Introduction
Teratomas are tumors of embryonic origin classified
within the group of non-seminomatous germ cell tumors (GCTs)
(1). While they are most frequently
detected in the ovaries or testes, they can also be found in the
mediastinum, retroperitoneum and central nervous system (2). Non-seminomatous GCTs can manifest as
pure teratomas; however, they are more commonly observed in mixed
forms that involve various non-seminomatous histologies. The only
histological element found in the residual lesions following
platinum-based treatment for metastatic mixed non-seminomatous GCTs
are teratomas. Mature cystic teratomas (MCTs), also known as
dermoid cysts, are benign GCTs comprised of mature tissues that
originate from the endoderm, mesoderm, or ectoderm layers of the
embryo. They comprise ~20% of all ovarian tumors and 95% of all
ovarian GCTs (2,3). These tumors contain totipotent stem
cells that can transform into a wide range of tissue types,
including mesodermal tissues such as bone and muscle, endodermal
tissues present in the lungs and gastrointestinal tract, and
ectodermal tissues such as hair and skin. The most widely accepted
theory regarding their origin suggests that teratomas arise from
primitive germ cells (2). Teratomas
are the most common type of testicular GCTs in children (3). In this age group, mature teratomas
usually present with a benign clinical course. However, in adults,
teratomas tend to exhibit metastatic potential. Clinically, mature
teratomas are generally slow-growing tumors, with their clinical
presentations varying significantly. Acute abdominal pain from
ovarian teratomas may occur as a result of ovarian torsion or
spontaneous rupture, as well as nonspecific abdominal-pelvic
discomfort and compressive effects on nearby organs (4,5). The
present study aimed to address this gap by presenting a rare case
and discussing its implications for pathophysiological
understanding and clinical management.
The differentiation into somatic-type tissues that
reflect either embryonic or adult developmental stages
distinguishes teratomas from other types of neoplasms (6,7). Males
who are prepubertal or postpubescent may develop testicular
teratomas; however, the biological behavior and prognosis of these
tumors vary significantly between these age groups. Teratomas in
children are usually diagnosed prior to the age of 4 years, are
often pure and are generally benign (8,9). These
prepubertal tumors fall under the category of non-GCTs and are not
linked to chromosomal abnormalities such as the presence of
isochromosome 12p (i12p) or germ cell neoplasia in situ
(GCNIS). Adult postpubertal teratomas, on the other hand, are
typically parts of mixed GCTs. They can be found in metastatic
locations, particularly after non-teratomatous GCT components have
received chemotherapy. This theory is supported by a high degree of
genetic concordance between teratomas and GCT metastases from the
same patient (10). Notably,
elevated serum levels of tumor markers such as β-human chorionic
gonadotropin (β-hCG) or alpha-fetoprotein (AFP) indicate the
presence of other co-existing GCT components rather than being
caused by the teratomatous elements themselves.
Teratomas have been divided into four subtypes for
both descriptive and diagnostic reasons, with an increasing focus
on understanding their pathophysiological basis in recent
literature. Since both types are prone to somatic-type malignant
transformation and can metastasize, this distinction has limited
clinical significance (10).
Organoid structures and differentiated somatic tissues comprise
mature teratomas, which are usually embedded in a fibrous or myxoid
stroma (6,7). Skin appendages, thyroid follicles,
pancreatic tissue, respiratory and gastrointestinal epithelium,
cartilage, as well as squamous epithelium are examples of common
constituents. Although they are common in the ovary, MCTs are
uncommon in the testis. These typically have no immature components
and are composed only of sebaceous glands and squamous epithelium.
Similarly, there may be epidermoid cysts, which are comprised
entirely of keratinized squamous epithelium and do not have adnexal
structures. Since teratomas are usually linked to this chromosomal
abnormality and have the potential to become malignant, the lack of
i12p can aid in differentiating these lesions from malignant
teratomas (11). With an average
presentation age of 24 years, a small subset of dermoid cysts and
teratomas identified after puberty appear benign (9). These tumors are tiny, cystic, unrelated
to GCNIS and are frequently found close to the testicular hilum.
