Introduction
Rubinstein-Taybi syndrome (RTS), also known as Broad
Thumb-Hallux syndrome, was initially described by Michail et
al in 1957(1) and further
characterized by Rubinstein and Taybi in 1963(2). This is a rare neurodevelopmental
disorder with an incidence of 1 in 125,000 individuals and is
characterized by wide spectrum of features, such as a short
stature, broad thumbs and halluces, facial abnormalities
(downslanted palpebral fissures, arched/thick eyebrows, beaked nose
with a hanging columella below the nostrils, microtia and low set
ears, micrognathia, ptosis, a high arched palate, cleft lip/palate,
atypical/grimacing smile, overcrowded teeth and talon cusps,
microcephaly), postnatal growth delay and intellectual disability
(3,4). Other common associated findings in RTS
are described in Table I (5).
 | Table ISummary of reported cases of cardiac
anomalies and dilated cardiomyopathy in patients with syndromes
identified in the literature. |
Table I
Summary of reported cases of cardiac
anomalies and dilated cardiomyopathy in patients with syndromes
identified in the literature.
| Study | Age of the
patients | Sex | Cardiac
anomalies | Dilated
cardiomyopathy | Outcome | (Refs.) |
|---|
| Case 1 | 5 years | Male | PDA (4 mm) | Not specified | Managed
conservatively | (11) |
| Cases2 | 2 years | Female | Tricuspid atresia,
pulmonary atresia | Present | Surgical intervention
required | (9) |
| Case 3 | 8 years | Male | VSD, ASD | Present | Ongoing management
for heart failure | (12) |
| Case 4 | 6 years | Male | Multiple congenital
defects | Not specified | Complications from
malignancy | (13) |
| Case 5 | 10 years | Female | Aortic
coarctation | Present | Surgical repair
performed | (14) |
| Current case | 32 years | Male | None | Present (LVEF
20%) | Responded well to IV
diuretics; referred for cardiology | Present study |
Although the exact underlying pathology remains
unclear, RTS is considered to be caused by mutations in the
cyclic-AMP-regulated enhancer binding protein (CREBBP) gene on
chromosome 16 and, less frequently, in the E1A binding protein p300
(EP300) gene on chromosome 22 (5,6). These
mutations are typically de novo with a minimal chance of
transmission from parents to the next generation (7). The syndrome usually requires management
through a multidisciplinary approach due to its various
health-related implications. Affected individuals often require
proper care throughout their lives. The present study describes the
first case of a clinically diagnosed 32-year-old male patient with
RTS in Pakistan, presenting with features of heart failure.
Case report
A 32-year-old male patient, born out of a
consanguineous marriage with no family history of RTS, presented to
the Outpatient Department of Ayub Teaching Hospital, Abbottabad,
Pakistan, with chief complaints of abdominal distension, shortness
of breath and generalized swelling. These symptoms developed
gradually over a few months. The case history provided by the
attendant revealed that the patient had been intellectually
disabled and deaf-mute since birth.
Clinical examination. General
condition of the patient
The patient had a short stature with a syndromic
face. The patient also had intellectual disability and congenital
deaf-mutism.
Cardiovascular condition. The patient was
found to have raised jugular venous pressure, a displaced apex beat
to the left and high-grade pansystolic murmur.
Abdominal findings. The patient exhibited
gross distension with positive shifting dullness and fluid thrill,
no palpable visceromegaly, bilateral pitting pedal edema up to the
knees and normal vital signs.
