Introduction
Sap is a plant fluid carried through the xylem
cells, which transport water and nutrients in the plant, or through
the phloem sieve tube elements (1). Sap primarily consists of water with a
small amount of mineral elements, sugars, hormones and other
nutrients (1). Acer mono
(A. mono) is an endemic Korean mono maple (2) whose sap may be ingested directly as a
beverage or concentrated into syrup by boiling it for use as a
sweetener (1,3). It has been suggested that the sap of
A. mono may be beneficial due its abundant calcium and
magnesium ion content. A solution of A. mono sap ameliorates
osteoporosis-like symptoms, and is therefore known as ‘bone-benefit
water’ (1).
Neutrophils, the most abundant type of white blood
cells, are phagocytes that play critical roles in combating acute
infection and innate immunity (4).
These cells have a unique capacity to engulf and eliminate microbes
and quickly congregate at the site of infection (5). The phagocytic responses (ingesting
microorganisms or particles) of neutrophils against pathogens
involve the polymerization and rearrangement of cellular actin
filaments (6). The phagocytozed
pathogens are then eliminated by microbicidal hydrolytic enzymes
and an oxidative burst caused by the formation of reactive oxygen
species (ROS) (5). Although
increased production of phagocyte-derived ROS may damage host cells
and tissues, ROS function as potent antimicrobial agents that
protect against infection and cellular signaling molecules under
certain conditions (7).
It has been reported that constituents extracted
from Acer okamotoanum (A. okamotoanum) leaves and
twigs have antioxidant activities (2). However, the effects of the sap on
phagocytic responses have not been studied. The objective of the
present study was to determine whether the sap of A.
okamotoanum affects phagocytic responses of peripheral blood
polymorphonuclear neutrophilic leukocytes (PMNs). For this, we
performed experiments using mouse PMNs compromised by treatment
with dexamethasone (DEX). DEX is a well-known glucocorticoid that
impairs the innate functions of phagocytes (8,9). We
also examined the in vitro effects of A. okamotoanum
sap on phagocytic capacity, oxidative burst activity (OBA) and
filamentous polymeric actin (F-actin) levels of mouse PMNs. Our
results showed that the sap of A. okamotoanum enhanced
phagocytic activity and OBA in mouse and canine neutrophils, thus
demonstrating its antimicrobial effect.
Materials and methods
Experimental animals and treatments
Male, 9-week-old ICR mice were obtained from KOATECH
(Gyeonggi, Korea). The animals were housed in polycarbonate cages
and acclimated in an environmentally controlled room (23±2°C;
relative humidity, 50±10%; frequent ventilation; 12-h light/dark
cycle) prior to use. The sap of A. okamotoanum was prepared
as previously described (1). To
assess the effect of the sap on DEX-treated PMNs, the mice were
randomly divided into eight groups. A total of 50 ml tap water or
25, 50 or 100% solutions of sap were administered to the mice at
ages 9–16 weeks. Mice in the control group received a physiological
saline (0.9% NaCl) solution and the other mice (treatment group)
received a DEX solution (Sigma-Aldrich Co., St. Louis, MO, USA).
Mice in the treatment group received three injections of DEX (1
mg/kg, subcutaneous) every 24 h. For the control group, an
equivalent volume of saline solution was injected at the same time
points. The experimental procedures were approved by the Ethics
Committee of Chungbuk National University (Chungbuk, Korea).
PMN isolation
PMNs were then isolated from rat and canine
peripheral blood vessels using a double density gradient
centrifugation method to immediately collect blood samples, as
described previously (10,11). Briefly, heparinized blood samples
were overlaid on a Histopaque-1077 solution (specific gravity,
1.077; Sigma-Aldrich Co.) and Histopaque-1119 solution (specific
gravity, 1.199; Sigma-Aldrich Co.) and centrifuged at 700 × g for
40 min at 20°C. The PMNs were subsequently harvested from the
interface between Histopaque-1077 and Histopaque-1119, and washed
three times with cold phosphate-buffered saline (PBS). To purify
the PMNs, residual erythrocytes were lysed by treatment with 0.83%
NH4Cl in a tri(hydroxymethyl)-aminomethane-based buffer
(pH 7.2) for 5 min. PMN purity in the final cell suspension was
verified to be >96% as determined by Wright-Giemsa staining
analysis of a blood film obtained by cytocentrifugation. The
viability of the PMNs, as determined by trypan blue dye exclusion,
was >97% in every case. The resulting PMNs were resuspended in
RPMI-1640 medium (Sigma-Aldrich Co.) supplemented with 2 mM
L-glutamine, 0.02 mg gentamicin/ml and 5% heat-inactivated fetal
bovine serum (Invitrogen, Grand Island, NY, USA).
