Introduction
Psammoma bodies (PBs) are round, layered calcified
structures considered to form through dystrophic calcification, a
process in which calcium is deposited locally in damaged or dying
tissues, despite normal blood calcium levels and no abnormalities
in the metabolism of calcium (1).
PBs are usually associated with papillary tumors and are most often
observed in papillary thyroid carcinoma. Consequently,
microcalcifications on an ultrasound are more frequently observed
in classic papillary thyroid carcinoma than in other thyroid cancer
types, and their histological presence is a hallmark feature of
papillary thyroid carcinoma (2). PBs
are present in 40-50% of paraffin-embedded sections and in 11-35%
of fine-needle aspiration smears of papillary thyroid carcinoma
(1). They most often occur on tumor
cells located at the tips of papillary structures, likely due to
the effects of vascular thrombosis (3). When present in thyroid areas remote
from a tumor, PBs are typically found between follicles or within
the interlobular septa, regions that contain the lymphatic and
vascular structures of the gland (4). However, although rare, PBs can be found
in a benign thyroid gland, and their presence without accompanying
papillary thyroid carcinoma can pose a diagnostic challenge
(5). The present study describes a
rare case of PBs found in a benign thyroid gland and also performed
a brief review of the literature. The references cited in the
present case report have been assessed for reliability (6), and the manuscript was prepared
according to the CaReL guidelines (7).
Case report
Patient information
A 28-year-old female patient presented to Smart
Health Tower (Sulaymaniyah, Iraq) with a 3-month history of weight
loss, decreased appetite and generalized weakness, without other
associated symptoms. An analysis of her past medical and surgical
history did not reveal any notable findings, with no family history
of similar conditions and no history of radiation exposure.
Clinical findings
An examination revealed a firm, mildly enlarged
thyroid gland without palpable cervical lymphadenopathy.
Diagnostic approach
Laboratory investigations revealed that the level of
thyroid-stimulating hormone was 0.85 µIU/ml (normal range, 0.8-6.0
µIU/ml) and the level of free T4 was 20.1 pmol/l (normal range,
12.8-27 pmol/l). A neck ultrasound revealed bilateral thyroid
nodules, both classified as TI-RADS 3 according to the American
College of Radiology TI-RADS system (https://www.acr.org/Clinical-Resources/Clinical-Tools-and-Reference/Reporting-and-Data-Systems/TI-RADS).
The right nodule measured 25x21x19 mm, and the left measured
17x15x13 mm. As part of the pre-operative assessment for total
thyroidectomy, vocal cord function was evaluated and was normal
bilaterally. Additional investigations revealed a thyroglobulin
level of 6.15 ng/ml (normal range, 3.5-77 ng/ml) and a serum
calcium level of 9.27 mg/dl (normal range, 8.6-10.0 mg/dl).
Therapeutic intervention
Under general anesthesia, a total thyroidectomy was
performed through a collar incision, with the careful preservation
of both recurrent laryngeal nerves and the parathyroid glands.
