1. Introduction
The selection of the appropriate venous access
device often depends on the condition of the patient, the duration
of therapy, the nature of the infusate, the potential risk of
complications and whether venous access is elective or emergent
(1,2). Each access device poses certain
benefits over others, and healthcare providers often select them
based on clinical factors, availability and anticipated
complications (Table I) (1). For instance, peripheral intravenous
catheters (PIVCs) are suitable for short-term therapies; however,
they are less durable than more invasive centrally inserted
catheters. Peripherally inserted central catheters (PICCs) have
prolonged dwelling times, rendering them more suitable for
long-term therapies, such as long-term antibiotic treatment
(3). Centrally inserted central
catheters (CICCs) are often preferred for patients in critical care
settings or during surgical procedures, given the ease of insertion
and larger lumens.
 | Table ISummary of various venous access
devices. |
Table I
Summary of various venous access
devices.
| Device type | Insertion site and
tip location | Dwell time | Notes |
|---|
| Peripheral IV
catheters | Peripheral
veins | 72-96 h | Ideal for
short-term therapies; high failure rate and increased risk of
phlebitis and mechanical complications with prolonged use. |
| Long peripheral
catheters | Peripheral
veins | Up to 4 weeks | Useful in patients
with poor IV access; extended dwell time compared to PIVCs. |
| Midline
catheters | Larger peripheral
veins (e.g., basilic, brachial, or cephalic) with tip in axillary
or subclavian vein | 6-14 days | Lower incidence of
phlebitis than PIVCs; contraindicated for vesicant
medications. |
| Peripherally
inserted central catheters | Peripheral veins
(typically basilic or cephalic) with tip positioned at the
cavoatrial junction | Several weeks to 6
months | Preferred for
long-term IV therapies (e.g., antibiotics); reduces hospitalization
costs, not recommended if therapy lasts <5 days. |
| Non-tunneled
centrally inserted central catheters | Inserted in central
veins (e.g., internal jugular, subclavian, or femoral) with tip in
the central circulation | Days to 2
weeks | Employed in
critical care settings for ease of insertion and multi-lumen
access. |
| Tunneled centrally
inserted central catheters | Inserted in central
veins (e.g., internal jugular, subclavian, or femoral) with tip in
the central circulation | Weeks to
months | Tunneled catheters
reduce infection risk and have more secure fixation. Can provide
long term dialysis access. |
| Implantable
ports | Surgically
implanted on the chest wall accessing the subclavian vein with tip
in central circulation | Months to
years | Commonly used in
oncology for chemotherapy; improve patient comfort and allow normal
daily activities. |
The present narrative review discusses the different
types of venous access devices, including PIVCs, long peripheral
catheters, midline catheters, CICCs and implantable ports. The aim
of the present review was to provide a comprehensive understanding
of the indications and current evidence-based recommendations for
venous access devices to assist junior healthcare providers in
making informed decisions through delineating the appropriate
clinical conditions in which each access device is indicated.
Vascular access in specific patient population, such as those
receiving chemotherapy, hemodialysis or undergoing hemodynamic
monitoring are also discussed. In addition, some of the common
adverse events and complications, such as infections and thrombosis
associated with venous catheters are discussed.
2. Data collection methods
For the purpose of the present narrative review
article, the ‘best-evidence synthesis’ set forth by Slavin
(4) was followed and the PubMed and
Google Scholar databases were searched to identify articles from
1990 through November 1, 2024. The following medical subject
headings were used: Venous access devices, venous catheters,
peripheral intravenous catheters, long peripheral catheters,
midline catheters, central venous access, peripherally inserted
central catheters, ultrasound guided venous access, central line
associated blood stream infection, catheter related blood stream
infection and catheter associated thrombosis. The snowball
technique was implemented, whereas the bibliographies of the
retrieved articles were analyzed for other relevant articles.
Search results were filtered for articles in the English language
only. Of note, two authors (MA and KTJ) performed the search. Of
the 882 articles identified, 94 articles were selected. Article
selection involved screening the titles and abstracts for
eligibility, followed by a full-text review to assess relevance to
venous access devices. Disagreements regarding study inclusion were
resolved by a consensus between the authors in consultation with a
third author (HA). For inclusion, only peer reviewed articles were
selected. Articles were selected based on relevance to current
clinical practice educational value and the degree of evidence.
Randomized clinical trials, large longitudinal observational
studies, and more recent articles were prioritized. The artwork was
independently created by the authors.
3. Peripheral venous access
Peripheral intravenous catheters
Short peripheral intravenous catheters (PIVCs),
commonly referred to as ‘peripheral IVs’ are a mainstay in
healthcare. Common insertion sites include the median antecubital,
cephalic or basilic veins (2). The
adoption of ultrasound-guided peripheral intravenous access has
markedly reduced the number of insertion attempts and has improved
the success rates by 2- or 3-fold when compared to the traditional
blind technique (2,5,6). A
universal Birmingham gauge-specific coloring system has been
adopted, which allows for the rapid identification of PIVC size,
which ranges from 14 to 30 gauge (5). Accordingly, flow rates differ
considerably, from 13 ml/min in a 26-gauge catheter to a robust 270
ml/min in a 14-gauge catheter, rendering a wide gauge PIVC an ideal
option for early resuscitation efforts. However, one must also
acknowledge that such catheters have their limitations in
resuscitation; for instance, catheters <23 gauge are associated
with hemolysis when blood is transfused (7). The benefits of short PIVCs include the
ease of insertion, the immediate initiation of therapy and
cost-effectiveness. However, the dwell time of short PIVCs is
relatively limited, typically between 72 to 96 h, after which the
risk of complications, such as phlebitis and mechanical
complications significantly increases (2,8).
Notably, ~19% of patients develop phlebitis (9). It is recommended that the short PIVC be
removed at the first sign of phlebitis (5).
The microbial colonization of peripheral catheters
can occur within 24 h of placement; however, this does not
necessarily reflect a clinical infection (8). Although the incidence of short
PIVC-associated infections is underreported, the incidence is
reported to be at ~2% (10). One
factor that affects the risk of short PIVC-associated infections is
the type of material catheters are made from. Catheters made from
teflon or polyurethane have lower rates of infection than those
made of polyvinyl chloride or polyethylene (11).
Short PIVCs are also susceptible to mechanical
complications, such as dislodgement, infiltration, extravasation,
or occlusion (2,5,8-10).
Infiltration refers to the unintentional leakage of a non-vesicant
solution into the surrounding tissue, while extravasation is the
leakage of a vesicant solution. Extravasation and infiltration are
caused by several factors, including the migration of the catheter
out of the vein over time, incomplete threading into the vein
during insertion, or passing through the vein and out into the
surrounding tissues (5,12). The management of infiltration and
extravasation usually depends on the pharmacological properties of
the infused solution and specific institutional guidelines
(12,13)
Integrated short peripheral catheters have been
introduced to overcome the mechanical complications associated with
short PIVC. Integrated catheters are described as closed system
catheters that are winged, non-ported, and equipped with
preassembled extension and preassembled needle-free connector that
have added safety mechanisms against needle stick injury prevention
(14). These catheters have a
prolonged dwell time of 6 days when compared to standard PIVC, as
well as a statistically significant reduction in the risk of
catheter failure as and occlusion (15).
Long peripheral catheters
Long peripheral catheters (LPCs) are 6-15 cm in
length and are typically inserted into the forearm or upper arm and
the distal catheter tip terminates before reaching the axilla
(16). They are recommended in
patients with difficult IV access due to the ease of their
insertion, where it has been reported that average insertion time
ranges from 8 to 16.8 min (17).
