Introduction
Human sparganosis is a food-borne zoonosis caused by
the plerocercoid larvae (sparganum) of tapeworms belonging to the
genus Spirometra. Although human sparganosis is relatively
infrequent globally, the majority of cases occur primarily in East
and Southeast Asian countries due to dietary habits and traditional
medical practices (1). The disease
poses a critical public health concern due to its transmission
through multiple pathways, including the ingestion of contaminated
water, the consumption of raw or undercooked freshwater frogs or
snakes, and the application of raw meat poultices to treat
infections. Sparganosis is principally characterized by a slowly
migrating subcutaneous nodule(s) and can invade the brain, eyes,
breasts, spinal cord and subcutaneous tissues, often resulting in
severe illness (2). In the first
week following parasite infection, the host may develop an
inflammatory cellular infiltrate. Tunnel-like structures appear 2
weeks thereafter, and fibroblast proliferation occurs 4 weeks
following infection.
The present study reports an extremely rare case of
sparganosis found in the mons pubis of a 26-year-old female patient
who was immunocompetent. The purpose of the present case report was
to enhance the understanding of sparganosis and emphasize that
parasitic infection should be considered as a differential
diagnosis when encountering a patient presenting with durable,
wandering skin nodules.
Case report
A 26-year-old Chinese female patient presented to
People's Hospital of Xindu District, Chengdu, Sichuan, China, in
November, 2023 with a subcutaneous nodule in the mons pubis. Of
note, 2 months prior, the patient complained that subcutaneous
nodules appeared on the abdomen, which were not treated due to her
history of lipoma and normal skin temperature and color. These
nodules disappeared spontaneously in ~10 days. However, a nodule
was later found at the mons pubis that persisted for >1 month,
prompting her to return to the hospital for treatment. During the
course of the disease, there was no obvious pain in the nodule, and
the patient did not suffer from headaches or nausea. The complete
blood count from laboratory tests revealed an increased in
peripheral eosinophilia. Routine blood tests were performed,
including a complete blood count, hematocrit level and platelet