Diagnostic criteria and features of
RTS
The clinical diagnosis of RTS should adhere to the
consensus criteria established by Lacombe et al (8). The criteria for RTS are as follows,
which were met by the patient in the present study (apart from
genetic testing):
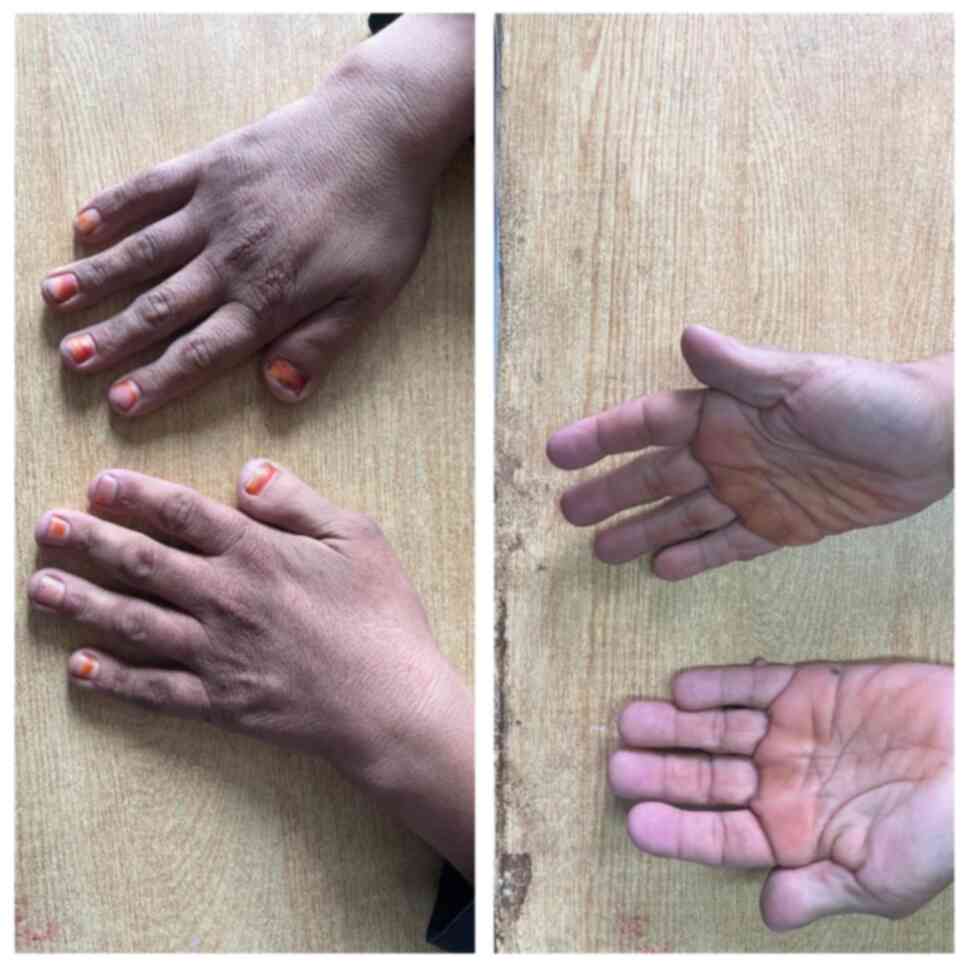
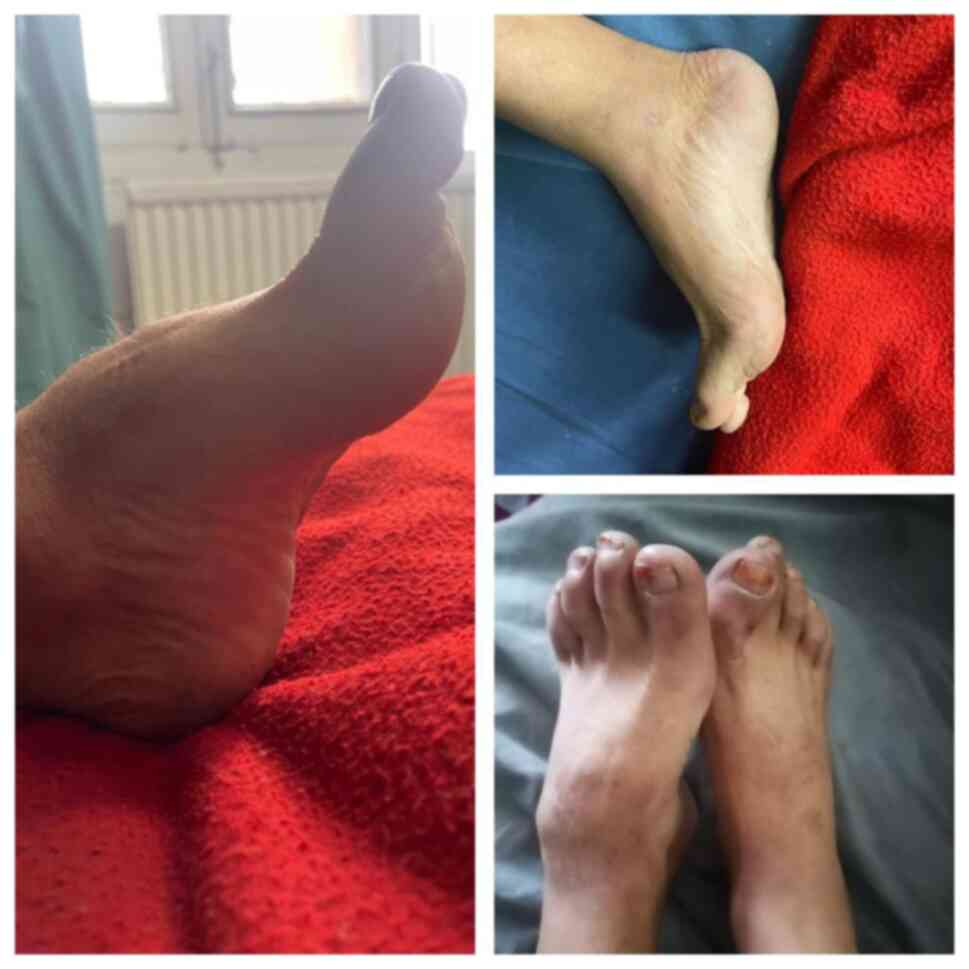
Essential criteria: Intellectual disability,
broad and angulated thumbs/halluces (Figs. 1 and 2), and pathogenic CREBBP or EP300 mutation
(if confirmed by genetic testing).
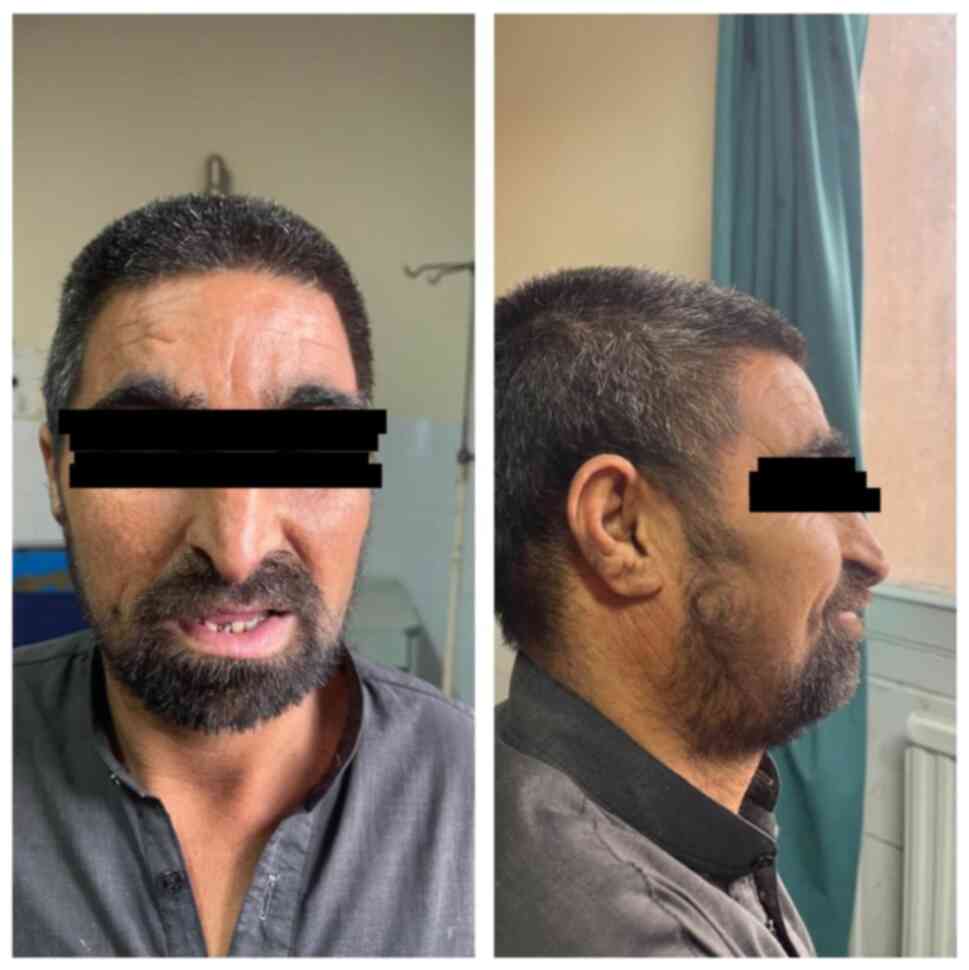