Dwell time is recommended to not exceed 4 weeks (18). Such catheters are a novelty in the
current era, and significant confusion exists regarding their
nomenclature (16). Nonetheless,
LPCs had lower failure rates and longer dwell times when compared
to PIVCs (17).
Midline catheters
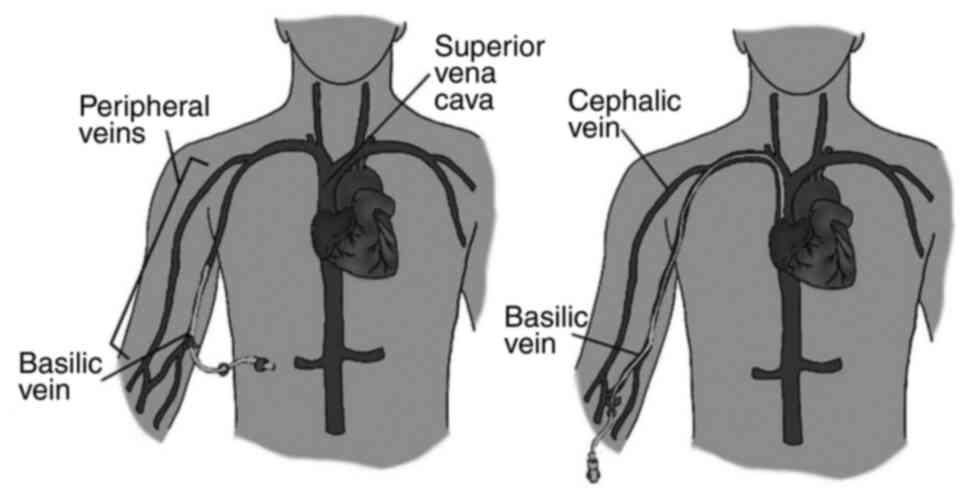
Midline catheters (MCs) are peripheral venous access
devices typically ranging from 15 to 25 cm in length (16). They are inserted into upper arm with
the catheter tip positioned at or slightly below the level of the
axillary vein, as illustrated in Fig.
1 (2,19). Such catheters are used primarily in
patients requiring intermediate term intravenous therapy of around
6-14 days (20). Their prevalence is
dependent on institutional protocols, clinician preferences and
healthcare settings (21). Such
catheters access larger veins, and are therefore advantageous for
those with difficult venous access.
Compared to PIVCs, MCs are associated with a lower
incidence of phlebitis, thereby enhancing patient satisfaction and
decreasing the need for catheter replacement (21). The rate of catheter-related
bloodstream infections (CRBSIs) in MCs aligns with that seen in
PIVCs but is significantly lower than that of CICCs (21). Additionally, patients with difficult
access to healthcare or poor adherence to infection control
protocols might not be ideal candidates for an MC (22).
Despite their advantages, MCs have specific
limitations. They are not recommended for the administration of
vesicant or highly irritating medications, such as chemotherapy
agents, which could potentially damage the peripheral veins
(2,21). A notable decline in their use has
been noted after the introduction of PICCs, which offer longer
dwell times and lower overall complication rates (23). However, midlines were associated with
a lower risk of bloodstream infection and occlusion compared with
PICC when duration of use is less than 10 days (21). Yet, one must acknowledge that the
lower complication risk of the midline is likely due to their lower
dwell time when compared to PICCs rather than an inherit catheter
property.
4. Central venous access
Central venous access devices (CVADs) are indwelling
catheters that are inserted into large, central veins. Catheter
diameters are measured using the French gauge system, which has
uniform increments of 1/3 mm between sizes, unlike the non-uniform
Birmingham gauge system developed for industrial wire sizes, and
adapted for medical use to describe the outer diameter of the
catheter. A lower French-gauge number indicates a larger catheter
diameter.
As per the World Conference on Vascular Access
(WoCoVA) definitions, the position of the tip of the catheter is
inside the superior vena cava, inside the right atrium, or inside
the inferior vena cava (1) The types
of CVADs include tunneled and non-tunneled CICCs, PICCs and
implantable ports. Notably, the terms CICC and Centrally Inserted
Venous Catheter (CIVC) are used interchangeably depending on the
area of practice. This type of access is typically utilized when
patients require multiple simultaneous intravenous therapies and
when central venous system access is required, such as in dialysis.
Other indications include the need for recurrent blood sample
collection when peripheral sampling is challenging, the
administration of parenteral nutrition, plasmapheresis and central
venous pressure monitoring (24).
Such devices are mainly placed at bedside under
sterile conditions. Following catheter implantation, a chest
radiograph is typically performed to verify the correct positioning
of the catheter and to identify any potential complications, such
as pneumothorax or mispositioning (24). However, the experience of one center
suggests against using postprocedural X-rays, given the exceedingly
rare complications and excessively high costs (25). The intraprocedural assessment of the
position of the catheter tip can be performed through fluoroscopy,
intracavitary ECG or echocardiography (26-28).
One commonly encountered indication for central
access has been vasopressor medication administration. Policies
requiring CVAD vary significantly across health care systems, and
mainly dependent on agents to be infused, availability of skillset,
and anticipated duration of therapy. A previous state-wide study
performed across hospitals in Michigan revealed that 73% of
hospitals required or preferred central access prior to initiation
of vasopressor medications (29).
Recently, the safe peripheral administration of vasopressors has
been demonstrated and was associated with expedited vasopressor
initiation (30). Another
multicenter prospective study revealed that implementing a protocol
for peripheral vasopressor administration leads to avoidance of
requiring central access in 51.6% (31). Peripheral vasopressor administration
up to 24 h has been shown to be safe with a low incidence of
extravasation (32).
PICC
A PICC is a slender (2-6 French) catheter ~50 to 60
cm in length that can have up to three lumens (33). It is usually inserted percutaneously
with ultrasound or fluoroscopy guidance into the basilic or
cephalic vein in the upper arm, as shown in Fig. 1 (19,33).
Due to the length of the catheter, PICCs can also be
inserted in more distal veins. For instance, the catheterization of
the great saphenous vein has been reported (34). PICCs reduce the procedural burden on
physicians, as specially trained nurses can commonly perform the
insertion at the patient's bedside, often utilizing ultrasound or
fluoroscopy guidance (33). Their
popularity has grown due to the potential to reduce hospitalization
costs by shortening the length of hospitalization stay of patients
by allowing for home intravenous therapy (2,33). These
devices are indicated in patients requiring several weeks to 6
months of intravenous therapy; notably, experts recommend against
their use when the proposed duration of use is <5 days (20). A contraindication is active IV
substance use disorder given the concerns of inappropriate use upon
discharge. However, mortality and catheter-related adverse events
in this group have been found to be comparable to non-users,
although, high quality evidence to inform practice is limited
(35).
A commonly encountered practice is requesting
multi-lumen PICCs in anticipation of future IV access requirements,
although these are associated with a higher risk of complications
compared to single-lumen PICCs (36).
CICCs
CICCs can be categorized based on whether they are
tunneled or non-tunneled (2,37). They are directly inserted into
central veins without traveling through the subcutaneous tissue,
hence the name ‘non-tunneled’. Conversely, tunneled catheters
travel under the skin and terminate away from the venous access
site, which decreases the risk of infection and ensures a more
secure access point (2,37). The introduction of subcutaneously
anchored securement devices has decreased the risk of catheter
dislodgement to <3%; however, these devices have not been shown
to reduce the risk of venous thrombosis or infections (38). These devices have also been used to
anchor PICC and midline catheters (39). The duration of use for CICCs can vary
depending on the catheter type, its intended purpose and
patient-specific factors (2,24,33,37).
Non-tunneled central venous catheters.