Simultaneous measurement of phagocytic
capacity and OBA
Phagocytic capacity and OBA were evaluated
simultaneously as previously described (12). Briefly, the isolated PMNs were
placed in 24-well plates at a density of 1×106
cells/ml/well and incubated for 2 h at 37°C with 5% CO2
in a humidified atmosphere. For the in vitro assay, the PMN
cells were isolated from rat and canine vessels (abdominal aorta
and median antebrachial vein) and incubated with A.
okamotoanum sap for 2 h with the previously indicated doses at
37°C in a 5% CO2-humidified atmosphere. A
carboxylate-modified polystyrene fluorescent microsphere suspension
(20 μl; 1.0 μm in size; TransFluoSpheres; Molecular Probes Inc.,
Eugene, OR, USA) adjusted to a concentration of 1×109
beads/ml was added to the wells for the final 1 h of culture. When
15 min of culture time remained, 1 μM dihydrorhodamine 123
(Sigma-Aldrich Co.) was added. The conversion of non-fluorescent
dihydrorhodamine 123 into fluorescent rhodamine 123 by
intracellular ROS was used to measure OBA. The phagocytic capacity
was determined by estimating the number of PMNs that had
phagocytized fluorescent microspheres in the gated cell population
of the sample.
The cultured cells were gently harvested,
centrifuged at 400 × g for 3 min at 4°C and washed three times with
PBS solution containing 3 mM EDTA. All steps performed following
the start of cultivation were conducted in the dark. The cells were
analyzed within 30 min using a multipurpose flow cytometer (FACS
Calibur system; Becton Dickinson Immunocytometry Systems, San Jose,
CA, USA) with an argon laser set at 488 nm and analysis software
(CELLQuest, version 3.3; Becton Dickinson Immunocytometry Systems).
The FL1 channel was set to 505–545 nm to detect green fluorescent
rhodamine 123 and the FL3 channel was set to 630–660 nm to detect
red fluorescent microspheres. The cells were gated on the basis of
their forward and side light-scattering characteristics. Phagocytic
capacity and OBA were expressed as percentages and mean
fluorescence intensities (MFIs, arbitrary units), respectively.
Measurement of total cellular F-actin
contents
Total cellular F-actin levels were measured as
described previously (13). The
isolated PMNs were seeded in 24-well plates at a density of
1×106 cells/ml/well, and then incubated with an extract
of A. okamotoanum sap for 80 min at 37°C in a 5%
CO2-humidified atmosphere. A carboxylate-modified
polystyrene fluorescent microsphere suspension (20 μl; 1.0 μm in
size) adjusted to a concentration of 1×109 beads/ml was
added to the wells for the final 20 min of culture. The cultured
cells were gently harvested, centrifuged at 400 × g for 3 min at
4°C and washed three times with PBS containing 3 mM EDTA. The
viability of the PMNs was verified to be >98% based on trypan
blue dye exclusion. The cells were fixed with fixation buffer (BD
Cytofix; Becton Dickinson Biosciences) at 4°C according to the
manufacturer’s instructions, washed three times with PBS and then
stained in the dark for 15 min at 37°C with 165 nM FITC-labeled
phalloidin (Sigma-Aldrich Co.) and 100 μg/ml
lysophosphatidylcholine. The cells were washed and analyzed within
30 min using a FACS Calibur system (Becton Dickinson
Immunocytometry Systems) and analysis software (CELLQuest, version
3.3; Becton Dickinson Immunocytometry Systems) with the argon laser
set at 488 nm. Samples from 10,000 cells were assayed in
triplicate. The FL1 channel was set to 505–530 nm to detect the
green fluorescing FITC molecule. The F-actin levels were expressed
as MFI.
