Introduction
Breast cancer (BCa) remains one of the most
prevalent tumors affecting women worldwide. According to the World
Cancer Research Fund, BCa is the most commonly occurring type of
cancer among women and the second most common type of cancer
overall, with over two million new cases recorded in 2018(1). In particular, the incidence rate of BCa
in Bangladesh according to GLOBOCAN is 105.6 per 100,000
individuals per year (2). This high
incidence rate implies the importance of effective treatment
modalities.
Ovarian ablation (OA), a critical therapeutic
approach, particularly in hormone-receptor-positive metastatic BCa,
functions by reducing estrogen production. In fact, estrogen plays
a crucial role in the proliferation of hormone-receptor-positive
BCa cells. OA can be achieved through several methods: Surgical
oophorectomy, radiation-induced OA or pharmacological agents,
leading to ovarian suppression. Each method has its distinct
mechanisms and implications (3).
Radiotherapy (RT), as a means of OA, has been used
for a number of decades due to its non-invasive nature and
potential efficacy. RT involves the application of ionizing
radiation to the ovarian tissue, leading to follicular destruction
and consequently, estrogen deprivation. Moreover, RT is often
preferred for OA in low-middle-income countries (LMICs) due to its
cost-effectiveness, particularly in comparison to surgical methods
or hormonal treatments, such as luteinising hormone-releasing
hormone analogs. In fact, RT provides a practical alternative in
settings where medical or surgical facilities are limited, reducing
the need for postoperative care. Additionally, the simplicity and
one-time expense of RT make it more feasible and financially viable
for both healthcare systems and patients in resource-constrained
environment.
However, the lack of clear guidelines on the optimal
dose and fractionation of RT poses challenges. The variability in
practice and the absence of a standardized protocol underscore the
need for comparative studies (3).
Given this background, the present study aimed to compare two
different RT protocols for OA in patients with metastatic BCa: A
dose of 15 Gy delivered in 5 fractions vs. 20 Gy in 10 fractions.
The present comparative analysis is intended to provide clearer
insight into the efficacy and safety of these protocols,
potentially guiding future clinical practice and standardizing
treatment approaches.
Patients and methods
Study design and setting
The present study was conducted at the Department of
Radiation Oncology, National Institute of Cancer Research and
Hospital, Dhaka, Bangladesh, from September 30, 2021 to June 30,
2022. The study aimed to evaluate the efficacy and safety of RT for
OA in patients with metastatic BCa. The present study was conducted
according to the guidelines of the Declaration of Helsinki and was
approved by Ethics Committee of the National Institute of Cancer
Research and Hospital, Dhaka, Bangladesh (NICRH/Ethics/2021/280;
dated September 30, 2021). Written informed consent was obtained
from all subjects involved in the study.
Patient selection
A total of 68 patients were enrolled in the study,
with 34 patients allocated to each study arm by purposive sampling.
The inclusion criteria were the following: Premenopausal female
patients with a confirmed diagnosis of hormone-receptor-positive,
Her-2 negative metastatic BCa, for whom OA was deemed clinically
necessary. The exclusion criteria included patients who were in
visceral crisis, or who had previous history of ovarian surgery or
pelvic RT, or had received chemotherapy within the past 1 year of
the study, or those with pre-existing conditions affecting ovarian
function.
Operational definitions
Patients were considered hormone receptor-positive
in the present study if, in available immunohistochemical reports
performed at another laboratory, they had ≥10% estrogen receptor
(ER)- or progesterone receptor (PR)-positive cells. Female patients
were deemed to be premenopausal and included in the present study
if they had: i) A normal menstrual period within 2 months
clinically; or ii) a normal menstrual period within the past 12
months, with serum follicle-stimulating hormone (FSH) and estradiol
levels within the premenopausal range. Patients were considered to
have developed amenorrhea if they had experienced an absence of
menstruation for 3 consecutive months without any subsequent
resumption. Serum FSH levels >22 mIU/ml and estradiol levels
<30 pg/ml were used as criteria for postmenopausal hormone
levels to observe the response. The Common Terminology Criteria for
Adverse Events (CTCAE) version 5.0 was used to evaluate
radiation-induced toxicities (4).
Postmenopausal symptoms were assessed using the standardized
Menopause Rating Scale (MRS), which measures health-related quality
of life regarding 11 symptoms related to the menopause transition.
The presence of a visceral crisis, considered as an exclusion
criterion for the study, was defined as extensive visceral
metastasis with profound symptomatic involvement, such as
lymphangitis carcinomatosis, bone marrow replacement, lung
metastases with severe symptoms, carcinomatous meningitis,
significant liver metastasis, or a rise in liver function markers
to three times above the upper limit.