Non-tunneled central catheters are commonly referred to as central
lines and are typically utilized in emergency departments,
operating rooms, and critical care settings (2,24). These
catheters are relatively straightforward to insert at the bedside,
with ultrasound guidance being standard of care (6). Intended dwell time ranges from 3 days
for femoral access to 2 weeks for jugular access; however, prompt
removal is key as ~3% of patients develop one or more serious
complications when catheters are left for ≥3 days (40,41).
They are categorized upon the site of venous access as follows:
a) Internal jugular vein. Access to the
internal jugular vein (IJV) provides several advantages. These
include a reduced risk of pneumothorax compared to subclavian
access, a short distance from skin to vessel and enhanced
visualization of the needle path (42,43). The
Trendelenburg position has been described as the standard of care
during insertion due to the increase in IJV size, which ultimately
decreases risk of developing complications; however, that
compromises comfort in conscious patients (44). A previous study and a recent
metanalysis revealed that further inclination from 10˚ does not
statistically benefit IJV size (45,46). The
IJV is the preferred access site for cardiac surgery and any
procedure where the chest will be in the sterile field. However,
using the IJV for venous access increases the risk of developing
immediate complications, such as arterial puncture due the fact
that the trajectory of the IJV often overlies the carotid artery.
Potential nerve injury can also arise due to the proximity of the
sympathetic chain. Although rare, Horner's syndrome has been
reported during the placement of CICCs in the IJV (47,48). In
addition, the risk of iatrogenic pneumothorax with IJV placement is
extremely low in the era of ultrasound, at around 0.1% (49). When compared to the subclavian vein,
the IJV imposes a higher risk of infection and thrombus formation
(50).
b) Femoral vein. Femoral vein access with
traditional catheters is classified as peripheral access as the
length of most catheters is ~20 cm, which leaves the tip of the
catheter located an iliac vein (either external or common)
(1). Therefore, Annetta et al
(51) suggested utilizing the term
‘femorally inserted central catheter’ whenever catheters culminate
in the central circulation. Femoral vein access is often selected
in emergency settings due to its easy accessibility and large
caliber, facilitating swift catheter placement (24). However, it carries a heightened risk
of infection and deep vein thrombosis compared to other sites
(52). One way to mitigate
infectious risks includes mid-thigh exit sites with
pseudo-tunneling (53). Ideally,
femoral catheters placed in emergency settings should be removed
and replaced within 48 h of insertion (51).
c) Subclavian and thoracic axillary veins.
Subclavian vein access is often favored in patients requiring
long-term catheterization. It has the lowest infection and
thrombosis rates among central catheter sites but potentially has
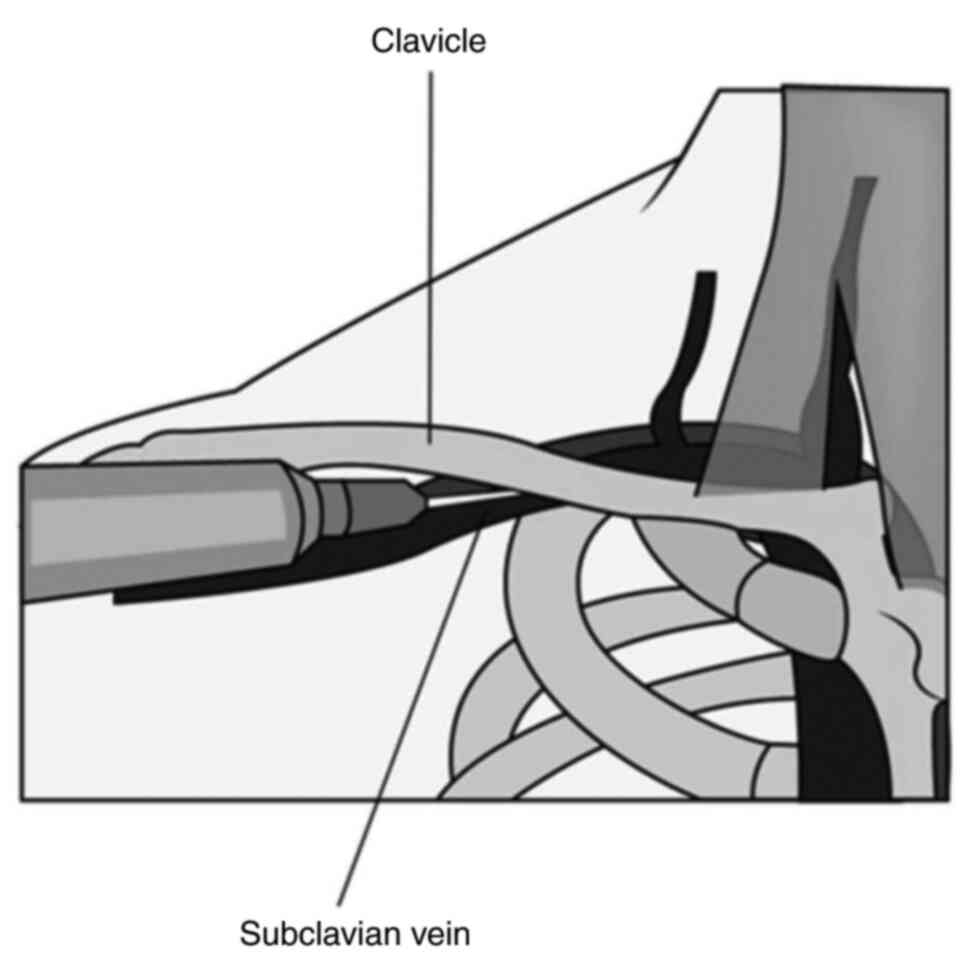
the highest rates of venous stenosis (24). Right-sided subclavian central
catheters are associated with a lower incidence of pneumothorax,
but an increased risk of catheter malposition, hemothorax and
subclavian artery puncture due to the anatomical position of the
subclavian vein (Fig. 2) (54). The catheterization of the
supraclavicular tributary of the subclavian vein, when compared to
the IJV, increased the first-attempt success proportion [relative
risk (RR), 1.22; 95% confidence interval (CI), 1.06-1.40] (55). Historically, ultrasound guidance was
linked with worse outcomes when compared to landmark or blind
technique, however, recently published studies revealed lower
complication rates and higher success rate with ultrasound guided
placement when compared to blind technique (56). Nonetheless, a suitable alternative
has been the long axillary vein as it resides outside the thoracic
cavity and is not hindered by the clavicle promoting easier
ultrasound visualization. A recent metanalysis of five randomized
controlled trials compared blind technique subclavian
catheterization to ultrasound guided axillary vein catheterization
(57). There was a statistically
significant increase the in overall catheterization success rates
(RR, 1.09; 95% CI, 1.04-1.15; P<0.01) and a decrease in adverse
events, including the risk of arterial puncture (RR, 0.18; 95% CI,
0.06-0.55; P<0.01) pneumo-and hemothorax (RR, 0.12; 95% CI,
0.02-0.64; P=0.01). However, all five studies in the metanalysis
compared blind technique subclavian catheterization to US guided
axillary vein (57).
Tunneled central venous catheters. These
catheters are indicated for patients who require long term IV
access. The exit site is in one location and is tunneled under the
skin away from the point of venous entry (2,37).
Tunneled catheters are typically inserted under ultrasound or
fluoroscopy guidance and are positioned on the chest wall to
facilitate catheter care. A unique feature of tunneled catheters is
the anchoring Dacron cuff that facilitates internal fixation once
tissue ingrowth occurs. Lower CRBSI rates for tunneled central
catheters, when compared to PICCs, have been reported, and they
have a longer time to the first infection (58). These catheters can be left in place
for an extended period, ranging from several weeks to months.