Additionally, every case examined using fluorescence in situ
hybridization (FISH) for i12p has yielded negative results
(10). In any case, these tumors
usually have a benign clinical course. The presence of embryonal or
poorly differentiated tissues characterizes immature teratomas.
Although primitive neuroepithelial elements are frequently regarded
as immature components, the standards for determining the degree of
immaturity are still unclear. Although these characteristics are
present, their clinical significance remains unclear, and adult
pathology reports frequently undervalue them (12). Histological characteristics
suggestive of somatic malignancy characterize malignant
transformation within teratomas. Large-scale growth of immature
neuroectodermal components that resemble embryonal tumors may be
one example of this (13). Malignant
transformation may be detected with the help of imaging modalities
like MRI and ultrasound. While MRI may show fat suppression or
enhancement patterns suggestive of neoplastic tissue, ultrasound
findings suggestive of malignancy include complex echotexture with
branching isoechoic elements (14).
Although they lack specificity, serum biomarkers such as
carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA
19-9) may bolster clinical suspicion of malignancy (15). Although it is less informative in
other histologic subtypes like intestinal adenocarcinoma, elevated
serum SCC antigen (SCC-Ag) may be seen in cases of transformation
to squamous cell carcinoma (SCC) and can be useful in the
diagnostic process (16,17). Definitive diagnosis requires
histopathological analysis, despite suggestive imaging and tumor
markers. In the present study, in reviewing the literature, case
reports published within the past 20 years were selected based on
the availability of histopathological confirmation and relevance to
postpubertal testicular teratomas.
Other than observation, localized testicular
teratomas usually do not require any treatment. Advanced cases,
however, require additional care. There is no standard chemotherapy
regimen available as malignant transformation is uncommon, and
there is limited reproducibility across reported protocols due to
heterogeneity in histological presentation. However, 5-fluorouracil
and platinum-based chemotherapy have shown promise in certain
instances (13,18). The identification of KRAS mutations
in certain patients raises the possibility that targeted EGFR
therapies could be an effective course of treatment (19). Tumor extension outside the testis,
invasion of the cyst wall, ascites, spontaneous or iatrogenic
rupture, adhesion to surrounding tissues and non-SCC malignant
histologies are all poor prognostic factors that are linked to
aggressive disease and poorer outcomes (20). It is extremely uncommon for
testicular GCTs to malignantly transform into adenocarcinomas. The
present study describes a rare instance of testicular metastatic
teratoma that transformed into intestinal-type mucinous
adenocarcinoma, a phenomenon that, to date, at least to the best of
our knowledge, has not been reported in the literature.
Case report
A 52-year-old male patient with no known history of
chronic illnesses or malignancies in his family presented to the
Urology Clinic at Ege University Hospital (Izmir, Turkey) with
swelling in the right testis, which had developed 21 years prior
(June, 2004). At that time, the levels of serum tumor markers were
elevated with an AFP level of 550 ng/ml, a β-HCG level of 1,826
IU/l and a lactate dehydrogenase (LDH) level of 1,313 U/l. A
scrotal Doppler ultrasound revealed a necrotic, vascularized mass
in the right testis, measuring ~6 cm in diameter. A whole-body CT
scan revealed peripancreatic, paraaortic and aortocaval
conglomerate lymphadenopathies (since these CT images were obtained
21 years ago and were obtained at an external center, the actual
images could not be retrieved; thus, only the report information
has been shared). The patient underwent a right radical
orchiectomy, and a histopathological examination (images not
available) revealed findings consistent with embryonal carcinoma.