RT technique
The RT technique employed was two-dimensional (2D)
radiation therapy. For treatment, patients were positioned in a
supine position on the treatment table, and radiopaque markers were
utilized to outline the pelvic region. Anteroposterior and
posteroanterior pelvic fields were designed to fully encompass the
ovaries. The delineation of field borders included the following:
The inferior border was set at the lower border of the obturator
foramen; superiorly, it extended to the inferior sacroiliac joint;
and laterally, it was placed 1.5 cm beyond the true pelvic brim. To
ensure precise ablation, the localization of the ovaries was
verified using ultrasonography. Of note, two different dosing
regimens were prescribed for the present study: Arm A received a
total dose of 15 Gy, delivered in 5 fractions over the course of 1
week, while arm B was administered 20 Gy in 10 fractions spread
over a period of 2 weeks, with treatments administered on
consecutive days.
Assessment of efficacy
The primary endpoint was the efficacy of RT in
inducing OA, assessed by the development and persistence of
amenorrhea and the attainment of postmenopausal levels of FSH and
estradiol. These hormonal levels were measured prior to the
commencement of RT and then at 4, 12, and 24 weeks post-RT.
Statistical analysis
Data were analyzed using appropriate statistical
methods to compare the efficacy and safety profiles of the two RT
regimens. The primary comparative analysis focused on the rate of
OA, while secondary analyses included the assessment of
radiation-induced toxicities and menopausal symptoms. The
statistical method used was the inferential statistical analysis
(Hypothesis testing). Analyses were performed using a two-way mixed