Statistical analyses
The analyses were performed with SigmaStat, version
2.0 (SPSS Inc., Chicago, IL, USA). Differences between the
treatment groups were evaluated by a one way analysis of variance
(ANOVA), followed by Dunnett’s post hoc test. Two-group comparisons
were performed with a two-sample t-test. Normality tests
(Kolmogorov-Smirnov) were performed to determine whether or not the
results had a standard normal distribution. P<0.05 was
considered to indicate a statistically significant difference.
Results are shown as the mean ± standard deviation (SD).
Results
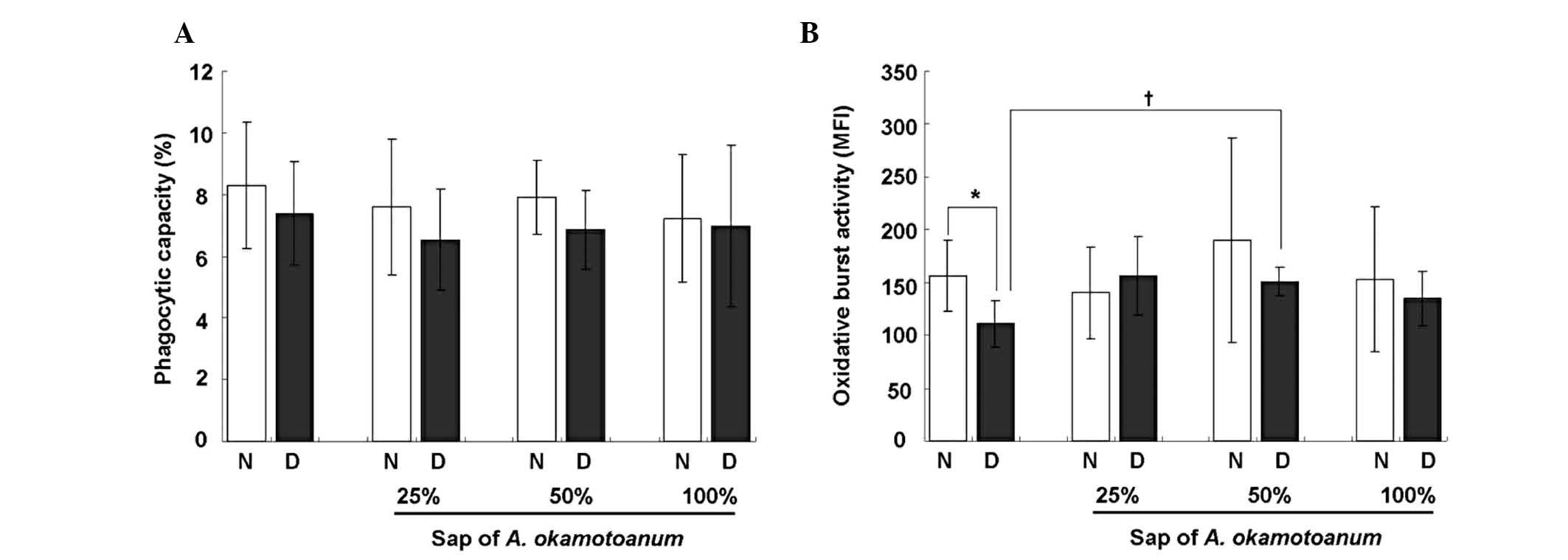
Sap of A. okamotoanum reverses
DEX-induced reduction of OBA in vivo
To investigate the effect of A. okamotoanum
sap on phagocytic responses, mature (9-week-old) mice were fed
increasing concentrations (0, 25, 50 and 100%) of A.
okamotoanum sap for 6 weeks and were then injected with DEX (1
mg/kg) for the last 3 days. Following treatment, PMNs were isolated
from the mice and the phagocytic capacity and OBA were evaluated.
The OBA of the PMNs from mice that received only DEX was
significantly decreased (P=0.004) compared with OBA in cells
obtained from the saline-treated group (Fig. 1B). By contrast, the phagocytic
capacity of the PMNs was not significantly altered by treatment
with DEX (P=0.426; Fig. 1A). The
reduction of OBA by DEX was reversed by pretreatment with A.
okamotoanum sap at a concentration of 50%, indicating that the
sap affected OBA in vivo.