Potential complications of tunneled catheters include thrombosis,
pneumothorax, extravasation, external fracture, and CLABSIs. Rare
complications that may occur include air embolism and pinch-off
syndrome (59). Commonly encountered
tunneled catheters include Hickman, Broviac, Hohn and Groshong
catheters.
Special patient populations. Oncology
patients and implantable ports
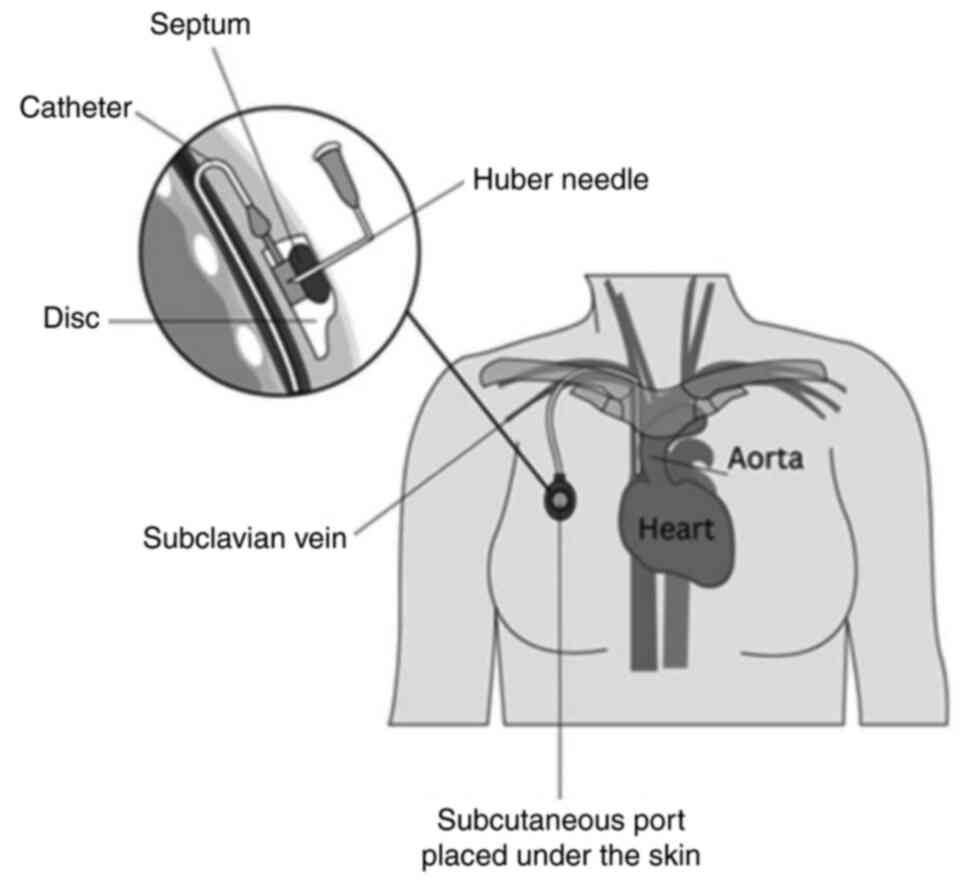
Implantable port catheters, known simply as ‘ports’,
provide superior patient comfort and mobility compared to external
catheters (2). Despite their extra
associated costs, they are extensively used in the field of
oncology for administration of chemotherapy as they have been
proven to improve patient satisfaction and quality of life
(60). Given their placement beneath
the skin, patients can engage in daily activities, such as
showering and swimming without fear of disrupting the device.
Placement of a port involves a surgical procedure, performed under
either local or general anesthesia (61). During this procedure, a subcutaneous
pocket is created in the chest wall and the catheter is typically
tunneled into the jugular vein. Other potential sites for
cannulation include the subclavian, cephalic, and innominate veins
(Fig. 3) (62).
A more cosmetically appealing approach has been arm
ports, which is appealing to some patients such as those undergoing
therapy for breast cancer especially if they are requiring
radiotherapy, flap transfer, or reconstructive surgeries (63). Furthermore, femoral placement of
ports has been described in patients with bilateral breast cancer
or hindered neck and chest accessibility (64,65).
Notably, implanted ports provide a reduced risk of infectious and
mechanical complications when compared to either PICC or tunneled
catheters (60,66).
Hemodialysis patients and dialysis catheters.
Central venous catheters offer a crucial alternative in
hemodialysis patients without a functioning arteriovenous (AV)
fistula or graft (67). This form of
access becomes particularly relevant when urgent hemodialysis needs
to be performed. Patients initiating hemodialysis with non-tunneled
catheters face significantly higher infection rates compared to
those using tunneled hemodialysis catheters (67,68). It
is important to note that while CICCs can provide immediate access
to hemodialysis, they should not be viewed as a long-term solution
as the recommended duration of use is <2 weeks (69) facilitate life-saving treatment;
however, their use should be minimized to decrease the risk of
central venous stenosis or occlusion, a severe complication that
can compromise future permanent access options (68). In terms of site selection for venous
access, the National Kidney Foundation's Kidney Disease Outcomes
Quality Initiative (KDOQI) guidelines suggest a hierarchy of
choice. The IJV is the most recommended site, followed by external
jugular, femoral veins and the subclavian vein is the least
preferred due to the inherent high risk of stenosis. Right sided
insertion is preferable due to more direct anatomy in the absence
of contraindications, such as central stenosis or previous
pacemaker insertion (69).
In those who require long term dialysis and do not
have a mature arteriovenous fistula, tunneled catheters are the
preferred venous access. In the most recent statement of the KDOQI,
no maximal dwell time was recommended; rather, the indefinite use
of tunneled catheters can be pursued if they are deemed to be the
most appropriate permanent dialysis access (69). Two separate catheters can be inserted
side by side (Tesio catheters) or a single dual-lumen catheter
(Permcath) can be used (70).
Hemodynamically monitored patients and Swan-Ganz
catheters. The Swan-Ganz catheter is a pulmonary artery
catheter used to monitor the hemodynamic status of critical
patients (71). It is used to
evaluate right-sided cardiac filling pressures, estimation of
cardiac output, intracardiac shunt evaluation, pulmonary artery and
occlusion pressures, and the calculation of vascular resistance.
The catheter is inserted into a central vein through an introducer
port until it reaches the pulmonary artery. The introducer port,
also known as the Cordis sheath, is a polyethylene sheath system
with a hemostatic valve assembly and side port extension, which can
act as a central vascular access point, theoretically eliminating
the need for other CVAD in such patients.
5. Potential complications of central venous
access
Infectious sequelae
Prolonged dwell time leads to a statistically
significant and exponential increase in infection rates, where the
incidence of infection was found to be 4.8 per 1,000 catheter/days
when dwell times were <10 days; such rates double when dwell
times extend over a period of 20 days (72). Factors such as poor aseptic technique
and catheter hub colonization also contribute to the risk of
infection (24,33,37).
Moreover, the experience level of the healthcare professional
inserting the device may impact the potential risk of complications
(73).
Central line associated blood stream infection
(CLABSI) is a primary bloodstream infection occurring in a patient
who has a central catheter in place at the time of (or within 48 h
before) the development of the infection, and who does not have an
infection at another site (74). The
diagnosis of CLABSI does not necessarily require the central
catheter to be the confirmed source of the bloodstream infection.
Instead, it is an epidemiological surveillance definition used by
the Centers for Disease Control and Prevention (CDC) to monitor the
rates of these infections across healthcare institutions. CRBSI is
a bloodstream infection where the catheter is confirmed as the
source of the infection (75). The
diagnosis of CRBSI typically requires more rigorous criteria
compared to CLABSI. This usually includes a comparison of culture
results from the catheter and a peripheral vein to demonstrate that
the infection is more likely to have been caused by the catheter.
