Martinez-Majander N, Uhl E, Ntaios G and Strbian D: Hemicraniectomy
for dominant vs. nondominant middle cerebral artery infarction: A
systematic review and meta-analysis. J Stroke Cerebrovasc Dis.
30(106102)2021.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Yao Z, Ma L, You C and He M: Decompressive
craniectomy for spontaneous intracerebral hemorrhage: A systematic
review and meta-analysis. World Neurosurg. 110:121–128.
2018.PubMed/NCBI View Article : Google Scholar
|
|
38
|
de Oliveira Manoel AL: Surgery for
spontaneous intracerebral hemorrhage. Crit Care.
24(45)2020.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Darkwah Oppong M, Golubovic J, Hauck EF,
Wrede KH, Sure U and Jabbarli R: Decompressive craniectomy in
aneurysmal subarachnoid hemorrhage: Who and when?-A systematic
review and meta-analysis. Clin Neurol Neurosurg.
199(106252)2020.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Granget E, Milh M, Pech-Gourg G, Paut O,
Girard N, Lena G and Scavarda D: Life-saving decompressive
craniectomy for acute disseminated encephalomyelitis in a child: A
case report. Childs Nerv Syst. 28:1121–1124. 2012.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Nguyen HS, Callahan JD and Cohen-Gadol AA:
Life-saving decompressive craniectomy for diffuse cerebral edema
during an episode of new-onset diabetic ketoacidosis: Case report
and review of the literature. Childs Nerv Syst. 27:657–664.
2011.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Iaccarino C, Kolias A, Adelson PD, Rubiano
AM, Viaroli E, Buki A, Cinalli G, Fountas K, Khan T, Signoretti S,
et al: Consensus statement from the international consensus meeting
on post-traumatic cranioplasty. Acta Neurochir (Wien). 163:423–440.
2021.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Iaccarino C, Kolias AG, Roumy LG, Fountas
K and Adeleye AO: Cranioplasty following decompressive craniectomy.
Front Neurol. 10(1357)2020.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Stefini R, Esposito G, Zanotti B,
Iaccarino C, Fontanella MM and Servadei F: Use of ‘custom made’
porous hydroxyapatite implants for cranioplasty: Postoperative
analysis of complications in 1549 patients. Surg Neurol Int.
4(12)2013.PubMed/NCBI View Article : Google Scholar
|