Hemostasis was successfully achieved, and the wound was closed in
layers, with a drain placed on the left side. The excised tissue
was sent for a histopathological examination. The sections
(5-µm-thick) were paraffin-embedded and fixed in 10% neutral
buffered formalin at room temperature for 24 h. They were then
stained with hematoxylin and eosin (H&E; Bio Optica Co.) for
1-2 min at room temperature. The sections were then examined under
a light microscope (Leica Microsystems GmbH). Post-operatively, the
patient remained stable with no complications, and serum calcium
levels were within the normal range (9.3 mg/dl). The
histopathological examination revealed thyroid follicular nodular
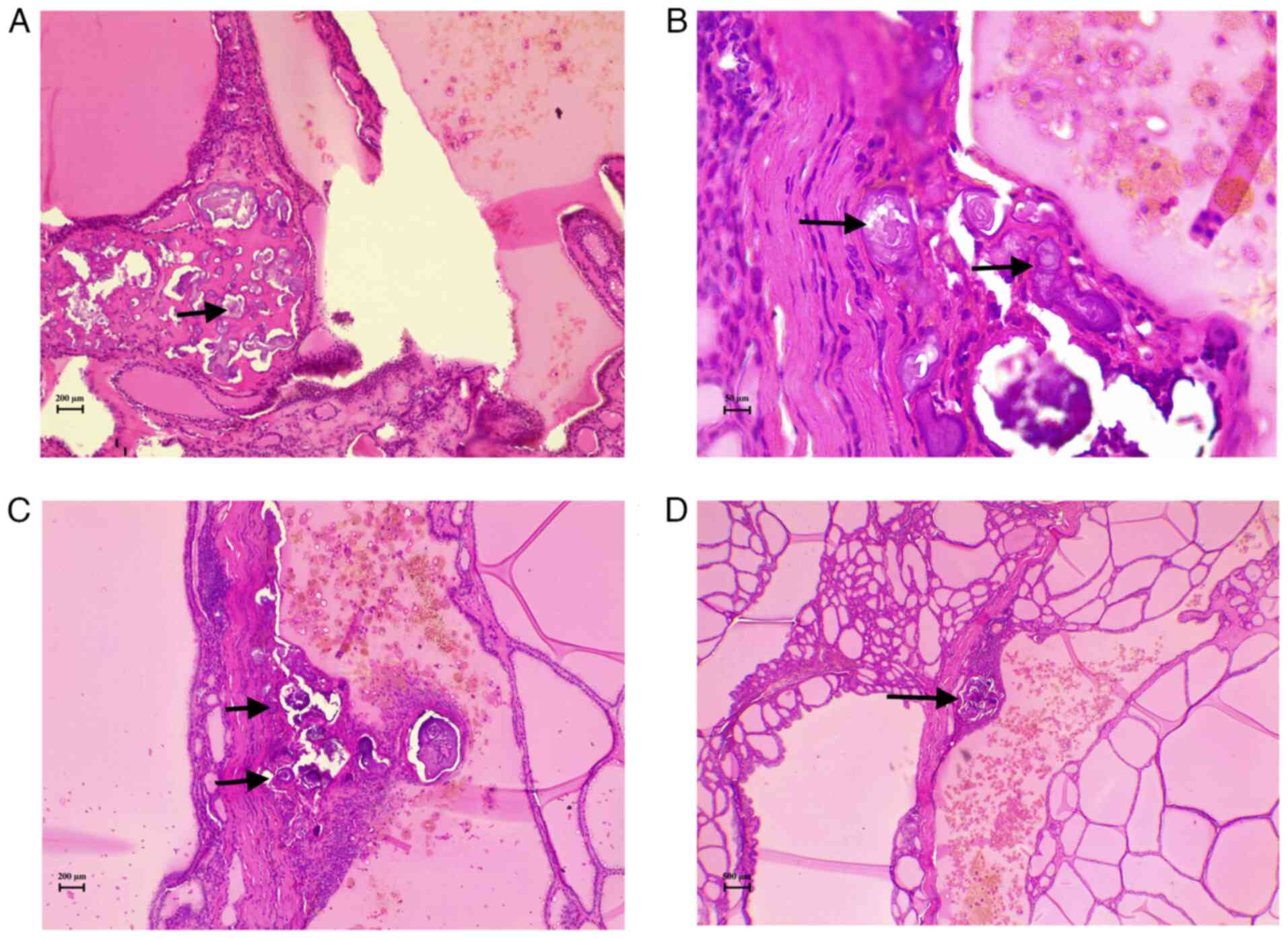
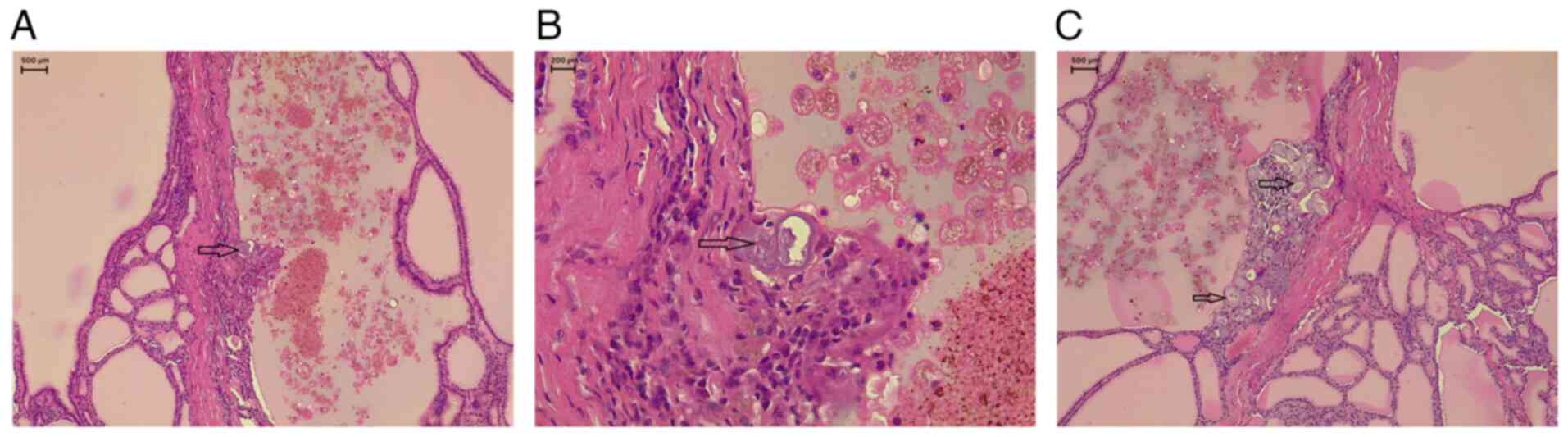
disease (TFND) with focal lymphocytic thyroiditis, and PBs were
identified without any evidence of suspicious epithelial cell
components (Figs. 1 and 2). To ensure a comprehensive evaluation,
the entire thyroid was submitted for additional sectioning to
exclude microscopic carcinoma, no malignancy was identified.
Follow-up and outcomes
The patient recovered well without complications and
was discharged 2 weeks following admission, and was prescribed
levothyroxine 100 µg once daily,, with a follow-up appointment
scheduled. A multidisciplinary team evaluated her and decided that
she would undergo regular follow-ups to monitor for any signs of
recurrence.
Discussion
It is well-established that PBs are commonly