The tumor, measuring 6 cm, exhibited widespread lymphovascular
invasion and invasion of the tunica albuginea. No evidence of
metastasis was found in the lungs or brain at initial staging,
although significant retroperitoneal involvement was noted. Due to
advanced-stage disease, the patient received four cycles of
bleomycin, etoposide and cisplatin (BEP) chemotherapy (typically
consisting of bleomycin 30 units/m² once weekly for 3 weeks,
etoposide 100 mg/m² daily for 3 consecutive days, and cisplatin 20
mg/m² daily for 5 consecutive days administered in 3-week cycles)
between July and October, 2004, during which partial regression of
the lymph nodes was observed. During chemotherapy, the patient
developed neutropenic fever and required hospitalization for
supportive care, including the immediate initiation of granulocyte
colony-stimulating factor via subcutaneous injection at a dose of 5
mcg/kg/day, continued daily until the absolute neutrophil count
recovered above 1,000/mm³. Following chemotherapy, a residual tumor
measuring 2.5 cm was found, prompting a retroperitoneal lymph node
dissection (RPLND), which revealed findings consistent with a
mature teratoma in November 2004. At 3 months following surgery
(February 2005), the patient developed elevated AFP levels, and a
2.5 cm retroperitoneal lymph node was detected, leading to the
administration of four cycles of VIP (etoposide, ifosfamide and
cisplatin) chemotherapy. The VIP regimen consists of etoposide 75
mg/m² administered intravenously daily on days 1 through 5,
ifosfamide 1,200 mg/m² intravenously daily on days 1 through 5 with
mesna uroprotection, and cisplatin 20 mg/m² intravenously daily on
days 1 through 5, repeated every 3 weeks. Partial regression was
again noted, and the patient was subsequently placed under
follow-up care. The VIP regimen was complicated by grade 2
neurotoxicity and transient renal function deterioration, both of
which resolved with dose adjustment and hydration protocols.
At 9 years post-operatively (November, 2013), the
patient experienced AFP progression, prompting a CT scan of the
thorax and abdomen, which again revealed a 2.5-cm retroperitoneal
lymph node (it should be noted that only the reports of the CT
images taken during this period are available for the patient. The
actual images could not be obtained because they were performed at
a private radiology laboratory in an external center). RPLND was
performed, and the pathological examination was consistent with a
mature teratoma. The immunohistochemical findings were as follows:
AFP, positive; CD30, negative; PLAP, positive; OCT-4, negative; and
D2-40, negative; this confirmed the diagnosis of a mature teratoma.
Samples were obtained from different retroperitoneal lymph node
regions during RPLND and were separately labeled and processed for
histopathological evaluation. In the pathology report, samples 1
and 3 exhibited cystic structures lined with single-layered or
cuboidal epithelium. Samples 2 and 4 revealed epithelial cysts and
chondroid areas, which were consistent with the teratomatous
component of the GCT. Samples 7 and 8 displayed areas of fat
necrosis, thrombus organization, giant cells and inflammatory cell
infiltration with vascular proliferation, which were interpreted as
secondary changes. A reactive lymph node was dissected from sample
5. Sample 4 exhibited solid epithelial tumors along with cystic
structures, and immunohistochemical analysis identified these as
compatible with the solid yolk sac component of the tumor. All
specimens contained only tumor tissue, with no surrounding lymphoid
tissue. Of note, the pathological blocks were assessed at a private
external laboratory by medical pathologists experienced in
uro-oncology. Therefore, detailed visual and pathological data
could not be provided. The information we share is based solely on
the pathology report. The patient then received an additional four
cycles of VIP chemotherapy between December, 2013 and March, 2014.