ANOVA test followed by the Bonferroni post hoc test and independent
t-test for continuous variables and the Chi-squared test and
Fisher's exact test for categorical variables. For associations,
the Chi-squared test of independence was used. All reported
P-values were two-sided and P-values <0.05 were considered to
indicate statistically significant differences. As statistical
software, IBM SPSS software version 25.0 for windows (IBM SPSS
Statistics for Windows, version 25.0; IBM Corp.) was used.
Results
Efficacy of RT in OA
The patient characteristics are detailed in Table I. In evaluating the efficacy of RT
for OA, the present study found no significant differences between
arm A (15 Gy in 5 fractions) and arm B (20 Gy in 10 fractions). The
rate of development and persistence of amenorrhea was comparable
between the two arms, with 85.7% in arm A and 89.5% in arm B
(P-value, not significant), excluding 28 (41.17%) patients who had
pre-RT amenorrhea with premenopausal hormonal levels. Similarly,
the achievement of postmenopausal estradiol levels exhibited no
significant differences between the groups, being 91.2% in arm A
and 94.1% in arm B (P-value, not significant). The rate of
attainment of postmenopausal FSH levels was also similar, with
79.4% in arm A and 88.2% in arm B (P-value, not significant)
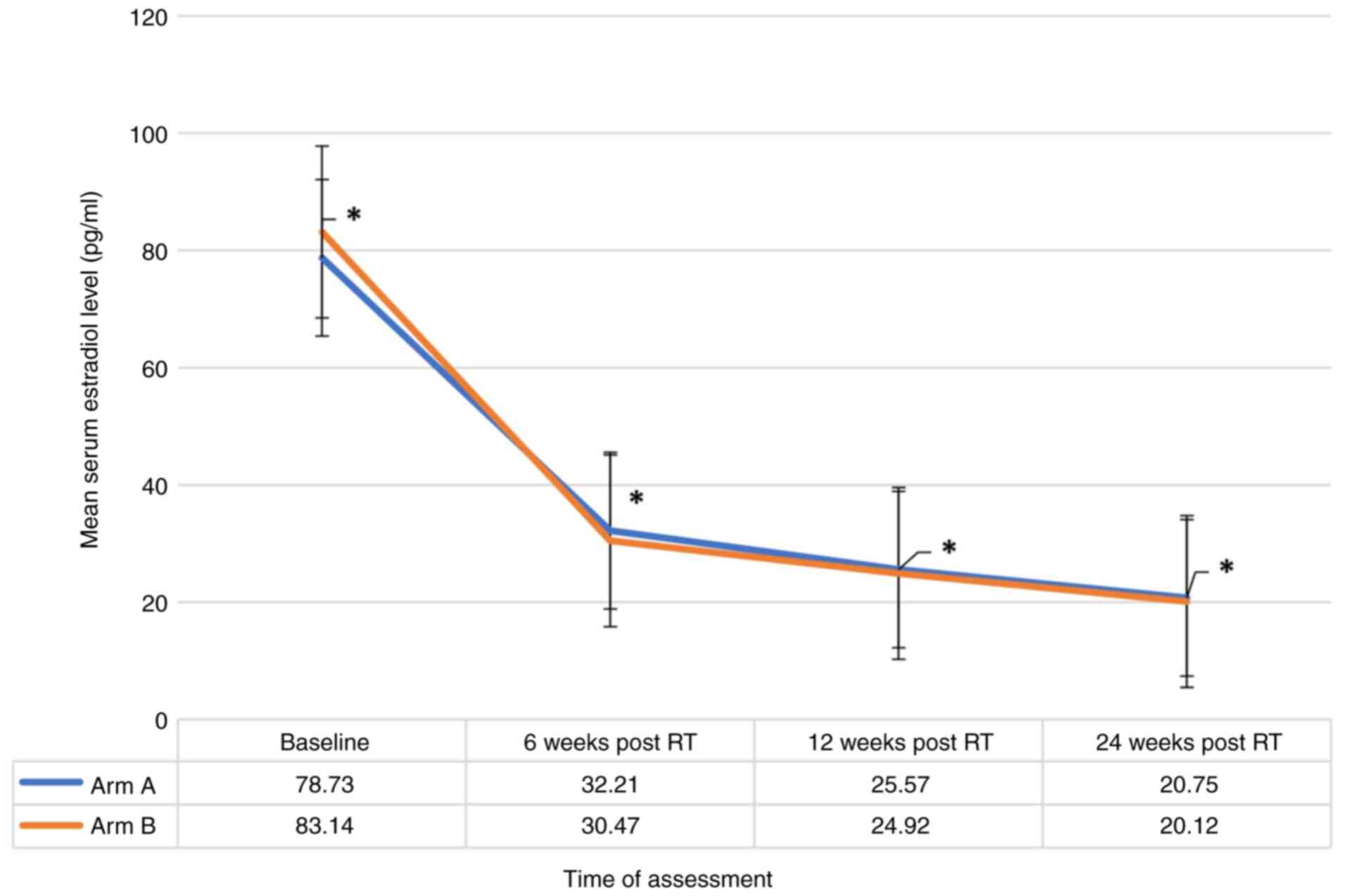
(Table II). A significant reduction
(P-value <0.001) in the mean estradiol level in all the patients
included in both groups across all four time points was observed
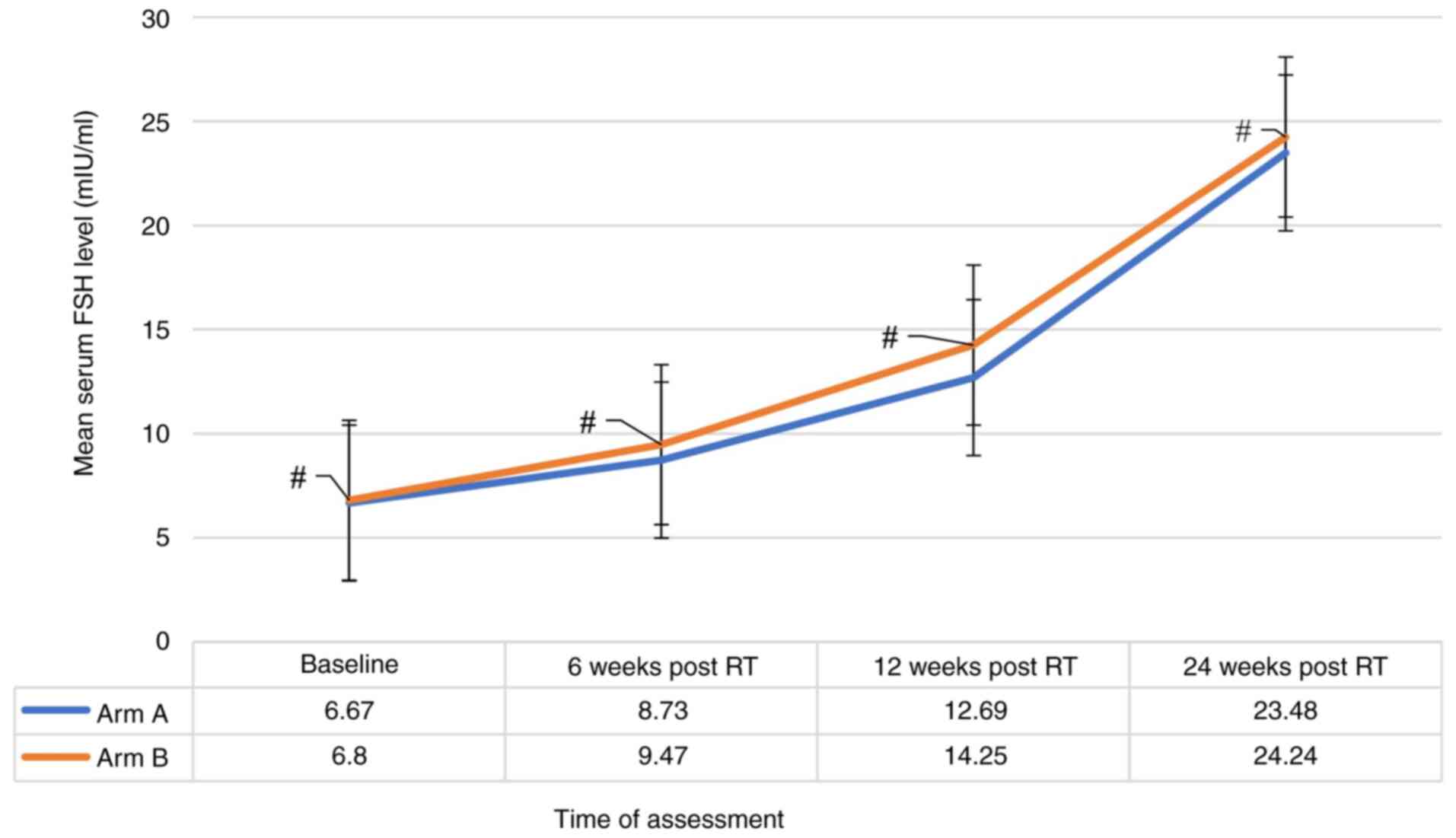
(Fig. 1), alongside a notable
consistent significant increase in the mean FSH level at all four
points (P-value <0.001) (Fig. 2).