It is pertinent to note that sending the catheter tip for culture
offers poor positive predictive value (76)
In the USA, it is estimated that ~250,000 cases of
CLABSI occur annually, contributing to the death of 12,000-28,000
patients and incurring billions in healthcare costs (75). Despite improvements in prevention,
CRBSIs and CLABSIs remain a significant cause of
healthcare-associated infections. The overall mortality rate
associated with CRBSIs ranges from 12-25%, although it can be as
high as 35% in intensive care units (77). Several risk factors for the
development of CRBSIs and CLABSIs have been identified. These
include the prolonged duration of catheterization, femoral or
internal jugular placement (vs. subclavian), immunosuppression,
inadequate staff education and training, poor adherence to
infection control practices and improper catheter handling. The
type of central access also accounts for infection risks; PICCs
have been found to be associated with lower infection rates and
longer time dwell times prior to colonization in critically ill
patients when compared to other CICCs (58).
The most common pathogens associated with CRBSIs and
CLABSIs include the following from the most common to the least
common: Gram-positive organisms (coagulase-negative staphylococci,
34%; enterococci, 16%; and Staphylococcus aureus, 9.9%) and
Gram-negative organisms (Klebsiella, 6%;
Enterobacter, 4%; Pseudomonas, 3%; Escherichia
coli, 3%; Acinetobacter, 2%) and yeast (Candida species,
12%) (72). The Infectious Disease
Society of America (IDSA) recommends empiric treatment for CLABSI
with vancomycin plus either piperacillin-tazobactam, cefepime, or a
carbapenem. Definitive therapy is based on culture and
susceptibility results. The duration of treatment varies based on
the pathogen, although is typically between 7-14 days (78). In general, any catheters suspected of
being the source of bacteremia or septicemia should be promptly
removed. However, in some cases (e.g., infections with
coagulase-negative staphylococci), catheter salvage may be
attempted under certain conditions by using a combination of
systemic antibiotics and antibiotic lock therapy. This is not
recommended if there is hemodynamic instability or metastatic
complications of infection. Consulting infectious disease
specialists is recommended if cultures are positive for
Staphylococcus aureus or Candida species, persistent
bacteremia or shock after 72 h of appropriate antimicrobial therapy
or presence of an intravascular prosthetic device (79).
Multiple strategies regarding mitigating the risk of
infectious sequalae have been suggested. For instance, the American
Society of Anesthesiologists Task Force on Central Venous Access
endorses aseptic techniques and the use of maximal barrier
precautions (80). Additionally, the
use of chlorhexidine for skin preparation before catheter insertion
has been shown to significantly reduce CRBSIs compared to
povidone-iodine (81). Additionally,
the use of chlorhexidine-containing dressings is currently
considered an ‘essential practice’ (82). The use of antimicrobial coated
catheters is highly recommended by multiple societies; this stems
from multiple studies and a meta-analysis that demonstrated
significant differences in the rate of CRBSIs per 1,000
catheter-days between antimicrobial-impregnated and standard CVADs
(RR, 0.70; 95% CI, 0.53-0.91; P=0.008) (83).
Catheter occlusion
Catheter occlusion is often encountered in CVADs and
is most commonly due to thrombosis of the catheter (84). Other causes of occlusion include
catheter malposition, migration, drug precipitation and mechanical
occlusion. Lock solutions, such as heparin and citrate are left in
catheters when not in use to decrease the risk of thrombosis.
Citrate chelates calcium ions decreasing the activation of the
coagulation cascade and platelet aggregation (85). Notably, the use of citrate is
preferred in patients on hemodialysis as it has been shown to
reduce the frequency of flow-related catheter exchanges. Citrate is
also preferred in patients who develop heparin-induced
thrombocytopenia. In cases of catheter thrombosis, alteplase or
other thrombolytic agents, such as tenecteplase are often used to
restore flow (84). Recently,
taurolidine-based catheter lock solutions have shown promising
results in decreasing both catheter-related thrombosis and
infections, but are not currently commercially available (86). The diagnosis of catheter-related
thrombosis is achieved with duplex ultrasonography. The treatment
of catheter-related thrombosis is individualized; however, the
mainstay of therapy is anticoagulation. Catheter removal is not
indicated if the catheter is functioning appropriately and is still
needed for patient care (87).
Emerging trends and technologies
Recent advancements in VADs are revolutionizing
clinical practice through improvements in VAD design and
management. For instance, the application of high-strength,
thromboresistant hydrogels has improved catheter flexibility and
durability (88). Novel advancements
in anti-infective coating, anti-infective VAD hubs, and novel
needleless connectors, have been shown to decrease both infectious
and mechanical complications (89).
For instance, antimicrobial (minocycline, rifampin and
chlorhexidine) Polycarbonate coating for intravenous connectors
leads to the cessation of microbial colonization (90). Hydrophobic catheter material has been
noted to be associated with a similar risk of VAD complications and
failure when compared to standard polyurethane; however,
hydrophilic catheter material has been shown to decrease thrombotic
and infectious complications and improve patient comfort (91,92).
Furthermore, artificial intelligence (AI) has been
employed to optimize catheter design; for example, AI-aided
geometric modifications have been shown to significantly impede
bacterial migration along catheter surfaces, reducing infection
rates by up to 100-fold (93).
AI-based models have also been used in accurately and reliably
determining CVC placement in chest X-rays (94).
6. Conclusion
The present review provides clinicians with
essential insight into selecting the most appropriate venous access
device based on current evidence-based recommendations. The present
review serves as a clinical decision-making aid through summarizing
best practices and addressing potential knowledge gaps. Different
access sites have both advantages and disadvantages based on the
duration of therapy, flow rates and risks of complications. The
selection of a vascular access device, as well as the insertion
site should be thoughtfully approached based on indications,
intended use, anticipated duration and specific patient factors.
Adverse events, such as bloodstream infections and thrombosis, are
key issues that can alter the trajectory of therapy and lead to
poor patient outcomes. As venous access carries inherent risks, the
prudent choice of device and meticulous procedural and
post-procedural care is paramount in ensuring patient safety and
successful therapy.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
MA was involved in the investigation of the
literature, as well as in the writing of the original draft of the
manuscript, in the reviewing of the manuscript and in referencing
software (Endnote) provision. OH was involved in the writing of the
original draft of the manuscript, and in the reviewing and editing
of the manuscript. HA was involved in the writing, reviewing and
editing of the manuscript and in image design. KTJ was involved in
the writing, reviewing and editing of the manuscript. TH was
involved in the writing, reviewing and editing of the manuscript
and in study supervision. All authors have read and approved the
final manuscript. Data authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Pittirut M, Van Boxtel T, Scoppettuolo G,
Carr P, Konstantinou E, Ortiz Miluy G, Lamperti M, Goossens GA,
Simcock L, Dupont C, et al: European recommendations on the proper
indication and use of peripheral venous access devices (the ERPIUP
consensus): A WoCoVA project. J Vasc Access. 24:165–182.
2023.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Moureau NL and Alexandrou E: Device
Selection. In: Vessel Health and Preservation: The Right Approach
for Vascular Access. Moureau NL (ed). Springer International
Publishing: Cham, pp23-41, 2019.
|
|
3
|
Pattnaik SK, Sahoo S and Hota B:
Establishment of peripherally inserted central catheter line clinic
at a tertiary care hospital: Review of first 100 Cases. Apollo Med.
20:341–345. 2023.
|
|
4
|
Slavin RE: Best evidence synthesis: An
intelligent alternative to meta-analysis. J Clin Epidemiol.