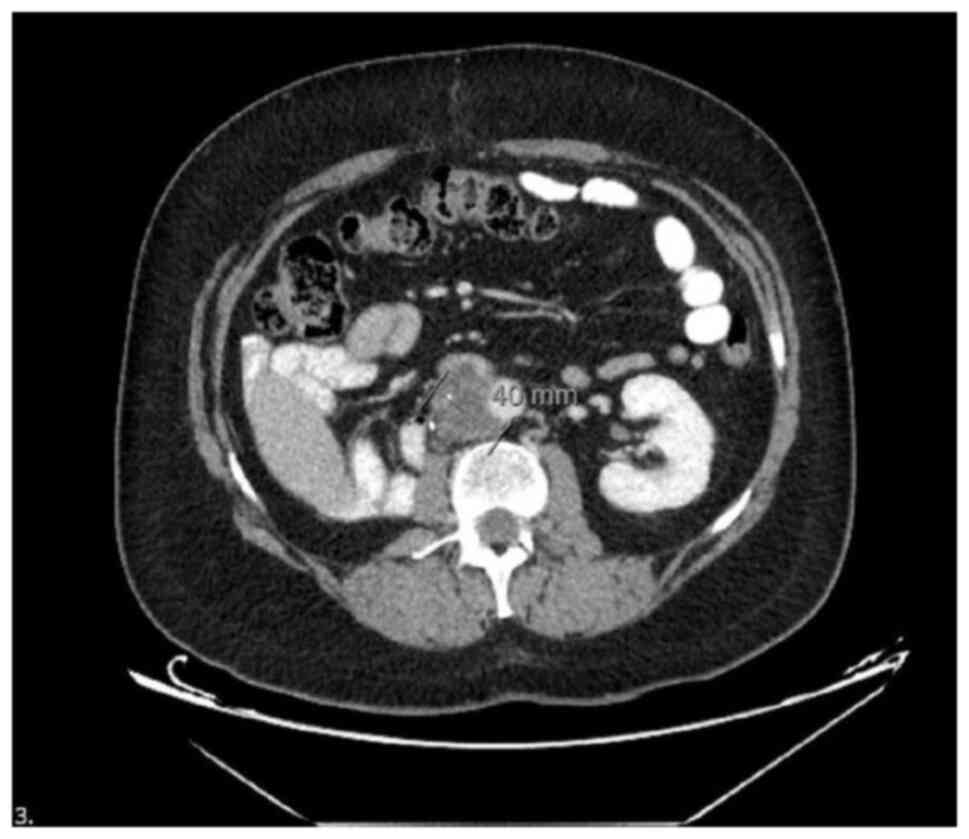
Follow-up imaging performed 8 years later revealed new abdominal
findings, including a 56 mm segment of a lobulated soft tissue mass
in the pericaval region, reaching a size of 4 cm. The mass caused
the obliteration of the vena cava lumen at the caudal level, with
no contrast observed within the lumen, indicating venous
compression and possible thrombosis. This vascular invasion led to
bilateral lower extremity edema and venous stasis symptoms,
requiring low-molecular-weight heparin therapy. These findings were
compared with prior imaging reports from June, 2018, which revealed
no such soft tissue mass. The current images revealed the
development of recurrent lymphadenopathy (LAP) metastasis and vena
cava invasion (Fig. 1).
The mass, which also infiltrated the abdominal
aorta, underwent an incisional biopsy by the cardiovascular surgery
team. During this period, the serum levels of tumor markers
including AFP (normal range, 0-10 ng/ml), β-HCG (normal range,
<5 IU/l) and LDH (normal range, 140-280 U/l) were within normal
limits. A pathological examination revealed that
immunohistochemical stains for AFP, CD30, glypican, OCT-4 and SAL-4
were all negative. The resected material exhibited a mucinous
adenocarcinoma component along with a benign chondroid component.
These findings raised the possibility of mucinous adenocarcinoma
arising from a teratoma. This represented a rare instance of
somatic-type malignant transformation, a known but infrequent
complication of mature teratomas. Following the pathological
diagnosis, a gastrointestinal tract screening was performed,
specifically an endoscopic colon examination, which revealed no
abnormal findings. Following radiation therapy targeting the
residual mass, the patient received four cycles of adjuvant
chemotherapy with an oxaliplatin + capecitabine-based regimen
(XELOX) from May to November, 2023. The XELOX regimen consisted of
oxaliplatin 130 mg/m² administered intravenously on day 1, and
capecitabine 1,000 mg/m² taken orally twice daily on days 1 through
14 of each 3-week cycle. Due to significant neuropathy symptoms,
treatment was completed with four cycles of capecitabine over a
period of 6 months. Despite cumulative treatment burden, the
patient maintained an adequate performance status and responded
well to supportive interventions, including neuroprotective agents.
In the third postoperative year, the patient remains under
surveillance with no evidence of recurrence.