However, these changes were not significantly different between the
two treatment groups when compared at baseline, 6 weeks, 12 weeks
and at 24 weeks indicating that both groups followed a similar
pattern of estradiol and FSH levels (Figs. 1 and 2). Additionally, there was no strong
evidence of a difference in the mean estradiol (P=0.856) and mean
FSH (P=0.056) levels between the two groups.
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| Characteristic | Arm A (15 Gy/5
fractions) | Arm B (20 Gy/10
fractions) | All patients |
|---|
| Age (years), median
(range) | 35 (26-48) | 37 (26-49) | 36, (26-49) |
| Previous
chemotherapy | 30 (88.2%) | 29 (85.3%) | 59 (86.76%) |
| Body mass index
(kg/m2), median (range) | 24 (20-32) | 23 (20-33) | 24 (20-33) |
 | Table IIDistribution of patients according to
the response to treatment after 24 weeks (n=68). |
Table II
Distribution of patients according to
the response to treatment after 24 weeks (n=68).
| Treatment
response | Arm-A (n=34) | Arm-B (n=34) | P-value |
|---|
| Development of
amenorrhea | n=34 | % | n=34 | % | 0.831
(NS)a |
| Prior to RT | 13 | 38.2 | 15 | 44.1 | |
| Amenorrhea after
RT | 18 | 52.9 | 17 | 50.0 | |
| Menstruating after
RT | 3 | 8.8 | 2 | 5.9 | |
| Estradiol level | | | | | 0.642
(NS)a |
| Postmenopausal level
(<30 pg/ml) | 31 | 91.2 | 32 | 94.1 | |
| Premenopausal level
(>30 pg/ml) | 3 | 8.8 | 2 | 5.9 | |
| FSH level | | | | | 0.323
(NS)b |
| Postmenopausal level
(>22 mIU/ml) | 27 | 79.4 | 30 | 88.2 | |
| Premenopausal level
(<22 mIU/ml) | 7 | 20.6 | 4 | 11.8 | |
Factors influencing efficacy of
OA
The analysis revealed that a younger age (<36
years), a higher body mass index (BMI), elevated baseline estradiol
levels, and the absence of prior chemotherapy were significantly
associated with a failure to achieve OA (P<0.05). These findings
suggest that patient-specific characteristics play a crucial role
in the response to RT for OA (Table
III, Table IV, Table V and Table VI).
 | Table IIIAssociation of amenorrhea with the age
of the patients (n=68). |
Table III
Association of amenorrhea with the age
of the patients (n=68).
| | Amenorrhea | |
|---|
| | Amenorrhea prior to
RT (n=28) | After 24 weeks of RT
(n=35) | No amenorrhea after
24 weeks of RT (n=5) | |
|---|
| Age group
(years) | No. of patients | % | No. of patients | % | No. of patients | % | P-value |
|---|
| 26-30 | 2 | 7.1 | 4 | 11.4 | 3 | 60.0 | 0.013a |
| 31-35 | 3 | 10.7 | 10 | 28.6 | 2 | 40.0 | |
| 36-40 | 12 | 42.9 | 9 | 25.7 | 0 | 0 | |
| 41-45 | 10 | 35.7 | 8 | 22.9 | 0 | 0 | |
| 45-50 | 1 | 3.6 | 4 | 11.4 | 0 | 0 | |
 | Table IVAssociation of post-RT hormone levels
with BMI in the patients (n=68). |
Table IV
Association of post-RT hormone levels
with BMI in the patients (n=68).