associated with carcinomas in different organs, such as papillary
thyroid carcinoma, ovarian serous tumors, meningioma and others,
and this association is considered diagnostically critical
(8). However, PBs may be found in
benign conditions, such as TFND with papillary hyperplasia,
follicular adenomas, including the oncocytic variant, Hashimoto's
thyroiditis and other less common condition (3). A total of 4 cases of PBs and
psammomatous calcifications associated with benign conditions were
identified in the literature and were reviewed herein; 2 cases were
male and 2 cases were female (Table
I) (5,9-11).
Half of the cases involved thyroid-region lesions and half were
pediatric patients (2/4). All 4 cases required surgical excision,
while fine-needle aspiration was only used in 1 out of the 4 cases.
Computed tomography was used in half the cases (2/4) and other
modalities in the remainder. Histopathological analysis confirmed
PBs or psammomatous calcifications in every case, and all patients
had favorable outcomes with no recurrence documented on follow-up
(100%). These findings emphasize that psammomatous calcifications
can occur in diverse benign conditions, are best identified by
histology, and are commonly managed successfully by surgical
resection.
 | Table IReview of some cases of PBs and
psammomatous calcifications associated with benign conditions in
the literature. |
Table I
Review of some cases of PBs and
psammomatous calcifications associated with benign conditions in
the literature.