Discussion
In primary testicular tumors, teratomas rarely
undergo somatic malignant transformation. Somatic malignancy
transformation refers to the development of a non-GCT within a
mature teratoma. While the malignant transformation of ovarian
cystic teratomas has been well-documented, with an incidence of
~2%, the occurrence of such transformations in mature teratomas
outside the ovaries, including in the testis, is exceedingly rare
(21). However, comparisons with
prior studies on malignant transformation in testicular teratomas
remain limited, highlighting a need for further research in this
area. In the existing literature, only a limited number of cases of
malignant transformation in primary testicular teratomas have been
reported. One of these cases involved colon transformation, while
the other two lacked specific pathological details. Notably, the
transformation of testicular teratomas into signet ring
adenocarcinoma has been described in only a single case (22).
The mechanism underlying the malignant
transformation of testicular mature teratomas remains incompletely
understood. Sarcoma is the most commonly reported malignancy;
however, various other tumor types, including enteric
adenocarcinoma, primitive neuroectodermal tumors and leukemia, have
also been observed (6,23-25).
Furthermore, a previous study reported the transformation of
testicular GCT metastasis into papillary renal cell carcinoma
following platinum-based chemotherapy (26). Of note, two main mechanisms have been
proposed for malignant transformation. One of these mechanisms
suggests that malignancy arises from the differentiation of
totipotent embryonal carcinoma cells into somatic phenotypes, while
the other points to the malignant transformation of mature teratoma
components themselves (27). Despite
these insights, stronger referencing to recent studies is required
to support these proposed mechanisms fully. Another crucial factor
in testicular teratoma malignancy transformation is the potential
secondary transformation due to chemotherapy or radiotherapy. The
literature indicates that only a few cases of testicular
retroperitoneal metastatic GCTs undergo malignant transformation
due to chemotherapy or radiotherapy, some of which exhibit
sarcomatoid changes (23). In
addition, there have been reports of malignant transformations in
untreated primary testicular teratomas (6,20,23).
Notably, the adenocarcinoma phenotype-related malignant
transformation in primary mature testicular teratomas remains
exceptionally rare.
It is an uncommon occurrence for somatic-type
cancers, specifically intestinal-type adenocarcinomas, to develop
within testicular teratomas. It is considered that endodermal
components that resemble the epithelium of the lower
gastrointestinal tract are the source of these cancers. Such tumors
often express markers of intestinal differentiation, such as CK20
and CDX2, according to immunohistochemical analyses (24). The identification of these markers
lends credence to the notion that intestinal epithelium-like
phenotypic characteristics are retained in the malignant
transformation taking place within the teratomatous elements. The
identification of these markers would benefit clinicians in
diagnostic and treatment planning. In order to correctly classify
the histological subtype of adenocarcinomas, immunohistochemical
profiling is essential. According to Park et al (28), CDX2 expression was found in ~60.9% of
gastric adenocarcinoma patients, highlighting the diagnostic value
of organ-specific immunohistochemical markers in differentiating
between adenocarcinoma origins. The immunohistochemical profile,
CDX2-positive, CK7-positive, TTF-1-negative and CK20-negative,
produced a positive predictive value of 85.7% for signet-ring cell
adenocarcinoma in a documented case of the cancer developing within
a teratoma, underscoring the usefulness of immunohistochemical
markers in determining the tissue of origin (29).
Koseoglu et al (30) demonstrated that adenocarcinoma
originating from colon glands in the testis exhibited CEA(+), CA
19-9(+), CK20(+) and CK7(-) based on immunohistochemical staining,
which was distinct from the findings of the case presented herein.
Asano et al (31) reported
that in patients with non-metastatic testicular malignant
transformation, no recurrence occurred following radical
orchiectomy. However, in cases with metastasis, the prognosis of
testicular malignant transformation is heavily influenced by the
histological phenotype. Testicular malignant transformation with
adenocarcinoma typically requires an aggressive treatment regimen.