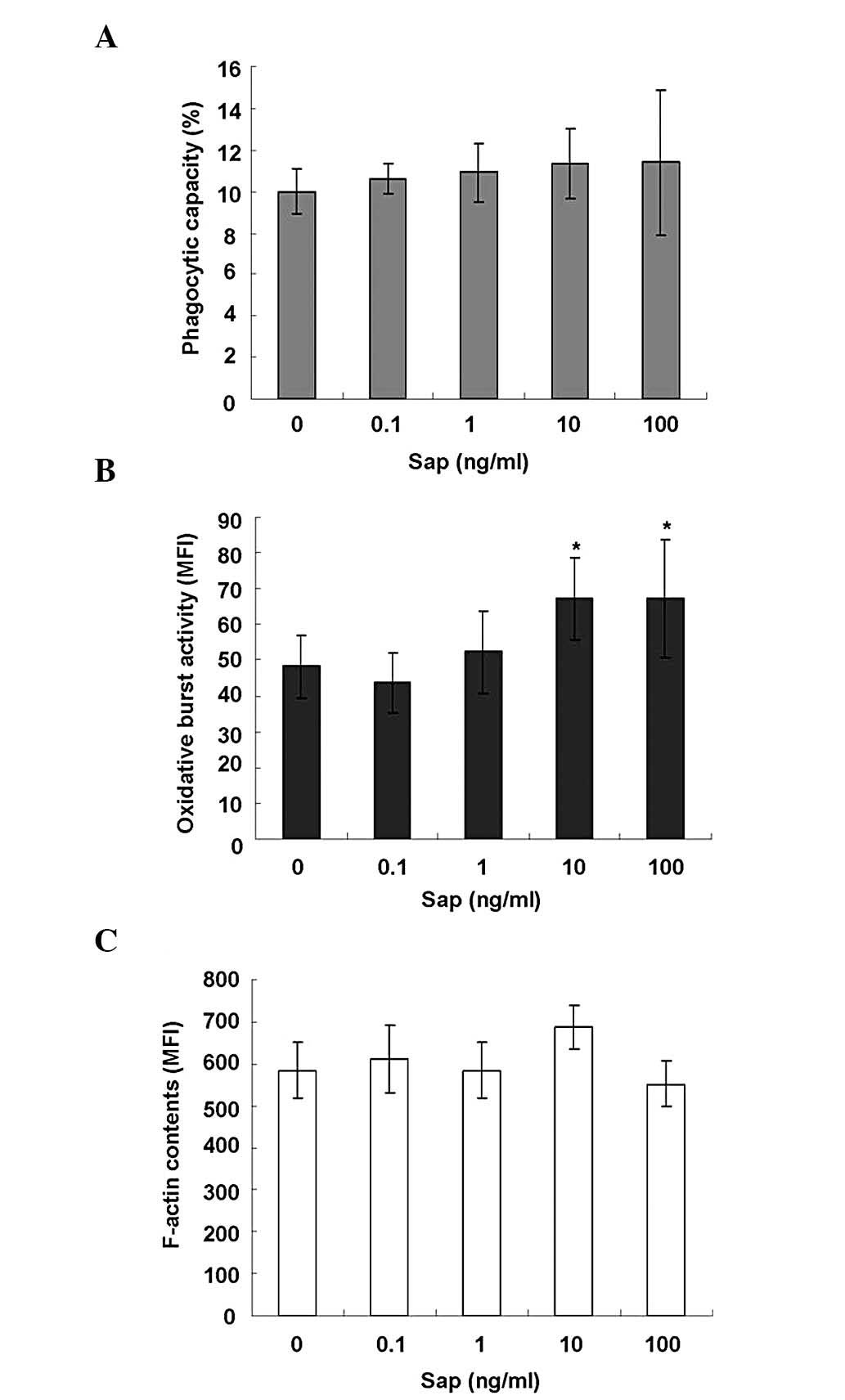
Sap of A. okamotoanum enhances OBA in rat
in vitro PMNs
The effects of A. okamotoanum sap on
phagocytic capacity, OBA and F-actin content was also determined
in vitro using rat PMNs. Similar to the results of the in
vivo study, the A. okamotoanum sap extract had no
significant effect on the phagocytic capacity (P=0.907; Fig. 2A). F-actin content (P=0.136;
Fig. 2C) of the rat PMNs was also
unaltered by this treatment. However, the OBA of the rat PMNs was
increased by treatment with the extract in a dose-dependent manner.