| First author, year of
publication | Age, years | Sex | Diagnosis | Clinical
findings | Imaging tests | Microscopic
examinations | Fine needle
aspiration | Treatment | Outcome | (Refs.) |
|---|
| Cardisciani,
2022 | 74 | F | Pseudonodular
hyperplastic oncocytic metaplasia in a background of chronic
lymphocytic Hashimoto thyroiditis. | History of
multinodular goiter pressing on the trachea. | Not mentioned. | Lymphocytic Hashimoto
thyroiditis with nodular oncocytic metaplasia, and PBs. IHC: -ve
HBME-1, galectin-3 and CK-19. | Not performed. | Total
thyroidectomy. | Is in stable
condition. | (5) |
| Mohammed, 2015 | 9 | M | Leiomyoma of the
thyroid gland. | Painless thyroid
mass. | X-ray: Tracheal
deviation to the left. | PBs and foci of
calcification. HPE: +ve SMA, vimentin and desmin, -ve cytokeratin
cocktail. | Benign smooth muscle
tumor. | Right
hemi-thyroidectomy. | No recurrence after 2
years. | (9) |
| Vemavarapu, 2011 | 46 | F | Benign Brenner tumor
of the ovary. | Progressively
enlarging pelvic mass. | US: Cystic lesion in
the left adnexa with punctate calcifications. CT: solid components
and calcifications. | Dystrophic
calcifications lining the cyst wall, obscuring the epithelium,
largely replacing it, with extensive stromal PC, and no nuclear
atypia or mitosis. | Not mentioned. | Total robotic
hysterectomy and bilateral salpingo-oophorectomy. | Is in stable
condition. | (10) |
| Ayala, 2003 | 3 | M | Benign thyroglossal
duct cyst. | Asthma symptoms. | CT: Hyoid bone
mass. | Thyroglossal duct
remnant with cystic spaces, and PC, no evidents of malignancy. | Not mentioned. | Excision of the
mass. | Is in stable
condition. | (11) |
Microcalcifications are observed in <5% of
adenomas on thyroid ultrasound, and these correspond to PB clusters
when examined cytologically or histologically (12). By contrast, macrocalcifications
appear on an ultrasound as bright echogenic spots that produce
shallow posterior acoustic shadowing (<1 mm deep), and are less
strongly linked to malignancy histologically. Coarse calcifications
upon microscopy are irregular dystrophic deposits usually resulting
from tissue necrosis. Distinguishing between microcalcifications
and macrocalcifications on ultrasound can be challenging, as it
depends not only on the level of experience of the operator, but
also on the technical capabilities of the imaging equipment
(2).
Rossi et al (3) evaluated cases of PBs in benign thyroid
lesions across five centers over a period of 14 years and
identified only 26 patients, with 16 of them being female. The most
common diagnoses were TFND and adenomas (12 patients each), of
which 11 cases were oncocytic adenomas and 1 case was a follicular
adenoma, as well as 2 cases of Hashimoto's thyroiditis. The precise
origin and clinical relevance of PBs in non-cancerous conditions
are still uncertain and continue to be investigated. The presence
of PBs in non-cancerous conditions raises the dilemma of whether
PBs in benign lesions should be observed as early signs of
malignancy and treated accordingly, or regarded as incidental
findings (3).
TFND, which was identified in the current case and
was previously referred to as multinodular goiter, is a benign,
non-inflammatory condition marked by an enlarged thyroid gland and
multifocal proliferation of thyroid follicular cells, resulting in
clonal and non-clonal nodules with diverse architectures. It is
more frequently observed in women, and iodine deficiency resulting
in an elevated thyroid-stimulating hormone release, which
stimulates hyperplasia of thyroid follicular epithelial cells, is
considered a primary contributing factor. TFND may present with
diverse histological features, that mimic well-differentiated
thyroid carcinomas. Distinguishing between concerning histological
changes resulting from a benign hyperplastic process and those
indicative of true neoplastic transformation is essential (13).