48:9–18. 1995.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Beecham GB and Tackling G: Peripheral Line
Placement. In: StatPearls. StatPearls Publishing, Treasure Island,
FL, 2025.
|
|
6
|
Franco-Sadud R, Schnobrich D, Mathews BK,
Candotti C, Abdel-Ghani S, Perez MG, Rodgers SC, Mader MJ, Haro EK,
Dancel R, et al: Recommendations on the use of ultrasound guidance
for central and peripheral vascular access in adults: A position
statement of the society of hospital medicine. J Hosp Med.
14:E1–E22. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Miller MA and Schlueter AJ: Transfusions
via hand-held syringes and small-gauge needles as risk factors for
hyperkalemia. Transfusion. 44:373–381. 2004.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Marsh N, Larsen EN, Ullman AJ, Mihala G,
Cooke M, Chopra V, Ray-Barruel G and Rickard CM: Peripheral
intravenous catheter infection and failure: A systematic review and
meta-analysis. Int J Nurs Stud. 151(104673)2024.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Drugeon B, Guenezan J, Pichon M, Devos A,
Fouassin X, Neveu A, Boinot L, Pratt V and Mimoz O: Incidence,
complications, and costs of peripheral venous catheter-related
bacteraemia: A retrospective, single-centre study. J Hosp Infect.
135:67–73. 2023.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zanella MC, Pianca E, Catho G, Obama B, De
Kraker MEA, Nguyen A, Chraiti MN, Sobel J, Fortchantre L, Harbarth
S, et al: Increased peripheral venous catheter bloodstream
infections during COVID-19 Pandemic, Switzerland. Emerg Infect Dis.
30:159–162. 2024.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Wassil SK, Crill CM and Phelps SJ:
Antimicrobial impregnated catheters in the prevention of
catheter-related bloodstream infection in hospitalized patients. J
Pediatr Pharmacol Ther. 12:77–90. 2007.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Tarpey A, Narechania S and Malesker M:
What is the optimal approach to infiltration and extravasation of
nonchemotherapy medications? Cleve Clin J Med. 90:292–296.
2023.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Gibian JT, Zakria D, March C, Schaheen B
and Drolet BC: Outcomes and management of peripheral intravenous
infiltration injuries. Hand (New York, N.Y.). 17:148–154.
2022.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Pinelli F and Pittiruti M: The integrated
short peripheral cannula: A new peripheral venous access device? J
Vasc Access. 24:353–357. 2023.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Gidaro A, Quici M, Giustivi D, Pinelli F,
Samartin F, Casella F, Cogliati C, Rizzi G, Salvi E, Bartoli A, et
al: Integrated short peripheral intravenous cannulas and risk of
catheter failure: A systematic review and meta-analysis. J Vasc
Access. 26:372–380. 2025.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Qin KR, Nataraja RM and Pacilli M: Long
peripheral catheters: Is it time to address the confusion? J Vasc
Access. 20:457–460. 2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Qin KR, Ensor N, Barnes R, Englin A,
Nataraja RM and Pacilli M: Long peripheral catheters for
intravenous access in adults and children: A systematic review of
the literature. J Vasc Access. 22:767–777. 2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Pacilli M, Bradshaw CJ and Clarke SA: Use
of 8-cm 22G-long peripheral cannulas in pediatric patients. J Vasc
Access. 19:496–500. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Garcilazo NHH, Hassanein M, Vachharajani
TJ and Anvari E: Can I place a peripherally inserted central
catheter in my patient with chronic kidney disease? Cleve Clin J
Med. 88:431–433. 2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Chopra V, Flanders SA, Saint S, Woller SC,
O'Grady NP, Safdar N, Trerotola SO, Saran R, Moureau N, Wiseman S,
et al: The michigan appropriateness guide for intravenous catheters
(MAGIC): Results from a multispecialty panel using the RAND/UCLA
appropriateness method. Ann Intern Med. 163 (Suppl 6):S1–S40.
2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Swaminathan L, Flanders S, Horowitz J,
Zhang Q, O'Malley M and Chopra V: Safety and outcomes of midline
catheters vs peripherally inserted central catheters for patients
with short-term indications: A multicenter study. JAMA Intern Med.
182:50–58. 2022.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Nickel B, Gorski L, Kleidon T, Kyes A,
DeVries M, Keogh S, Meyer B, Sarver MJ, Crickman R, Ong J, et al:
Infusion therapy standards of practice, 9th edition. J Infus Nurs.
47 (1S Suppl 1):S1–S285. 2024.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Thomsen SL, Boa R, Vinter-Jensen L and
Rasmussen BS: Safety and efficacy of midline vs peripherally
inserted central catheters among adults receiving IV therapy: A
Randomized clinical trial. JAMA Netw Open.
7(e2355716)2024.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Kolikof J, Peterson K, Williams C and
Baker AM: Central Venous Catheter Insertion, In: StatPearls
[Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan.
2025 Feb 4.
|
|
25
|
Chui J, Saeed R, Jakobowski L, Wang W,
Eldeyasty B, Zhu F, Fochesato L, Lavi R and Bainbridge D: Is
Routine Chest X-Ray after ultrasound-guided central venous catheter
insertion choosing wisely?: A population-based retrospective study
of 6,875 patients. Chest. 154:148–156. 2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Corradi F, Guarracino F, Santori G,
Brusasco C, Tavazzi G, Via G, Mongodi S, Mojoli F, Biagini RUD,
Isirdi A, et al: Ultrasound localization of central vein catheter
tip by contrast-enhanced transthoracic ultrasonography: A
comparison study with trans-esophageal echocardiography. Crit Care.
26(113)2022.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Ryu HG, Bahk JH, Kim JT and Lee JH:
Bedside prediction of the central venous catheter insertion depth.
Br J Anaesth. 98:225–227. 2007.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Erskine B, Bradley P, Joseph T, Yeh S and
Clements W: Comparing the accuracy and complications of
peripherally inserted central catheter (PICC) placement using
fluoroscopic and the blind pushing technique. J Med Radiat Sci.
68:349–355. 2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Munroe E, Claar D, Tamae-Kakazu M, Tatem
G, Blamoun J, McSparron JI and Prescott HC: Hospital policies on
intravenous vasopressor administration and monitoring: A survey of
michigan hospitals. Ann Am Thorac Soc. 19:1769–1772.
2022.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Munroe ES, Heath ME, Eteer M, Gershengorn
HB, Horowitz JK, Jones J, Kaatz S, Tamae Kakazu M, McLaughlin E,
Flanders SA and Prescott HC: Use and outcomes of peripheral
vasopressors in early sepsis-induced hypotension across michigan
hospitals: A retrospective cohort study. Chest. 165:847–857.
2024.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Yerke JR, Mireles-Cabodevila E, Chen AY,
Bass SN, Reddy AJ, Bauer SR, Kokoczka L, Dugar S and Moghekar A:
Peripheral administration of norepinephrine: A prospective
observational study. Chest. 165:348–355. 2024.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Zichichi A, Wallace R, Daniell J, Rouse G,
Ahearn P and Ammar M: Safety of peripherally infused
sympathomimetic vasopressors in the intensive care unit and
emergency department. Ann Pharmacother. 59:397–405. 2025.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Gonzalez R and Cassaro S: Percutaneous
Central Catheter, In: StatPearls. StatPearls Publishing, Treasure
Island, FL, 2025.
|
|
34
|
Jin MF, Thompson SM, Comstock AC, Levy ER,
Reisenauer CJ, McPhail IR and Takahashi EA: Technical success and
safety of peripherally inserted central catheters in the great
saphenous and anterior accessory great saphenous veins. J Vasc
Access. 23:280–285. 2022.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Suzuki J, Johnson J, Montgomery M, Hayden
M and Price C: Outpatient parenteral antimicrobial therapy among
people who inject drugs: A review of the literature. Open Forum
Infect Dis. 5(ofy194)2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Bozaan D, Skicki D, Brancaccio A, Snyder
A, Friebe S, Tupps M, Paje D and Chopra V: Less lumens-less risk: A
pilot intervention to increase the use of single-lumen peripherally
inserted central catheters. J Hosp Med. 14:42–46. 2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Flick AI and Winters R: Vascular Tunneled
Central Catheter Access. In: StatPearls. StatPearls Publishing,
Treasure Island, FL, 2025.