Kasai et al (27) described a
patient who succumbed to malignant transformation 8 months
following orchiectomy despite receiving cisplatin-based
chemotherapy. In the case in the present study, metastatic lesions
continued to progress despite combination chemotherapy based on
cisplatin. These observations highlight the importance of
discussing potential confounding factors and limitations in
treatment efficacy. Thus, the early detection of malignant
transformation is crucial for improving patient outcomes. Moreover,
incorporating a dedicated section on study limitations and
potential confounders would strengthen the discussion and provide a
more balanced interpretation of the findings. Previous research has
shown that somatic-type malignancies arising in metastatic GCTs are
associated with a significantly worse overall survival compared to
those confined to the testis, with carcinomatous subtypes carrying
the highest risk for mortality (32). Somatic-type malignancies are observed
in ~2.5 to 8% of testicular GCTs, most commonly arising within
teratomas and often presenting in metastatic sites, where they are
associated with poor clinical outcomes (33,34).
The present study has several limitations that
should be acknowledged. First, as a single case report, the
findings cannot be generalized to all patients with testicular
teratomas undergoing malignant transformation. The rarity of such
transformations, particularly into adenocarcinoma, limits the
ability to establish standardized treatment protocols or prognostic
markers. Second, although immunohistochemical analysis was utilized
to identify the histological subtype and probable origin of the
malignancy, genomic or molecular profiling was not performed, which
could have provided deeper insights into the mechanisms of
transformation. Third, the temporal association between
chemotherapy and malignant transformation could not be definitively
established, leaving causality speculative. Finally, due to the
retrospective and descriptive nature of this report, long-term
outcomes beyond the presented follow-up period remain unknown.
These limitations underscore the need for multicenter data
collection and molecular studies to better understand the
pathogenesis and clinical behavior of malignant transformations in
testicular GCTs.
In conclusion, the malignant transformation of
testicular teratomas, particularly into adenocarcinoma, represents
an exceptionally rare and diagnostically challenging entity with
significant therapeutic implications. While somatic malignancies,
such as sarcomas and enteric adenocarcinomas have been reported in
mature teratomas, such transformations in testicular teratomas are
exceedingly rare. The mechanisms driving these transformations,
including differentiation of embryonal carcinoma cells or secondary
changes induced by chemotherapy and radiotherapy, remain under
investigation. Given the rarity of such cases, further research is
warranted to elucidate the molecular and genetic pathways involved
in somatic transformation, particularly those leading to
adenocarcinomatous differentiation. The presence of somatic
malignancies complicates clinical management, requiring detailed
histological and immunohistochemical evaluation. The prognosis
depends on the histological subtype, with adenocarcinoma variants
often necessitating more aggressive treatment. The present case
report highlights the practical importance of vigilant long-term
follow-up, the integration of histopathological expertise and
timely intervention, particularly in patients with recurrent
retroperitoneal masses despite normalized tumor markers. Early
detection through tumor marker monitoring and imaging is crucial,
and a multidisciplinary approach is essential for optimizing
treatment outcomes and improving survival rates.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
All authors (OÖ, EG, AG and PP) were involved in the
conception of the study. OÖ and EG designed the study and were also
involved in the collection and processing of the patient's data. OÖ
and EG treated and examined the patient, while OÖ and EG were
responsible for collecting and processing the patient's imaging
data. OÖ and EG were also involved in the analysis and
interpretation of the patient's data. OÖ, EG and PP were involved
in the literature search. OÖ, EG, AG and PP were involved in the
writing of the study. OÖ and EG confirm the authenticity of all the
raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
Consent was obtained from the patient whose case is
described in the present study.
Patient consent for publication
Consent was obtained from the patient for the use of
the CT images and clinical medical information.
Competing interests
The authors declare that they have no competing
interests.
Use of artificial intelligence tools
During the preparation of this work, AI tools were
used to improve the readability and language of the manuscript or
to generate images, and subsequently, the authors revised and
edited the content produced by the AI tools as necessary, taking
full responsibility for the ultimate content of the present
manuscript.
References
|
1
|
Sagae S and Kudo R: Surgery for germ cell
tumors. Semin Surg Oncol. 19:76–81. 2000.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Kim MJ, Kim NY, Lee DY, Yoon BK and Choi
D: Clinical characteristics of ovarian teratoma: Age-focused
retrospective analysis of 580 cases. Am J Obstet Gynecol.