Ultrastructural studies of PBs of the thyroid found
in both tumor and non-tumor areas, suggests that they likely
represent the final stage of two biological processes: The first
involves the basement membrane in the vascular core of neoplastic
papillae becoming thickened, followed by vascular thrombosis,
calcification and tumor cell death. Secondly, necrosis and
calcification occur within the intralymphatic tumor thrombi, which
are located either near the tumor or in the opposite lobe of the
thyroid (1). PBs should be
differentiated from calcified colloid and dystrophic calcification
(4). There is an increased presence
of PBs and a higher frequency of lymph node metastases in tumors
with rearranged during transfection (RET) rearrangements. This
indicates a potential complex association between RET pathway
alterations, PB formation and nodal involvement (14). PBs located outside the tumor in
patients with classic type papillary thyroid carcinoma may be
linked to a greater likelihood of multifocal tumors, spread beyond
the thyroid, and lymph node metastasis compared to tumors that
contain PBs only within the tumor itself. Non-tumor PBs in patients
with lymph node involvement also exhibit a notably increased rate
of viable tumor cells present within lymphatic vessels compared to
those without such findings (14).
For the histopathological analysis of PBs, the
tissue is fixed in 10% buffered formaldehyde, embedded in paraffin,
sectioned into 5-µm-thick slices, and subsequently stained with
hematoxylin and eosin (3). PBs are
frequently observed to be encircled by cells and typically display
a concentric pattern, appearing either acidophilic or basophilic
when stained with Papanicolaou stain (8). PBs are less commonly detected in
fine-needle aspiration cytology, although their presence strongly
indicates carcinoma. However, they are not dependable indicators
unless accompanied by other distinct cytological features such as
nuclear grooves, nuclear pseudoinclusions and true papillary
formations (12). Ellison et
al (15) reported that PBs were
identified in only eight of 313 fine-needle aspirates, PBs alone
had a 50% positive predictive value for papillary carcinoma,
whereas combined cytological features were 100% predictive. PBs
were found in 80% of papillary thyroid cases when combined features
were present compared with 14% detection of PB alone in their study
(15).
The presence of PBs in benign lesions remains a
topic of diagnostic uncertainty. Submitting the entire thyroid
tissue for histological analysis, particularly in lobectomy
specimens, is advised to rule out microscopic papillary thyroid
carcinoma (4). Multiple and deeper
sections of thyroid parenchyma need to be examined to search for
occult carcinoma. However, some cases remain tumor-free, supporting
the occurrence of PBs in benign conditions (3). Hunt and Barnes (4) evaluated 29 patients (22 females) with
PBs not initially associated with tumors. A histological
examination, however, revealed carcinoma in the thyroid gland in 27
patients, and 12 of these were observed alongside microscopic,
incidental thyroid carcinomas, emphasizing the importance of
thoroughly sampling the entire surgical specimen. In their study,
23 patients had PBs associated with high-risk features according to
the AMES criteria, as well as the presence of the tall cell variant
as an added risk factor (4). A total
of 5 patients experienced disease recurrence, but no deaths were
attributed to thyroid cancer. In the total of 29 patients, a total
of 19 patients had a total thyroidectomy, while ten others
underwent lobectomy. The 2 patients without any carcinoma underwent
a lobectomy, and the entire surgical specimen was not sent for a
histopathological examination (4).
In the study by Rossi et al (3), 24 out of the 26 patients with PBs
underwent a total thyroidectomy.
Since the patient in the present study underwent a
simple total thyroidectomy and PBs were identified afterward,
intraoperative neuroendoscopy and magnetic resonance imaging were
not performed. It should be noted that sonographic categorization
is subject to both operator expertise and equipment variability,
which may influence nodule classification and reproducibility,
including this case.
In conclusion, PBs may occur in benign TFND and can
mimic malignancy on imaging and cytology, definitive diagnosis
relies on thorough histopathological sampling.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
AMA and AMS were major contributors to the
conception of the study, as well as to the literature search for
related studies. TOS, HAA and FHK were involved in the conception
and design of the study, in hte literature review and in the
writing of the manuscript. SFA, IJH and SHH were involved in the
literature review, in the design of the study, in the critical
revision of the manuscript and in the processing of the table. RMA
and HAY were the pathologists who performed the diagnosis of the
case. FHK and AMA confirm the authenticity of all the raw data. All
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
Written informed consent was obtained from the
patient for their participation in the present study.