Major criteria: Characteristic craniofacial
dysmorphism such as thick/arched eyebrows, hypertelorism,
downslanting palpebral fissures, beaked nose and grimacing smile
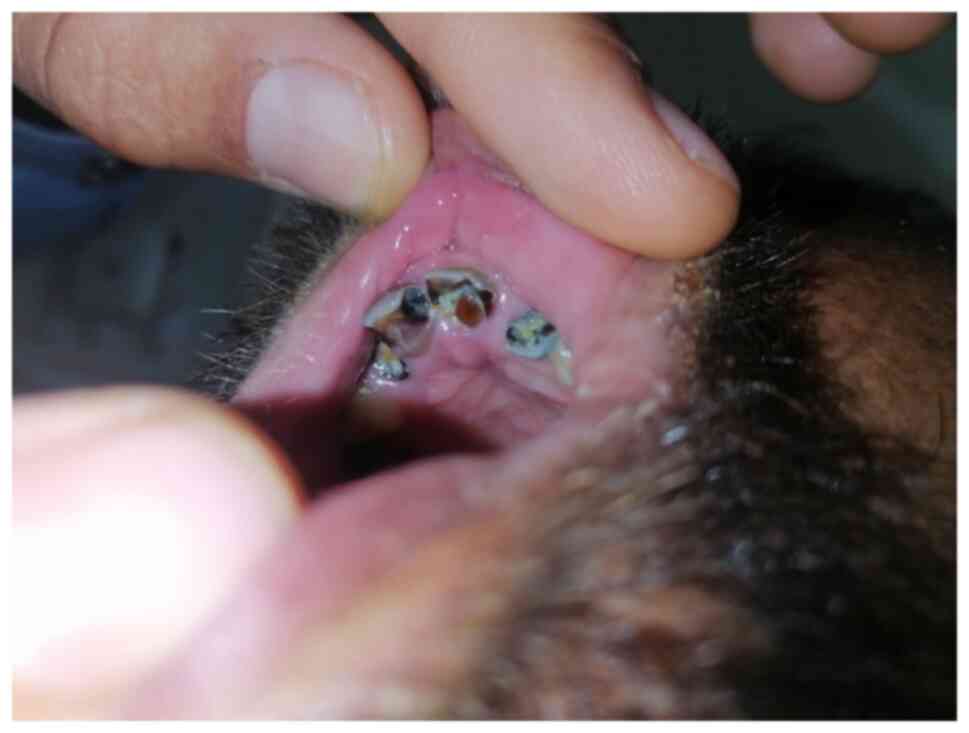
(Fig. 3), dental anomalies such as
talon cusps, high-arched palate, micrognathia, malocclusion
(crowding, anterior open bite), hypodontia, microdontia, delayed
eruption, narrow dental arches and increased susceptibility to
dental caries (Fig. 4) and
cryptorchidism (in males).
Minor criteria: A short stature, microcephaly
and congenital hearing impairment.
Cardiac involvement: Dilated
cardiomyopathy (DCM)
The patient was found to suffer from DCM. He had
severe left ventricular dysfunction [left ventricular ejection
fraction (LVEF) of 20%], mitral regurgitation, dilated ventricles
with global hypokinesis, an increased left ventricular
end-diastolic diameter (70 mm), an increased left ventricular
end-systolic diameter (60 mm), normal interventricular septal
thickness (10 mm), left atrial enlargement (diameter, 45 mm) and no
septal defects on echocardiography.