| A, Estradiol |
|---|
| | Estradiol level | |
|---|
| | <30 pg/ml
(n=63) | >30 pg/ml
(n=5) | |
|---|
| BMI
(kg/m2) | n=63 | % | n=5 | % | P-value |
|---|
| 18.5-24.9
(normal) | 41 | 65.1 | 1 | 20 | 0.009a,b |
| 25.0-29.9
(overweight) | 19 | 30.2 | 2 | 40 | |
| ≥30.0 (obese) | 3 | 4.8 | 2 | 40 | |
| B, FSH |
| | FSH level | |
| | >22 mIU/ml
(n=57) | <22 mIU/ml
(n=11) | |
| BMI
(kg/m2) | n=57 | % | n=11 | % | P-value |
| 18.5-24.9
(normal) | 37 | 64.9 | 5 | 45.5 | 0.467
(NS)a |
| 25.0-29.9
(overweight) | 16 | 28.1 | 5 | 45.5 | |
| ≥30.0 (obese) | 4 | 7 | 1 | 9.1 | |
 | Table VAssociation of post-RT hormone levels
with a history of systemic therapy. |
Table V
Association of post-RT hormone levels
with a history of systemic therapy.
| A, Estradiol |
|---|
| | Estradiol
level | |
|---|
| | <30 pg/ml
(n=63) | >30 pg/ml
(n=5) | |
|---|
| Systemic
therapy | n=63 | % | n=5 | % | P-value |
|---|
| Previously received
chemotherapy | | | | |
0.001** |
|
Yes | 57 | 90.5 | 2 | 40 | |
|
No | 6 | 9.50 | 3 | 60 | |
| Previously received
hormone therapy | | | | | 0.056
(NS)** |
|
Yes | 40 | 63.5 | 1 | 20 | |
|
No | 23 | 36.5 | 4 | 80 | |
| B, FSH |
| | FSH level | |
| | >22 mIU/ml
(n=57) | <22 mIU/ml
(n=11) | |
| Systemic
therapy | n=57 | % | n=11 | % | P-value |
| Previously received
chemotherapy | | | | | 0.001a,b |
|
Yes | 53 | 93.0 | 6 | 54.5 | |
|
No | 4 | 7.0 | 5 | 45.5 | |
| Previously received
hormone therapy | | | | | 0.076
(NS)c |
|
Yes | 37 | 64.9 | 4 | 36.4 | |
|
No | 20 | 35.1 | 7 | 63.6 | |
 | Table VIAssociation of post-RT hormonal
levels with mean baseline hormonal level. |
Table VI
Association of post-RT hormonal
levels with mean baseline hormonal level.
| A, Estradiol |
|---|
| | Post-RT estradiol
level | |
|---|
| | <30 pg/ml
(n=63) | >30 pg/ml
(n=5) | |
|---|
| Baseline hormonal
level | Mean | ± SD | Mean | ± SD | P-value |
|---|
| Mean FSH
(mIU/ml) | 6.62 | ±2.26 | 8.2 | ±2.4 | 0.141 (NS) |
| Range
(min-max) | 4.0 | -15.0 | 5.5 | -12.0 | |
| Mean estradiol
(pg/ml) | 78.095 | ±23.87 | 116.8 | ±35.513 | 0.001a |
| Range
(min-max) | 40.0 | -150 | 84.0- | 160.0 | |
| B, FSH |
| | Post-RT FSH
level | |
|
| >22 mIU/ml
(n=57) | <22 mIU/ml
(n=11) | |
| | Mean | ± SD | Mean | ± SD | P-value |
| Mean FSH
(mIU/ml) | 6.68 | ±2.32 | 7.0 | ±2.26 | 0.682 (NS) |
| Range
(min-max) | 4.0 | -15.0 | 4.1 | -12.0 | |
| Mean estradiol
(pg/ml) | 78.965 | ±24.10 | 91.18 | ±36.62 | 0.164 (NS) |
| Range
(min-max) | 40.0 | -150 | 49.0- | 160.0 | |
Toxicity and postmenopausal
symptoms
As regards safety, the treatment was well tolerated
in both arms. No grade ≥3 radiation induced toxicities were
observed. The most common postmenopausal symptoms reported by the
patients in both study arms were hot flushes and irritability, with
no significant difference in their incidence or severity between
the two treatment groups (Tables
VII and VIII).
 | Table VIIDistribution of the patients in the
present study according to toxicity following radiotherapy
(n=68). |
Table VII
Distribution of the patients in the
present study according to toxicity following radiotherapy
(n=68).