Patient consent for publication
Written informed consent was obtained from the
patient for the publication of the present case report and any
accompanying images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Das DK: Psammoma body: A product of
dystrophic calcification or of a biologically active process that
aims at limiting the growth and spread of tumor? Diagn Cytopathol.
37:534–41. 2009.PubMed/NCBI View
Article : Google Scholar
|
|
2
|
O'Connor E, Mullins M, O'Connor D, Phelan
S and Bruzzi J: The relationship between ultrasound
microcalcifications and psammoma bodies in thyroid tumours: A
single-institution retrospective study. Clin Radiol. 77:e48–e54.
2022.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Rossi ED, Agarwal S, Erkilic S, Hang JF,
Jalaly JB, Khanafshar E, Ladenheim A and Baloch Z: Psammoma bodies
in thyroid: Are they always indicative of malignancy? A
multi-institutional study. Virchows Arch. 485:853–858.
2024.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Hunt JL and Barnes EL:
Non-tumor-associated psammoma bodies in the thyroid. Am J Clin
Pathol. 119:90–94. 2003.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Cardisciani L, Policardo F, Tralongo P,
Fiorentino V and Rossi ED: What psammoma bodies can represent in
the thyroid. What we recently learnt from a story of lack of
evidence. Pathologica. 114:373–375. 2022.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Abdullah HO, Abdalla BA, Kakamad FH, Ahmed
JO, Baba HO, Hassan MN, Bapir R, Rahim HM, Omar DA, Kakamad SH, et
al: Predatory publishing lists: A review on the ongoing battle
against fraudulent actions. Barw Med J. 2:26–30. 2024.
|
|
7
|
Prasad S, Nassar M, Azzam AY,
García-Muro-San José F, Jamee M, Sliman RKA, Evola G, Mustafa AM,
Abdullah HO, Abdalla BA, et al: CaReL guidelines: A consensus-based
guideline on case reports and literature review (CaReL). Barw Med
J. 2:13–19. 2024.
|
|
8
|
Parwani AV, Chan TY and Ali SZ:
Significance of psammoma bodies in serous cavity fluid: A
cytopathologic analysis. Cancer. 102:87–91. 2004.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Mohammed AZ, Edino ST and Umar AB:
Leiomyoma of the thyroid gland with psammoma bodies. Niger Med J.
56:71–73. 2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Vemavarapu L, Alatassi H and
Moghadamfalahi M: Unusual presentation of benign cystic Brenner
tumor with exuberant psammomatous calcifications. Int J Surg
Pathol. 19:120–122. 2011.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Ayala C, Healy GB, Robson CD and Vargas
SO: Psammomatous calcification in association with a benign
thyroglossal duct cyst. Arch Otolaryngol Head Neck Surg.
129:241–243. 2003.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Triggiani V, Guastamacchia E, Licchelli B
and Tafaro E: Microcalcifications and psammoma bodies in thyroid
tumors. Thyroid. 18:1017–1018. 2008.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Flora R and Mukhopadhyay S: Multinodular
goitre: Pitfalls in the interpretation of thyroid follicular
nodular disease. Diagnostic Histopathology. 30:301–307. 2024.
|
|
14
|
Gubbiotti MA, Baloch Z, Montone K and
Livolsi V: Non-tumoral psammoma bodies correlate with adverse
histology in classic type papillary thyroid carcinoma. Arch
Otolaryngol Head Neck Surg. 6(3)2022.
|
|
15
|
Ellison E, Lapuerta P and Martin SE:
Psammoma bodies in fine-needle aspirates of the thyroid: predictive
value for papillary carcinoma. Cancer. 84:169–175. 1998.PubMed/NCBI View Article : Google Scholar
|