Investigations and management. Baseline
investigations yielded normal findings, apart from a slightly
increased alanine aminotransferase (ALT) level. The following
laboratory investigations were performed for the patient:
Electrolyte levels were normal (sodium, 140 mEq/l; potassium, 4.3
mEq/l; and chloride, 102 mEq/l). The serum albumin level was 3.6
g/dl, and the total bilirubin level was 1.8 mg/dl. Liver function
tests (LFTs) revealed an alkaline phosphatase level of 98 U/l and
an alanine aminotransferase (ALT) level of 31 U/l, both within
normal limits apart from the mildly elevated bilirubin level.
Renal function tests (RFTs) revealed a blood urea
nitrogen level of 15 mg/dl and serum creatinine level of 1.0 mg/dl,
indicating normal renal function. Fluid R/E was unremarkable. The
activated partial thromboplastin time (APTT) was 33 sec, and the
PT/INR was 12 sec/1.0, both normal. Urinalysis with microscopy
revealed a clear appearance with no protein, glucose, RBCs, or
casts. The C-reactive protein (CRP) level was 3 mg/l, the
phosphorus level was 3.7 mg/dl, the serum calcium level was 9.3
mg/dl, and the serum amylase level was 62 U/l. Overall, all other
parameters were within normal limits, with only a mildly elevated
total bilirubin and slightly low-normal albumin noted. Ascitic
fluid analysis revealed a SAAG level >1.1, normal ADA levels. An
echocardiography confirmed the presence of DCM with detailed
measurements.
The patient was treated with intravenous furosemide
(40 mg, twice daily), leading to significant diuresis, and the
resolution of ascites and limb edema within 2 days. He exhibited
clinical improvement with reduced dyspnea and an increased urine
output. He was subsequently referred to a cardiologist for the
further management of heart failure. A follow-up with a surgeon was
also arranged to address cryptorchidism.
Discussion
RTS is a genetic condition marked by distinct
physical traits, cognitive impairment and various congenital
abnormalities, including heart defects. DCM has been increasingly
observed among the cardiac issues linked to RTS. In the present
study, a review of the literature was also performed to compile
existing cases of RTS with DCM, highlighting the range of clinical
presentations, related heart anomalies and patient outcomes.
RTS results from mutations in either the CREBBP or
EP300 genes, which play crucial roles in gene expression regulation
and chromatin modification. The syndrome is typified by wide thumbs
and toes, facial irregularities and varying levels of intellectual
disability. Heart abnormalities are noted in approximately
one-third of patients with RTS, often presenting as isolated septal
defects or patent ductus arteriosus (PDA) (9). While less common, the occurrence of DCM
in RTS has been documented, suggesting a need for a more in-depth
exploration of the underlying mechanisms and treatment approaches.
The review performed herein identified various cases of RTS
associated with DCM and other cardiac abnormalities, including a
newly reported case (Table I).
The identified cases demonstrate the diverse cardiac
manifestations observed in patients with RTS. Some individuals
exhibit simple defects such as PDA (Case 1, Table I) or ventricular septal defect (VSD)
(Case 3, Table I), while others
exhibit more intricate abnormalities, including tricuspid and
pulmonary atresia (Case 2, Table I).
In the present study, the newly reported case of a 32-year-old male
patient with RTS and DCM sheds light on the adult presentation of
this syndrome, which is less frequently documented. This patient
experienced marked heart failure symptoms, such as abdominal
swelling and dyspnea, requiring diuretic treatment.
The occurrence of DCM in patients with RTS indicates
a possible connection between the genetic underpinnings of the
syndrome and heart muscle function. The case described herein
underscores the need to recognize RTS in adults, as its clinical
manifestation may differ from that in children. The management of
DCM in patients with RTS may be complex, often necessitating a
collaborative approach involving pediatric cardiologists,
geneticists and surgeons. Surgical procedures may be required for
marked cardiac anomalies, while ongoing monitoring and supportive
care are vital for addressing heart failure symptoms associated
with DCM.
RTS is a rare congenital multiple anomaly syndrome.