205:32.e1–e4. 2011.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Snir OL, DeJoseph M, Wong S, Buza N and
Hui P: Frequent homozygosity in both mature and immature ovarian
teratomas: A shared genetic basis of tumorigenesis. Mod Pathol.
30:1467–1475. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Pohl HG, Shukla AR, Metcalf PD, Cilento
BG, Retik AB, Bagli DJ, Huff DS and Rushton HG: Prepubertal testis
tumors: Actual prevalence rate of histological types. J Urol. 172
(6 Pt 1):2370–2372. 2004.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Caspi B, Lerner-Geva L, Dahan M, Chetrit
A, Modan B, Hagay Z and Appelman Z: A possible genetic factor in
the pathogenesis of ovarian dermoid cysts. Gynecol Obstet Invest.
56:203–206. 2003.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Motzer RJ, Amsterdam A, Prieto V,
Sheinfeld J, Murty VV, Mazumdar M, Bosl GJ, Chaganti RS and Reuter
VE: Teratoma with malignant transformation: Diverse malignant
histologies arising in men with germ cell tumors. J Urol.
159:133–138. 1998.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Park CH, Jung MH and Ji YI: Risk factors
for malignant transformation of mature cystic teratoma. Obstet
Gynecol Sci. 58:475–480. 2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Cong L, Wang S, Yeung SY, Lee JHS, Chung
JPW and Chan DYL: Mature cystic teratoma: An integrated review. Int
J Mol Sci. 24(6141)2023.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Salzillo C, Imparato A, Fortarezza F,
Maniglio S, Lucà S, La Verde M, Serio G and Marzullo A: Gonadal
teratomas: A state-of-the-art review in pathology. Cancers (Basel).
16(2412)2024.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Cheng L, Zhang S, MacLennan GT, Poulos CK,
Sung MT, Beck SD and Foster RS: Interphase fluorescence in situ
hybridization analysis of chromosome 12p abnormalities is useful
for distinguishing epidermoid cysts of the testis from pure mature
teratoma. Clin Cancer Res. 12:5668–5672. 2006.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Zhang C, Berney DM, Hirsch MS, Cheng L and
Ulbright TM: Evidence supporting the existence of benign teratomas
of the postpubertal testis: A clinical, histopathologic, and
molecular genetic analysis of 25 cases. Am J Surg Pathol.
37:827–835. 2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Moraru L, Mitranovici MI, Chiorean DM,
Coroș M, Moraru R, Oală IE and Turdean SG: Immature teratoma:
Diagnosis and management-a review of the literature. Diagnostics
(Basel). 13(1516)2023.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Giannatempo P, Pond GR, Sonpavde G, Albany
C, Loriot Y, Sweeney CJ, Salvioni R, Colecchia M, Nicolai N, Raggi
D, et al: Treatment and clinical outcomes of patients with teratoma
with somatic-type malignant transformation: An international
collaboration. J Urol. 196:95–100. 2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Kawaguchi M, Kato H, Furui T, Noda Y,
Hyodo F, Miyazaki T and Matsuo M: MRI findings of malignant
transformation arising from mature cystic teratoma of the ovary:
Comparison with benign mature cystic teratoma. Jpn J Radiol.
42:500–507. 2024.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Chiang AJ, Chen MY, Weng CS, Lin H, Lu CH,
Wang PH, Huang YF, Chiang YC, Yu MH and Chang CL: Malignant
transformation of ovarian mature cystic teratoma into squamous cell
carcinoma: A Taiwanese Gynecologic Oncology Group (TGOG) study. J
Gynecol Oncol. 28(e69)2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Hackethal A, Brueggmann D, Bohlmann MK,
Franke FE, Tinneberg HR and Münstedt K: Squamous-cell carcinoma in
mature cystic teratoma of the ovary: Systematic review and analysis
of published data. Lancet Oncol. 9:1173–1180. 2008.PubMed/NCBI View Article : Google Scholar
|
|
17
|
De Bruijn HWA, Hollema H, Willemse KA,
Hoor T and Boonstra H: Raised serum squamous cell carcinoma antigen
levels in malignant transformation of mature cystic ovarian
teratoma. Int J Gynecol Cancer. 6:76–79. 1996.
|
|
18
|
El Mesbahi O, Terrier-Lacombe MJ,
Rebischung C, Theodore C, Vanel D and Fizazi K: Chemotherapy in
patients with teratoma with malignant transformation. Eur Urol.