| | Arm-A (n=34) | Arm-B (n=34) | |
|---|
| Acute toxicity | n | % | n | % | P-value |
|---|
| Skin toxicity | | | | | 0.546
(NS)a |
|
Grade 1 | 3 | 8.8 | 4 | 11.8 | |
|
Grade 2 | 0 | 0.0 | 1 | 2.9 | |
|
Absent | 31 | 91.2 | 29 | 85.3 | |
| Vomiting | | | | | 0.535
(NS)a |
|
Grade 1 | 8 | 23.5 | 10 | 29.4 | |
|
Grade 2 | 2 | 5.9 | 4 | 11.8 | |
|
Absent | 24 | 70.6 | 20 | 58.8 | |
| Nausea | | | | | 0.965
(NS)a |
|
Grade 1 | 10 | 29.4 | 11 | 32.4 | |
|
Grade 2 | 3 | 8.8 | 3 | 8.8 | |
|
Absent | 21 | 61.8 | 20 | 58.8 | |
| Abdominal pain | | | | | 0.400
(NS)b |
|
Grade 1 | 8 | 23.5 | 10 | 29.4 | |
|
Grade 2 | 3 | 8.8 | 6 | 17.6 | |
|
Absent | 23 | 67.6 | 18 | 52.9 | |
| Diarrhea | | | | | 0.639
(NS)a |
|
Grade 1 | 4 | 11.8 | 6 | 17.6 | |
|
Grade 2 | 1 | 2.9 | 2 | 5.9 | |
|
Absent | 29 | 85.3 | 26 | 76.5 | |
| Urinary
toxicity | | | | | 0.554
(NS)a |
|
Grade 1 | 4 | 11.8 | 5 | 14.7 | |
|
Grade 2 | 0 | 0.0 | 1 | 2.9 | |
|
Absent | 30 | 88.2 | 28 | 82.4 | |
 | Table VIIIPostmenopausal symptoms of the study
patients (according menopause rating scale) (n=68). |
Table VIII
Postmenopausal symptoms of the study
patients (according menopause rating scale) (n=68).
| | Grade | |
|---|
| Postmenopausal
symptoms | 0 | 1 | 2 | 3 | 4 |
P-valuea |
|---|
| Hot
flush/sweating | | | | | | |
|
Arm-A | 9 | 10 | 11 | 4 | 0 | 0.941 (NS) |
|
Arm –B | 11 | 9 | 11 | 3 | 0 | |
| Heart
discomfort/palpitation | | | | | | |
|
Arm-A | 14 | 12 | 8 | 0 | 0 | 0.835 (NS) |
|
Arm-B | 15 | 13 | 6 | 0 | 0 | |
| Irritability | | | | | | |
|
Arm-A | 9 | 10 | 10 | 5 | 0 | 0.950 (NS) |
|
Arm-B | 8 | 12 | 10 | 4 | 0 | |
| Vaginal
dryness | | | | | | |
|
Arm-A | 16 | 9 | 6 | 3 | 0 | 0.994 (NS) |
|
Arm-B | 15 | 10 | 6 | 3 | 0 | |
| Anxiety | | | | | | |
|
Arm-A | 10 | 9 | 10 | 5 | 0 | 0.852 (NS) |
|
Arm-B | 11 | 8 | 12 | 3 | 0 | |
| Dyspareunia/sexual
problem | | | | | | |
|
Arm-A | 9 | 16 | 6 | 3 | 0 | 0.994 (NS) |
|
Arm-B | 10 | 15 | 6 | 3 | 0 | |
| Bladder
problem | | | | | | |
|
Arm-A | 25 | 9 | 0 | 0 | 0 | 0.500 (NS) |
|
Arm-B | 24 | 10 | 0 | 0 | 0 | |
| Physical &
mental exhaustion | | | | | | |
|
Arm-A | 10 | 9 | 10 | 5 | 0 | 0.991 (NS) |
|
Arm-B | 9 | 10 | 10 | 5 | 0 | |
| Joint &
muscular discomfort | | | | | | |
|
Arm-A | 26 | 8 | 0 | 0 | 0 | 0.500 (NS) |
|
Arm-B | 25 | 9 | 0 | 0 | 0 | |
| Insomnia/sleep
problem | | | | | | |
|
Arm-A | 9 | 10 | 11 | 4 | 0 | 0.960 (NS) |
|
Arm-B | 10 | 11 | 9 | 4 | 0 | |
| Mood
change/depressive mood | | | | | | |
|
Arm-A | 15 | 13 | 6 | 0 | 0 | 0.835 (NS) |
|
Arm-B | 14 | 12 | 8 | 0 | 0 | |
Discussion
The present study aimed to assess the efficacy and
safety of RT for OA in patients with metastatic BCa. It primarily
investigated the role of RT in inducing OA, assessed through the
development and persistence of amenorrhea, and the attainment of
postmenopausal levels of FSH and estradiol within 24 weeks of
treatment. Using a 2D RT technique, 68 patients were treated with
one of two different dose regimens: 15 Gy delivered in 5 fractions
(arm A) or 20 Gy delivered in 10 fractions (arm B). The
tolerability of these protocols treatments was measured through the
CTCAE version 5.0 for radiation-induced toxicities, and the MRS for
evaluating post-menopausal symptoms.
The results indicated no significant differences
between the two arms in terms of the rate of amenorrhea
development, and the achievement of postmenopausal estradiol and
FSH levels. In fact, both groups demonstrated a significant
decrease in mean estradiol levels and an increase in mean FSH
levels compared to baseline, without significant differences
between the groups. Furthermore, a younger age, a higher BMI, a
high estradiol level, and the absence of prior chemotherapy were
significantly associated with the failure to achieve OA. Notably,
the study observed no grade ≥3 toxicity, and the most common
postmenopausal symptoms were hot flushes and irritability, with no
significant differences between the study arms.