First described in 1957 by Michail et al (1), followed by Rubinstein and Taybi in
1963(2). The underlying cause is not
known, although research has shown genetic mutations in the CREBBP
gene and EP300 gene as a possible cause (5). The vast majority of cases occur due to
spontaneous mutations with a rare chance of transmission from
parents to offspring (7). There are
no precise and defined diagnostic criteria for RTS; however,
diagnosis can be made by genetic studies, although this is mostly
performed based on clinical features. It is characterized
clinically by retarded growth, intellectual disability, typical
facial features, broad and short thumbs, and halluces (3,4).
Cardiac defects in RTS include atrial septal defect,
ventricular septal defect, PDA, coarctation of the aorta, pulmonic
stenosis, bicuspid aortic valve, pseudo-truncus, aortic stenosis,
dextrocardia, vascular rings, conduction disorders and
occasionally, hypoplastic left heart (5). In 1995, a study was performed to
evaluate the frequency and type of cardiac defects and their
significance in RTS (10). Among the
138 patients in that study, 40 (32.6%) had a known cardiac
abnormality; 27 patients had single defects including atrial septal
defect, VSD, PDA, coarctation of the aorta, pulmonic stenosis, or
bicuspid aortic valve; 16 patients had complex congenital heart
defects or two or more abnormalities; and 5 patients had conduction
abnormalities (10). Little to no
literature is available to establish an association between RTS and
DCM; the purpose of the present case report is to provide insight
into this condition. RTS is linked to a range of cardiac
abnormalities, including DCM. The variability in cardiac
involvement requires careful assessment and the personalized
management of each patient.
A limitation of the present study is the lack of
molecular confirmation. Genetic testing for CREBBP and EP300
mutations could not be performed due to resource constraints in the
authors' setting. Therefore, the diagnosis was established
clinically according to the Lacombe et al (8) consensus criteria. This limits the
ability to directly associate the patient's phenotype with specific
genotypic findings. Additional research is crucial in order to
elucidate the mechanisms connecting RTS to cardiac dysfunction and
to develop effective treatment approaches.
In conclusion, RTS is a rare condition with diverse
clinical features, requiring further studies to refine diagnostic
guidelines. The present case report aimed to update the RTS
guidelines, particularly its association with heart failure and
DCM, which has not been previously published. RTS should be
considered in patients with broad thumbs, halluces and
characteristic facial features, as it may be underreported in
Pakistan, with only one documented case.
Following the diagnosis of RTS, comprehensive
evaluations, including cardiac, ophthalmic, renal, endocrine,
orthopedic and tumor screening, are essential for early
intervention. The case described herein is unique due to the rare
coexistence of RTS and severe DCM (LVEF 20%), a condition typically
linked to congenital heart defects rather than cardiomyopathy.
Potential mechanisms include genetic predisposition, chronic volume
overload, and metabolic dysfunction.
Long-term management involves heart failure therapy,
with adherence challenges due to intellectual disability, requiring
caregiver education and close monitoring. A multidisciplinary
approach is crucial, emphasizing the need for further research into
the genetic and metabolic links of RTS with cardiomyopathy.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
AS, HQ, ZE and MM were primarily involved in the
clinical management and treatment of the patient, including
examination, diagnosis and therapeutic decision-making. AR and AMAK
were mainly involved in the writing, research, and literature
review aspects of the study. AR was responsible for the study
methodology and provided clinical resources, including patient
records and diagnostic data. ZE and AMAK provided software support
(EndNote for reference management and Microsoft Word for manuscript
preparation). AR and AMAK contributed to the formal analysis and
investigative aspects of the study, including the review of imaging
and laboratory results. AS and AMAK were involved in data curation.
AS drafted the initial version of the manuscript, while ZE and AMAK
contributed to writing, review, and editing. HQ was involved in
visualization and project administration. AR supervised the study.
AS managed funding acquisition. MM additionally contributed to
clinical management, interpretation of data, and manuscript
revision. AS and AR confirm the authenticity of all the raw data.
All authors have read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
Written informed consent was obtained from the
patient's legal guardian for his participation in the present case
report.
Patient consent for publication
Written informed consent was obtained from the
patient's legal guardian for the publication of the present case
report and for any accompanying images.