51:1306-1311; discussion 1311-1312. 2007.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Li Y, Zhang R, Pan D, Huang B, Weng M and
Nie X: KRAS mutation in adenocarcinoma of the gastrointestinal type
arising from a mature cystic teratoma of the ovary. J Ovarian Res.
7(85)2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Comiter CV, Kibel AS, Richie JP, Nucci MR
and Renshaw AA: Prognostic features of teratomas with malignant
transformation: A clinicopathological study of 21 cases. J Urol.
159:859–863. 1998.PubMed/NCBI
|
|
21
|
Rim SY, Kim SM and Choi HS: Malignant
transformation of ovarian mature cystic teratoma. Int J Gynecol
Cancer. 16:140–144. 2006.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Michal M, Hes O and Kazakov DV: Primary
signet-ring stromal tumor of the testis. Virchows Archiv.
447:107–110. 2005.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Guo CC, Punar M, Contreras AL, Tu SM,
Pisters L, Tamboli P and Czerniak B: Testicular germ cell tumors
with sarcomatous components: An analysis of 33 cases. Am J Surg
Pathol. 33:1173–1178. 2009.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Clark ME and Will MD: Intestinal-type
adenocarcinoma arising in a mature cystic teratoma of the ovary.
Int J Gynecol Pathol. 35:352–356. 2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Ganjoo KN, Foster RS, Michael H, Donohue
JP and Einhorn LH: Germ cell tumor associated primitive
neuroectodermal tumors. J Urol. 165:1514–1516. 2001.PubMed/NCBI
|
|
26
|
Zeh N, Wild PJ, Bode PK, Kristiansen G,
Moch H, Sulser T and Hermanns T: Retroperitoneal teratoma with
somatic malignant transformation: A papillary renal cell carcinoma
in a testicular germ cell tumour metastasis following
platinum-based chemotherapy. BMC Urol. 13(9)2013.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Kasai T, Moriyama K, Tsuji M, Uema K,
Sakurai N and Fujii Y: Adenocarcinoma arising from a mature cystic
teratoma of the testis. Int J Urol. 10:505–509. 2003.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Park SY, Kim BH, Kim JH, Lee S and Kang
GH: Panels of immunohistochemical markers help determine primary
sites of metastatic adenocarcinoma. Arch Pathol Lab Med.
131:1561–1567. 2007.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Kuo CY, Wen MC, Wang J and Jan YJ:
Signet-ring stromal tumor of the testis: A case report and
literature review. Hum Pathol. 40:584–587. 2009.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Koseoglu RD, Parlaktas BS, Filiz NO,
Erdemir F, Uluocak N and Tulunay O: Adenocarcinoma originating from
a mature teratoma of the testis. Kaohsiung J Med Sci. 23:265–268.
2007.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Asano T, Kawakami S, Okuno T, Tsujii T,
Nemoto T, Kageyama Y and Kihara K: Malignant transformation in a
mature testicular teratoma left untreated for more than 50 years
since childhood. Scand J Urol Nephrol. 37:177–178. 2003.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Hwang MJ, Hamza A, Zhang M, Tu SM, Pisters
LL, Czerniak B and Guo CC: Somatic-type malignancies in testicular
germ cell tumors: A clinicopathologic study of 63 cases. Am J Surg
Pathol. 46:11–17. 2022.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Chu B and Huang J: Neuroendocrine tumor as
a somatic-type malignancy in metastatic teratoma. Int J Surg
Pathol: May 30, 2025 (Epub ahead of print).
|
|
34
|
Stephanie ES and Acosta AM: Somatic-type
malignancies of germ cell origin: Molecular, pathologic, and
clinical features. Surg Pathol Clin. 18:101–117. 2025.PubMed/NCBI View Article : Google Scholar
|