count tests, liver function tests and renal function tests, all of
which fell within the normal reference range; the results did not
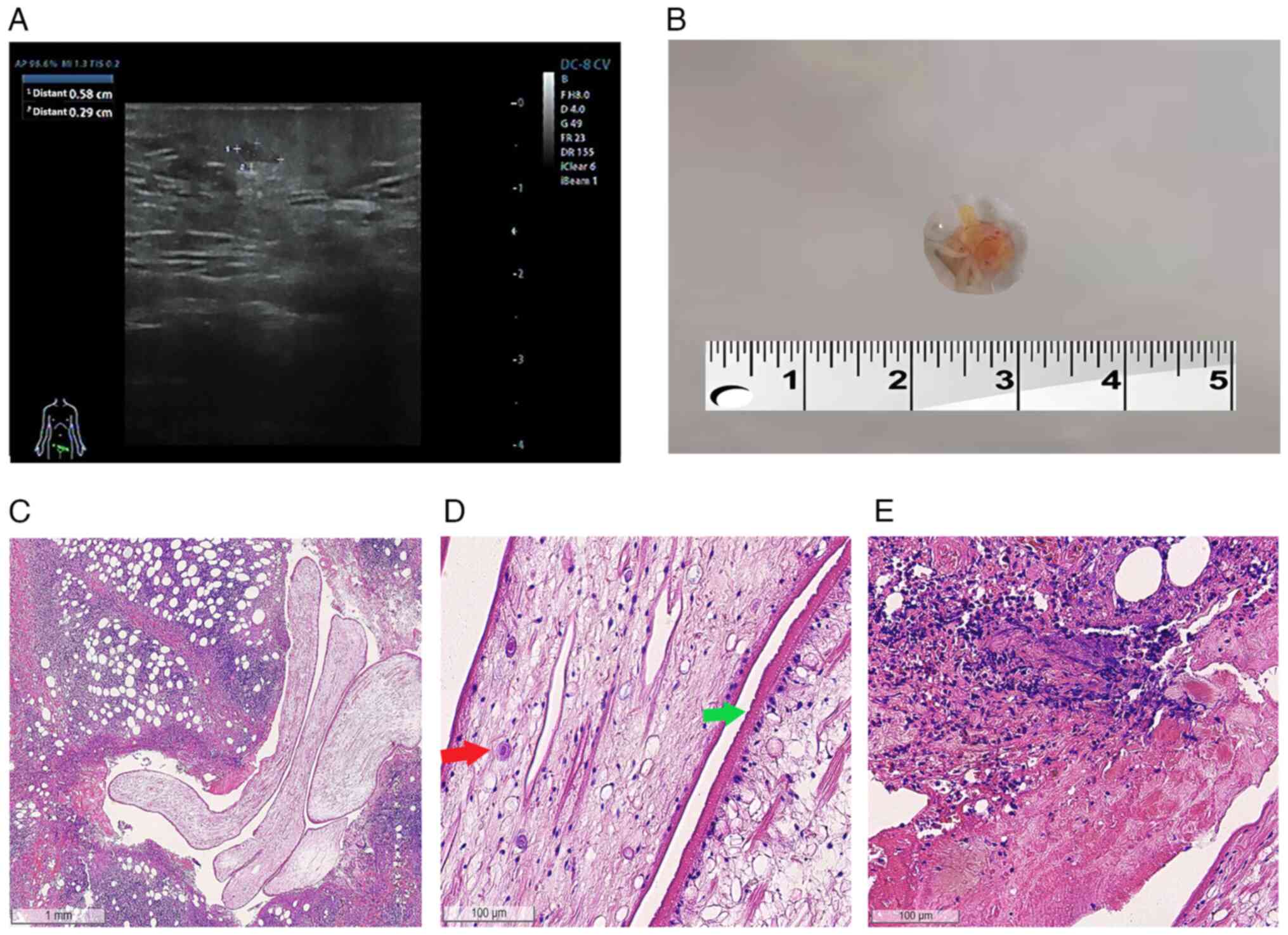
reveal anything uncommon. An ultrasonography revealed hypoechoic
nodules detectable subcutaneously at the mons pubis mass, measuring
~0.6x0.3 cm in size, with clear borders and a regular morphology
(Fig. 1A). No obvious blood flow
signal was detected in the above lesions on color Doppler flow
imaging (CDFI).
The nodule located on the mons pubis was completely
surgically resected, and filamentous wormlike objects were found
within the resected tissue. The precise length of the sample could
not be determined due to fragmentation during extraction; however,
it was >0.4 cm (Fig. 1B). A
histological examination revealed parasites characterized by a
deep-folded tegument and calcareous corpuscles, as well as
peripheral inflammatory changes in the parasite body, accompanied
by inflammatory cell infiltration. Hematoxylin and eosin (H&E)
staining for all sections presented in Fig. 1C-E was independently performed by the
Department of Pathology, People's Hospital of Xindu District,
Chengdu, China. The detailed procedures were as follows: Tissues
were fixed in formalin at room temperature for 24 h, followed by
processing into paraffin blocks after sampling. Paraffin sections
were cut at a thickness of 4 µm, which were then floated and
flattened on warm water at 45˚C. The sections were retrieved onto
glass slides and baked in an oven at 65˚C. For subsequent H&E
staining, paraffin sections were first dewaxed and rehydrated. They
were then stained with hematoxylin solution (MilliporeSigma) for
3-5 min, rinsed thoroughly with running water, and blued with warm
water. Following this, the sections were stained with eosin
solution (MilliporeSigma) for 2 min. Finally, the sections
underwent dehydration, clearing, and mounting procedures before
being observed under an optical microscope (LEICA DM3000; Leica
Microsystems GmbH). In conjunction with the epidemiological history
and the pathological examination, the patient was ultimately
diagnosed with subcutaneous sparganosis (Fig. 1C). Subsequently, anti-sparganum IgG
was detected in the serum of the patient using enzyme-linked
immunosorbent assay (ELISA) (data not shown).