The results of the present study can be compared
with those of previous studies. In the meta-analysis by Asiri et
al (5), the efficacy of
RT-induced OA was assessed in terms of amenorrhea rates,
progression-free survival and overall survival. Their study
concluded that RT-OA was effective, with doses of 15 Gy in 5
fractions, 15 Gy in 4 fractions, 16 Gy in 4 fractions, and 20 Gy in
10 fractions demonstrating high amenorrhea rates (5). Comparatively, the present study used
doses of 15 Gy in 5 fractions and 20 Gy in 10 fractions, aligning
closely with two regimens evaluated in the aforementioned
meta-analysis. Both studies found no significant differences in the
efficacy of OA between different dose regimens, suggesting that a
lower dose may be sufficient for effective OA.
In their retrospective evaluation, Bese et al
(6) reported a high rate of
amenorrhea (96%) with various doses ranging from 5 Gy in a single
fraction to 36 Gy in 18 fractions. Their study did not report any
severe acute or late complications attributable to RT (6). This aligns with the findings of the
present study, where no significant difference in amenorrhea rates
was observed between the two study arms, and no severe toxicity was
noted. The broad range of doses used in the study by Bese et
al (6) suggests a potential for
flexibility in dosing without compromising the efficacy of OA.
Hughes et al (7) reported 75% successful OA using a dose
of 20 Gy in 10 fractions, with no reported grade 3 or 4 toxicities.
This is consistent with the findings of the present study. In fact,
both analyses highlight the relatively low toxicity profile of
RT-OA, supporting its safety and tolerability. One of the most
notable aspects of the present study is its setting in a LMIC,
distinct from the majority of prior studies conducted predominantly
in high-income countries (8-12).
In fact, the effectiveness and tolerability of
medical therapies, including OA, can be greatly influenced by the
treatment setting. This is particularly true in LMICs, where unique
challenges, such as the prevalence of specific comorbidities,
issues related to malnutrition and the utilization of less advanced
medical technologies, including obsolete RT techniques, could
potentially affect the outcomes of such treatments. These factors
may influence not only the efficacy of the treatment, but also its
tolerability, patient compliance and overall outcomes.
Despite these potential limitations, the present
study achieved results consistent with those obtained in
high-income settings (8-12).
This finding is crucial, as it suggests that OA, even when
conducted under the constraints typical of a LMIC setting, can be
effective and well-tolerated. This is significant, particularly in
the context of resource-limited healthcare environments, where
access to the latest medical technologies and treatments is often
challenging. Moreover, in Bangladesh, radiotherapy-induced ovarian
ablation (RT-induced OA) can be performed at a cost as low as $25.
This cost is extremely low compared to the $1,200 required for 2
years of ovarian suppression using hormonal agents, or the $600
needed for surgical oophorectomy.
Furthermore, as demonstrated herein, the equivalence
in the efficacy of OA administered in 5 sessions, as opposed to 10,
holds particular importance in low resource settings. In fact, the
scarcity of RT equipment in a number of LMICs often leads to
extended waiting lists. Therefore, a shorter treatment regimen not
only reduces the burden on healthcare resources, but also improves
patient access to timely treatment and compliance to the prescribed
treatment. This can be a critical factor in the management of
metastatic BCa, where timely intervention can significantly impact
patient outcomes and quality of Life.
In conclusion, the findings of the present study not
only align with international research (5-7) but
also extend its applicability to LMIC contexts. They underscore the
potential for adapting and optimizing cancer treatment protocols in
resource-limited settings, thereby enhancing the global equity in
cancer care.
The present study, while providing key insight into
the efficacy of OA using RT, had certain limitations that need to
be mentioned. The small sample size and the specific demographic
characteristics of the participants may affect the generalizability
of the findings to a broader population. Another constraint was the
short follow-up period, which limited the authors' ability to
assess long-term efficacy in terms of persistence of clinical and
hormonal response as well as survival and also late-onset
toxicities. Furthermore, being a quasi-experimental study, there
may be inherent biases in data collection and analysis methods,
potentially affecting the reliability of the results.
On the other hand, the present study also had
several strengths. It highlighted the feasibility of a shorter
treatment regimen, potentially useful in reducing treatment wait
times and improving patient access to care. Moreover, the
assessment approach, using both clinical and biochemical markers,
provided a comprehensive understanding of the treatment impact.
Notably, the safety profile of the treatment regimens using
conventional 2D technique was a key finding, with no severe
toxicities reported in either treatment arm. This aspect is
particularly significant in the settings of metastatic BCa, where
patient tolerance and quality of life are paramount.
In summary, while the present study has some
limitations typical of quasi-experimental designs, its strengths
lie in its practical applicability, comprehensive outcome
assessment, and demonstrated safety profile.