|
|
38
|
Pinelli F, Pittiruti M, Van Boxtel T,
Barone G, Biffi R, Capozzoli G, Crocoli A, Elli S, Elisei D,
Fabiani A, et al: GAVeCeLT-WoCoVA Consensus on subcutaneously
anchored securement devices for the securement of venous catheters:
Current evidence and recommendations for future research. J Vasc
Access. 22:716–725. 2021.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Brescia F, Pittiruti M, Roveredo L, Zanier
C, Morabito A, Santarossa E, Da Ros V, Montico M and Fabiani F:
Subcutaneously anchored securement for peripherally inserted
central catheters: Immediate, early, and late complications. J Vasc
Access. 24:82–86. 2023.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Teja B, Bosch NA, Diep C, Pereira TV,
Mauricio P, Sklar MC, Sankar A, Wijeysundera HC, Saskin R, Walkey
A, Wijeysundera DN and Wunsch H: Complication rates of central
venous catheters: A systematic review and meta-analysis. JAMA
Intern Med. 184:474–482. 2024.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Kehagias E, Galanakis N and Tsetis D:
Central venous catheters: Which, when and how. Br J Radiol.
96(20220894)2023.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Millington SJ, Lalu MM, Boivin M and
Koenig S: Better with ultrasound: Subclavian central venous
catheter insertion. Chest. 155:1041–1048. 2019.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Parienti JJ, Mongardon N, Mégarbane B,
Mira JP, Kalfon P, Gros A, Marqué S, Thuong M, Pottier V, Ramakers
M, et al: Intravascular complications of central venous
catheterization by insertion site. N Engl J Med. 373:1220–1229.
2015.PubMed/NCBI View Article : Google Scholar
|
|
44
|
McGee DC and Gould MK: Preventing
complications of central venous catheterization. N Engl J Med.
348:1123–1133. 2003.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Judickas Š, Gineitytė D, Kezytė G,
Gaižauskas E, Šerpytis M and Šipylaitė J: Is the Trendelenburg
position the only way to better visualize internal jugular veins?
Acta Med Litu. 25:125–131. 2018.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Garcia-Leal M, Guzman-Lopez S,
Verdines-Perez AM, de Leon-Gutierrez H, Fernandez-Rodarte BA,
Alvarez-Villalobos NA, Martinez-Garza JH, Quiroga-Garza A and
Elizondo-Omaña RE: Trendelenburg position for internal jugular vein
catheterization: A systematic review and meta-analysis. J Vasc
Access. 24:338–347. 2023.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Yamamoto M, Yamada K, Horikawa M and Kondo
H: Horner syndrome caused by central venous port placement via the
internal jugular vein: A case report. Interv Radiol
(Higashimatsuyama). 5:14–18. 2020.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Khan Z and Bollu PC: Horner Syndrome. In:
StatPearls. StatPearls Publishing, Treasure Island, FL, 2025.
|
|
49
|
Adrian M, Borgquist O, Kröger T, Linné E,
Bentzer P, Spångfors M, Åkeson J, Holmström A, Linnér R and Kander
T: Mechanical complications after central venous catheterisation in
the ultrasound-guided era: A prospective multicentre cohort study.
Br J Anaesth. 129:843–850. 2022.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Heidenreich D, Hansen E, Kreil S, Nolte F,
Jawhar M, Hecht A, Hofmann WK and Klein SA: The insertion site is
the main risk factor for central venous catheter-related
complications in patients with hematologic malignancies. Am J
Hematol. 97:303–310. 2022.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Annetta MG, Elli S, Marche B, Pinelli F
and Pittiruti M: Femoral venous access: State of the art and future
perspectives. J Vasc Access. 26:361–371. 2025.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Hafeez SB, Ahmed A, Akhtar A, Ishtiaq W,
Javed NUS, Abbas K, Khan M, Zafar H and Jawed A: Catheter-related
bloodstream infection with femoral central access versus internal
jugular access in patients admitting to medical intensive care
unit. Cureus. 14(e29416)2022.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Elli S, Cannizzo L, Giannini L, Romanato
F, Trimarco C, Pessina M, Lucchini A, Foti G and Rondelli E:
Femorally inserted central catheters with exit site at mid-thigh: A
low risk alternative for central venous catheterization. J Vasc
Access. 25:808–812. 2024.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Deere M, Singh A and Burns B: Central
Venous Access of the Subclavian Vein. In: StatPearls. StatPearls
Publishing, Treasure Island, FL, 2025.
|
|
55
|
Imai E, Kataoka Y, Watanabe J, Okano H,
Namekawa M, Owada G, Matsui Y and Yokozuka M: Ultrasound-guided
central venous catheterization around the neck: Systematic review
and network meta-analysis. Am J Emerg Med. 78:206–214.
2024.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Sidoti A, Brogi E, Biancofiore G, Casagli
S, Guarracino F, Malacarne P, Tollapi L, Borselli M, Santori G,
Corradi F and Forfori F: Ultrasound-versus landmark-guided
subclavian vein catheterization: A prospective observational study
from a tertiary referral hospital. Sci Rep. 9(12248)2019.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Zhou J, Wu L, Zhang C, Wang J, Liu Y and
Ping L: Ultrasound guided axillary vein catheterization versus
subclavian vein cannulation with landmark technique: A
PRISMA-compliant systematic review and meta-analysis. Medicine
(Baltimore). 101(e31509)2022.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Pitiriga V, Bakalis J, Theodoridou K,
Kanellopoulos P, Saroglou G and Tsakris A: Lower risk of
bloodstream infections for peripherally inserted central catheters
compared to central venous catheters in critically ill patients.
Antimicrob Resist Infect Control. 11(137)2022.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Xu L, Qin W, Zheng W and Sun X:
Ultrasound-guided totally implantable venous access ports via the
right innominate vein: A new approach for patients with breast
cancer. World J Surg Oncol. 17(196)2019.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Chrisanthopoulou P, Iconomou G,
Assimakopoulos K, Vlachopoulos G, Makatsoris T, Koutras A,
Karnabatidis D and Katsanos K: Health-related quality of life in
patients with solid tumors receiving implantable venous access
ports for chemotherapy: A prospective randomized controlled trial.
Eur J Oncol Nurs. 67(102445)2023.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Akelma H, Salık F, Bıçak M and Erbatur ME:
Local anesthesia for port catheter placement in oncology patients:
An alternative to landmark technique using ultrasound-guided
superficial cervical plexus block-A prospective randomized study. J
Oncol. 2019(2585748)2019.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Hodson J: The case for using implanted
ports. Br J Nurs. 28 (Sup14a):S3–S10. 2019.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Li G, Zhang Y, Ma H and Zheng J: Arm port
vs chest port: A systematic review and meta-analysis. Cancer Manag
Res. 11:6099–6112. 2019.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Ding X, Ding F, Wang Y, Wang L, Wang J, Xu
L, Li W, Yang J, Meng X, Yuan M, et al: Shanghai expert consensus
on totally implantable access ports 2019. J Interv Med. 2:141–145.