Competing interests
The authors declare that they have not competing
interests.
References
|
1
|
Michail J, Matsoukas J and Theodorou S:
Arched, clubbed thumb in strong abduction-extension & other
concomitant symptoms. Rev Chir Orthop Reparatrice Appar Mot.
43:142–146. 1957.PubMed/NCBI(In French).
|
|
2
|
Rubinstein JH and Taybi H: Broad thumbs
and toes and facial abnormalities. A possible mental retardation
syndrome. Am J Dis Child. 105:588–608. 1963.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Bartsch O, Labonté J, Albrecht B,
Wieczorek D, Lechno S, Zechner U and Haaf T: Two patients with
EP300 mutations and facial dysmorphism different from the classic
Rubinstein-Taybi syndrome. Am J Med Genet A. 152A:181–184.
2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Negri G, Magini P, Milani D, Colapietro P,
Rusconi D, Scarano E, Bonati MT, Priolo M, Crippa M, Mazzanti L, et
al: From whole gene deletion to point mutations of EP300-positive
rubinstein-taybi patients: New insights into the mutational
spectrum and peculiar clinical hallmarks. Hum Mutat. 37:175–183.
2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Milani D, Manzoni FM, Pezzani L, Ajmone P,
Gervasini C, Menni F and Esposito S: Rubinstein-Taybi syndrome:
Clinical features, genetic basis, diagnosis, and management. Ital J
Pediatr. 41(4)2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
López M, García-Oguiza A, Armstrong J,
García-Cobaleda I, García-Miñaur S, Santos-Simarro F, Seidel V and
Domínguez-Garrido E: Rubinstein-Taybi 2 associated to novel EP300
mutations: Deepening the clinical and genetic spectrum. BMC Med
Genet. 19(36)2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kamenarova K, Simeonov E, Tzveova R,
Dacheva D, Penkov M, Kremensky I, Perenovska P, Mitev V and Kaneva
R: Identification of a novel de novo mutation of CREBBP in a
patient with Rubinstein-Taybi syndrome by targeted next-generation
sequencing: A case report. Hum Pathol. 47:144–149. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Lacombe D, Bloch-Zupan A, Bredrup C,
Cooper EB, Houge SD, García-Miñaúr S, Kayserili H, Larizza L, Lopez
Gonzalez V, Menke LA, et al: Diagnosis and management in
Rubinstein-Taybi syndrome: First international consensus statement.
J Med Genet. 61:503–519. 2024.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Loomba RS and Geddes G: Tricuspid atresia
and pulmonary atresia in a child with Rubinstein-Taybi syndrome.
Ann Pediatr Cardiol. 8:157–160. 2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Stevens CA and Bhakta MG: Cardiac
abnormalities in the Rubinstein-Taybi syndrome. Am J Med Genet.
59:346–348. 1995.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Desai K, Taksande A, Meshram R and Jain A:
Beaked nose with syndactyly: A rare case of Rubenstein-Taybi
syndrome. Med Sci. 27:1–5. 2023.
|
|
12
|
Scaglia F, Towbin JA, Craigen WJ, Belmont
JW, Smith EO, Neish SR, Ware SM, Hunter JV, Fernbach SD, Vladutiu
GD, et al: Clinical spectrum, morbidity, and mortality in 113
pediatric patients with mitochondrial disease. Paediatrics.
114:925–931. 2004.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Taylor MD, Mainprize TG, Rutka JT, Becker
L, Bayani J and Drake JM: Medulloblastoma in a child with
Rubenstein-Taybi syndrome: Case report and review of the
literature. Pediatr Neurosurg. 35:235–238. 2001.PubMed/NCBI View Article : Google Scholar
|
|
14
|
van Voorden AJ, Keijser R, Veenboer GJM,
Lopes Cardozo SA, Diek D, Vlaardingerbroek JA, van Dijk M,
Ris-Stalpers C, van Pelt AMM and Afink GB: EP300 facilitates human
trophoblast stem cell differentiation. Proc Natl Acad Sci USA.
120(e2217405120)2023.PubMed/NCBI View Article : Google Scholar
|