Acknowledgements
The authors sincerely thank the secretary at Alma
Mater Studiorum University of Bologna, Ms. Federica Busi, for her
secretarial and technical support in the drafting of this
manuscript.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
TH, EG and AGM were involved in the conception and
design of the study. TH, SA and NTH were involved in research and
data collection. TH, EG, AH, AFMKU, SB, QMH and NTH were involved
in the analysis and interpretation of the data. TH, EG and AGM were
involved in the writing of the manuscript. All authors have read
and approved the final manuscript. TH, AH, NTH and SB confirm the
authenticity of all the raw data.
Ethics approval and consent to
participate
The present study was conducted according to the
guidelines of the Declaration of Helsinki and approved by Ethics
Committee of National Institute of Cancer Research and Hospital,
Dhaka, Bangladesh (NICRH/Ethics/2021/280; dated: September 30,
2021). Written informed consent was obtained from all subjects
involved in the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Arnold M, Morgan E, Rumgay H, Mafra A,
Singh D, Laversanne M, Vignat J, Gralow JR, Cardoso F, Siesling S
and Soerjomataram I: Current and future burden of breast cancer:
Global statistics for 2020 and 2040. Breast. 66:15–23.
2022.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Ferlay J, Ervik M, Lam F, Laversanne M,
Colombet M, Mery L, Piñeros M, Znaor A, Soerjomataram I and Bray F:
Global Cancer Observatory: Cancer Today. International Agency for
Research on Cancer, Lyon, 2024. Available from: https://gco.iarc.who.int/today. Accessed March 2,
2024.
|
|
3
|
Celio L, Bajetta E, Toffolatti L, Catena
L, Beretta E and Buzzoni R: Ovarian ablation for premenopausal
early-stage breast cancer: An update. Tumori. 86:191–194.
2000.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Department of Health and Human Services,
National Institutes of Health, National Cancer Institute. Common
Terminology Criteria for Adverse Events (CTCAE). Version 5.0.
Published November 27, 2017. Available from: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_5x7.pdf.
|
|
5
|
Asiri MA, Tunio MA and Abdulmoniem R: Is
radiation-induced ovarian ablation in breast cancer an obsolete
procedure? Results of a meta-analysis. Breast Cancer (Dove Med
Press). 8:109–116. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Bese NS, Iribas A, Dirican A, Oksuz D,
Atkovar G and Ober A: Ovarian ablation by radiation therapy: Is it
still an option for the ablation of ovarian function in endocrine
responsive premenopausal breast cancer patients? Breast.
18:304–308. 2009.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Hughes LL, Gray RJ, Solin LJ, Robert NJ,
Martino S, Tripathy D, Ingle JN and Wood WC: Eastern Cooperative
Oncology Group; Southwest Oncology Group et al. Efficacy of
radiotherapy for ovarian ablation: Results of a breast intergroup
study. Cancer. 101:969–972. 2004.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Boccardo F, Rubagotti A, Perrotta A,
Amoroso D, Balestrero M, De Matteis A, Zola P, Sismondi P, Francini
G, Petrioli R, et al: Ovarian ablation versus goserelin with or
without tamoxifen in pre-perimenopausal patients with advanced
breast cancer: Results of a multicentric Italian study. Ann Oncol.
5:337–342. 1994.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Ejlertsen B, Mouridsen HT, Jensen MB,
Bengtsson NO, Bergh J, Cold S, Edlund P, Ewertz M, de Graaf PW,
Kamby C and Nielsen DL: Similar efficacy for ovarian ablation
compared with cyclophosphamide, methotrexate, and fluorouracil:
From a randomized comparison of premenopausal patients with
node-positive, hormone receptor-positive breast cancer. J Clin
Oncol. 24:4956–4962. 2006.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Thürlimann B, Price KN, Gelber RD,
Holmberg SB, Crivellari D, Colleoni M, Collins J, Forbes JF,
Castiglione-Gertsch M, Coates AS, et al: Is chemotherapy necessary
for premenopausal women with lower-risk node-positive, endocrine
responsive breast cancer? 10-year update of international breast
cancer study group trial 11-93. Breast Cancer Res Treat.
113:137–144. 2009.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Thomson CS, Twelves CJ, Mallon EA and
Leake RE: Scottish Cancer Trials Breast Group and Scottish Cancer
Therapy Network. Adjuvant ovarian ablation vs cmF chemotherapy in
premenopausal breast cancer patients: Trial update and impact of
immunohistochemical assessment of ER status. Breast. 11:419–429.
2002.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Adjuvant Breast Cancer Trials
Collaborative Group. Ovarian ablation or suppression in
premenopausal early breast cancer: Results from the international
adjuvant breast cancer ovarian ablation or suppression randomized
trial. J Natl Cancer Inst. 99:516–525. 2007.PubMed/NCBI View Article : Google Scholar
|