2019.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Almasi-Sperling V, Hieber S, Lermann J,
Strahl O, Beckmann MW, Lang W and Sagban TA: Femoral placement of
totally implantable venous access ports in patients with bilateral
breast cancer. Geburtshilfe Frauenheilkd. 76:53–58. 2016.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Neville JJ, Aye HM and Hall NJ: Tunnelled
external versus implanted port central venous catheters in
paediatric oncology: A systematic review and meta-analysis. Arch
Dis Child. 108:975–981. 2023.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Beecham GB and Aeddula NR: Dialysis
Catheter. In: StatPearls. StatPearls Publishing, Treasure Island,
FL, 2025.
|
|
68
|
El Khudari H, Ozen M, Kowalczyk B,
Bassuner J and Almehmi A: Hemodialysis catheters: Update on types,
outcomes, designs and complications. Semin Intervent Radiol.
39:90–102. 2022.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Lok CE, Huber TS, Lee T, Shenoy S, Yevzlin
AS, Abreo K, Allon M, Asif A, Astor BC, Glickman MH, et al: KDOQI
clinical practice guideline for vascular access: 2019 update. Am J
Kidney Dis. 75 (4 Suppl 2):S1–S164. 2020.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Ling XC, Lu HP, Loh EW, Lin YK, Li YS, Lin
CH, Ko YC, Wu MY, Lin YF and Tam KW: A systematic review and
meta-analysis of the comparison of performance among step-tip,
split-tip, and symmetrical-tip hemodialysis catheters. J Vasc Surg.
69:1282–1292. 2019.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Rodriguez Ziccardi M and Khalid N:
Pulmonary Artery Catheterization. In: StatPearls. StatPearls
Publishing, Treasure Island, FL, 2025.
|
|
72
|
Pitiriga V, Bakalis J, Kampos E,
Kanellopoulos P, Saroglou G and Tsakris A: Duration of central
venous catheter placement and central line-associated bloodstream
infections after the adoption of prevention bundles: A two-year
retrospective study. Antimicrob Resist Infect Control.
11(96)2022.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Safety Committee of Japanese Society of
Anesthesiologists. Practical guide for safe central venous
catheterization and management 2017. J Anesth. 34:167–186.
2019.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Sopirala MM, Estelle CD and Houston L:
Central line-associated bloodstream infection
misclassifications-rethinking the centers for disease control and
prevention's central line-associated bloodstream infection
definition and its implications. Crit Care Med. 52:357–361.
2024.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Haddadin Y, Annamaraju P and Regunath H:
Central Line-Associated Blood Stream Infections. In: StatPearls.
StatPearls Publishing, Treasure Island, FL, 2025.
|
|
76
|
Lai YL, Adjemian J, Ricotta EE, Mathew L,
O'Grady NP and Kadri SS: Dwindling utilization of central venous
catheter tip cultures: An analysis of sampling trends and clinical
utility at 128 US Hospitals, 2009-2014. Clin Infect Dis.
69:1797–1800. 2019.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Toor H, Farr S, Savla P, Kashyap S, Wang S
and Miulli DE: Prevalence of central line-associated bloodstream
infections (CLABSI) in intensive care and medical-surgical units.
Cureus. 14(e22809)2022.PubMed/NCBI View Article : Google Scholar
|
|
78
|
O'Grady NP, Alexander M, Burns LA,
Dellinger EP, Garland J, Heard SO, Lipsett PA, Masur H, Mermel LA,
Pearson ML, et al: Guidelines for the prevention of intravascular
catheter-related infections. Clin Infect Dis. 52:e162–e193.
2011.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Baang JH, Inagaki K, Nagel J, Ramani K,
Stillwell TL, Mack M, Wesorick D, Mack M, Wesorick D and Proudlock
A: Michigan Medicine Clinical Care Guidelines, in Inpatient
Diagnosis and Treatment of Catheter-Related Bloodstream Infection.
2023, Michigan Medicine University of Michiga, Ann Arbor, MI,
2023.
|
|
80
|
Practice guidelines for central venous
access 2020: An updated report by the American Society of
anesthesiologists task force on central venous access.
Anesthesiology. 132:8–43. 2020.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Lai NM, Lai NA, O'Riordan E,
Chaiyakunapruk N, Taylor JE and Tan K: Skin antisepsis for reducing
central venous catheter-related infections. Cochrane Database Syst
Rev. 7(CD010140)2016.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Buetti N, Marschall J, Drees M, Fakih MG,
Hadaway L, Maragakis LL, Monsees E, Novosad S, O'Grady NP, Rupp ME,
et al: Strategies to prevent central line-associated bloodstream
infections in acute-care hospitals: 2022 Update. Infect Control
Hosp Epidemiol. 43:553–569. 2022.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Wang H, Tong H, Liu H, Wang Y, Wang R, Gao
H, Yu P, Lv Y, Chen S, Wang G, et al: Effectiveness of
antimicrobial-coated central venous catheters for preventing
catheter-related blood-stream infections with the implementation of
bundles: A systematic review and network meta-analysis. Ann
Intensive Care. 8(71)2018.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Hicks MA, Popowicz P and Lopez PP: Central
Line Management. In: StatPearls. StatPearls Publishing, Treasure
Island, FL, 2025.
|
|
85
|
Deng Y, Xing J, Tan Z, Ai X, Li Y and
Zhang L: Clinical application of 4% sodium citrate and heparin in
the locking of central venous catheters (excluding dialysis
catheters) in intensive care unit patients: A pragmatic randomized
controlled trial. PLoS One. 18(e0288117)2023.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Agarwal AK, Roy-Chaudhury P, Mounts P,
Hurlburt E, Pfaffle A and Poggio EC: Taurolidine/Heparin lock
solution and catheter-related bloodstream infection in
hemodialysis: A Randomized, double-blind, active-control, phase 3
study. Clin J Am Soc Nephrol. 18:1446–1455. 2023.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Wall C, Moore J and Thachil J:
Catheter-related thrombosis: A practical approach. J Intensive Care
Soc. 17:160–167. 2016.PubMed/NCBI View Article : Google Scholar
|
|
88
|
LeRoy KJ and Donahue DT: Trackability of a
high-strength thromboresistant hydrogel catheter: An In vitro
analysis comparing venous catheter forces in a simulated use
pathway. J Mech Behav Biomed Mater. 139(105670)2023.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Crnich CJ and Maki DG: The promise of
novel technology for the prevention of intravascular device-related
bloodstream infection. I. Pathogenesis and short-term devices. Clin
Infect Dis. 34:1232–1242. 2002.PubMed/NCBI View
Article : Google Scholar
|
|
90
|
Truong YL, Gerges BZ, Rosenblatt J and
Raad II: 2123. Novel antimicrobial polycarbonate coating for
intravenous connectors. Open Forum Infect Dis. 10 (Suppl
2)(ofad500.1746)2023.
|
|
91
|
Moureau N: Hydrophilic biomaterial
intravenous hydrogel catheter for complication reduction in PICC
and midline catheters. Expert Rev Med Devices. 21:207–216.
2024.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Ullman AJ, August D, Kleidon TM, Walker
RM, Marsh N, Bulmer AC, Pearch B, Runnegar N, Leema J, Lee-Archer
P, et al: A Comparison of Peripherally Inserted Central Catheter
Materials. N Engl J Med. 392:161–172. 2025.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Zhou T, Wan X, Huang DZ, Li Z, Peng Z,
Anandkumar A, Brady JF, Sternberg PW and Daraio C: AI-aided
geometric design of anti-infection catheters. Sci Adv.
10(eadj1741)2024.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Stroeder J, Multusch M, Berkel L, Hansen
L, Saalbach A, Schulz H, Heinrich MP, Elser Y, Barkhausen J and
Sieren MM: Optimizing catheter verification: an understandable AI
model for efficient assessment of central venous catheter placement
in chest radiography. Invest Radiol. 60:267–274. 2025.PubMed/NCBI View Article : Google Scholar
|