A pathological examination further revealed that the
walls of these parasites were multilayered with a thick
eosinophilic microvillous noncellular tegument, and numerous small
clear vesicles were noted under the tegument. Moreover, numerous
secretory canaliculi, subcutaneous cells and calcareous bodies were
observed within the cytoplasm (Fig.
1D). Scolex, suckers, a digestive tract and reproductive organs
were not present. A pathological examination of the largest lesion
surrounding the worm revealed granulomatous inflammation and
tunnel-like necrosis with eosinophilic, neutrophilic and
lymphocytic infiltration (Fig. 1E).
Sparganosis was confirmed based upon these results. The nodule was
completely surgically resected, and wound healing proceeded without
complications. No oral anthelminthic treatment was administered,
and no subsequent lesions developed. Serological tests for
anti-sparganum IgG were negative at 8 months following surgery, and
no recurrence was observed.
Discussion
Due to the constant occurrence of sparganosis in
China, measures are required to prevent and control the disease.
First, food safety and prevention measures for this disease should
be emphasized and improved. The crucial point in preventing human
sparganosis is to cease the consumption of raw or undercooked frog,
snake and contaminated water, and the application of raw frog flesh
or skin to open wounds.
Sparganosis is a zoonotic parasitic disease caused
by the larvae (spargana) of the genus Spirometra, which is widely
distributed globally and threatens human health (3,4). Humans
serve as accidental secondary intermediate hosts, acquiring
infection through the consumption of raw or undercooked meat from
intermediate hosts, such as frogs or snakes, or by ingesting water
contaminated with infected copepods. While cases have been
sporadically documented across the globe, the disease is more
prevalent in Asian regions, particularly in China, Korea, and
Thailand (5,6). Sparganosis infections are primarily
associated with the consumption of undercooked frogs or snakes,
which serve as key sources of transmission. Following ingestion by
humans, the larvae preferentially migrate into soft subcutaneous
tissues; however, they may occasionally migrate into vital organs,
such as the brain, spinal cord, or eyes, causing deleterious
effects.
The clinical diagnosis of sparganosis involves a
physical examination of cutaneous and other nodules, a computed
tomography (CT) assessment of the location and dimension of the
nodule, and magnetic resonance imaging (MRI) for the demonstration
of a mass lesion or vasculopathy. Despite their obvious value,
these procedures lack specificity and do not provide a definitive
diagnosis (7). Laboratory methods
for the diagnosis of sparganosis include the macro- and microscopic
identification of spargana recovered through tissue biopsy,
biochemical tests, serological assays and molecular techniques. In
rare cases, next-generation sequencing has been used to identify
some uncommon parasite species (8).
The sparganum larvae are characterized as white, wrinkled and
ribbon-shaped, varying from a few millimeters to 50 cm in length
(4). Histopathological analysis
reveals fibrous cystic tunnels containing live worms, eosinophilic
granulomas, and intact worm structures exhibiting loose stroma,
smooth muscle, secretory tegument and calcareous bodies.
Biochemical tests may reveal pronounced peripheral eosinophilia
(9). By contrast, the diagnosis of
visceral or cerebral sparganosis poses significant challenges, and
serological testing, particularly ELISA for anti-sparganum
antibodies, serves as the primary diagnostic tool due to its high
sensitivity and specificity.
In the case in the present study, the involvement of
the mons pubis was extremely rare. Precisely due to its unique
location, the initial diagnosis invariably leaned toward
gynecological conditions. Relevant differential diagnoses were
excluded through examinations, including HPV-DNA testing, ELISA and
tissue biopsy. The presence of subcutaneous mobile masses in the
medical history suggested that parasitic infection should be highly
considered.
Treatment is primarily based on the surgical removal
of the larva. No medical treatment standard has been defined for
patients with masses not suitable for surgical resection, and
high-dose praziquantel (repeated cycles of 50-75 mg/kg body
weight/day for 10 days) has been used to treat infection (10,11). To
the best of our knowledge, mons pubis sparganosis has rarely been
reported. In the case described herein, the patient underwent
successful surgical removal. Following treatment, the patient
exhibited a reduction in eosinophil levels, and serological testing
for anti-sparganum IgG yielded negative results, indicating the
efficacy of surgical intervention for mons pubis sparganosis. The
patient recovered well following the procedure.
In conclusion, the present case report, the young
female patient had a subcutaneous nodule of the mons pubis.
Sparganosis was diagnosed by ELISA and tissue biopsy. This rare
case of sparganum infection is a reminder that when patients
presenting with persistent and migratory skin nodules are
encountered, regardless of the location, parasitic infection should
be considered as a differential diagnosis. Furthermore, it is
considered that health education on avoiding the consumption of
undercooked frogs and unboiled water is of utmost importance in
preventing parasitic infections.
Acknowledgements
Not applicable.
Funding
Funding: The present study was financially supported by the
Chengdu Health Commission Medical Research Project (grant no.
2024040).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
JZ and QL contributed equally to the design of the
study, in the collection of clinical specimens and information, and
manuscript preparation. QT contributed to the analysis of the
patient's data and data from the literature. QT and JZ were
responsible for the patient's treatment. JZ and QL confirm the
authenticity of all the raw data.
Ethics approval and consent to
participate
The patient provided written informed consent for
her inclusion in the present case report.
Patient consent for publication
The patient provided written informed consent for
the publication of the present case report and any related
images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Liu Q, Li MW, Wang ZD, Zhao GH and Zhu XQ:
Human sparganosis, a neglected food borne zoonosis. Lancet Infect
Dis. 15:1226–1235. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Li MW, Song HQ, Li C, Lin HY, Xie WT, Lin
RQ and Zhu XQ: Sparganosis in mainland China. Int J Infect Dis.
15:e154–e156. 2011.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Zhang X, Duan JY, Shi YL, Jiang P, Zeng
DJ, Wang ZQ and Cui J: Comparative mitochondrial genomics among
Spirometra (Cestoda: Diphyllobothriidae) and the molecular
phylogeny of related tapeworms. Mol Phylogenet Evol. 117:75–82.
2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Kuchta R, Kołodziej-Sobocińska M, Brabec
J, Młocicki D, Sałamatin R and Scholz T: Sparganosis (spirometra)
in Europe in the molecular era. Clin Infect Dis. 72:882–890.
2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Shin EH, Guk SM, Kim HJ, Lee SH and Chai
JY: Trends in parasitic diseases in the Republic of Korea. Trends
Parasitol. 24:143–150. 2008.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Liu W, Gong T, Chen S, Liu Q, Zhou H, He
J, Wu Y, Li F and Liu Y: Epidemiology, diagnosis, and prevention of
sparganosis in Asia. Animals (Basel). 12(1578)2022.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Zhu Y, Ye L, Ding X, Wu J and Chen Y:
Cerebral sparganosis presenting with atypical postcontrast magnetic
resonance imaging findings: A case report and literature review.
BMC Infect Dis. 19(748)2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Du B, Tao Y, Ma J, Weng X, Gong Y, Lin Y,
Shen N, Mo X and Cao Q: Identification of sparganosis based on
next-generation sequencing. Infect Genet Evol. 66:256–261.
2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Lin Q, Ouyang JS, Li JM, Yang L, Li YP and
Chen CS: Eosinophilic pleural effusion due to Spirometra mansoni
spargana: A case report and review of the literature. Int J Infect
Dis. 34:96–98. 2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zhang P, Zou Y, Yu FX, Wang Z, Lv H, Liu
XH, Ding HY, Zhang TT, Zhao PF, Yin HX, et al: Follow-up study of
high-dose praziquantel therapy for cerebral sparganosis. PLoS Negl
Trop Dis. 13(e0007018)2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Hong D, Xie H, Wan H, An N, Xu C and Zhang
J: Efficacy comparison between long-term high-dose praziquantel and
surgical therapy for cerebral sparganosis: A multicenter
retrospective cohort study. PLoS Negl Trop Dis.
12(e0006918)2018.PubMed/NCBI View Article : Google Scholar
|