These increases were significant at concentrations of 10 and 100
ng/ml A. okamotoanum sap (P=0.008; Fig. 2B).
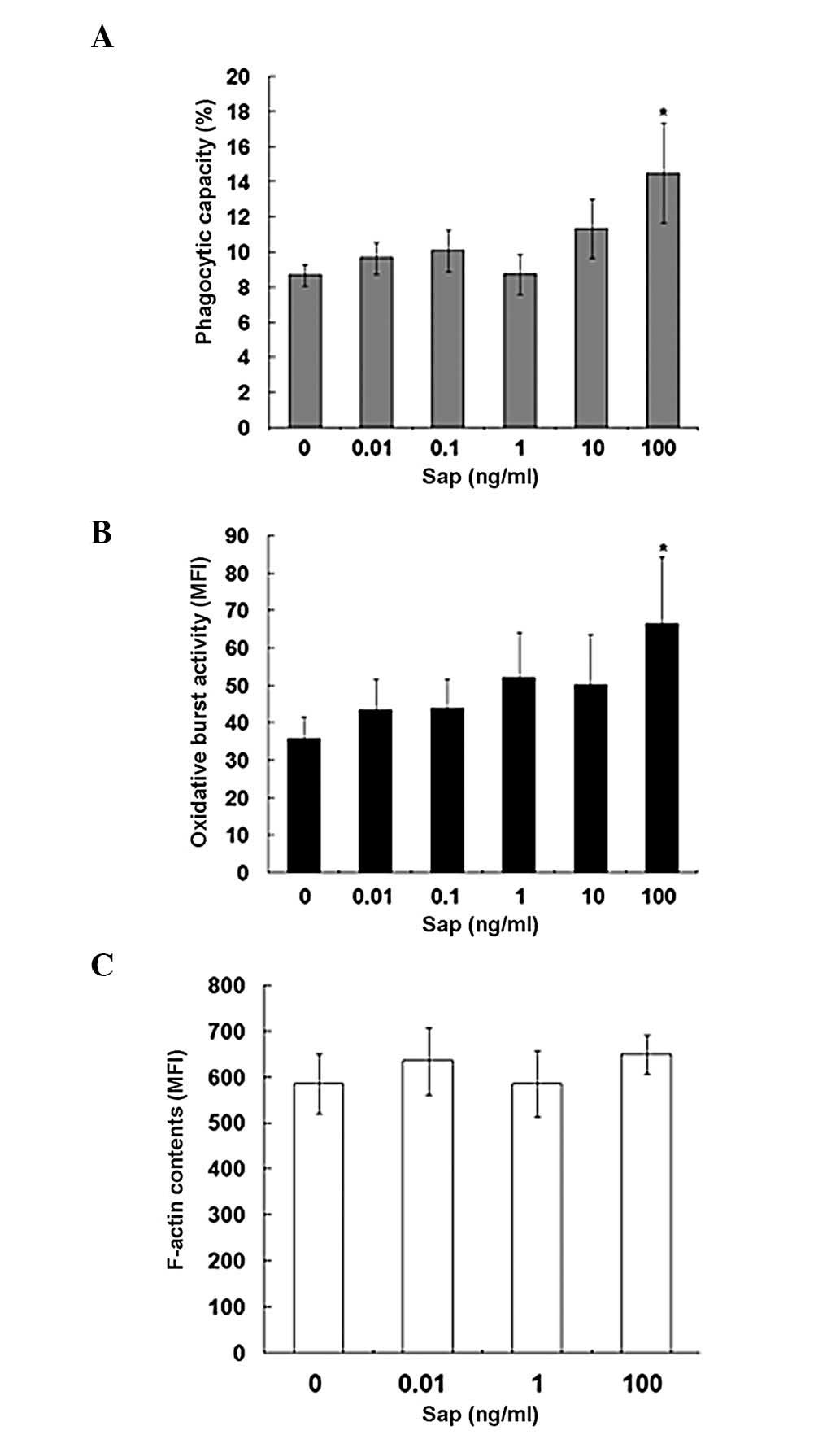
Sap of A. okamotoanum increases
phagocytic activity and OBA in canine in vitro PMNs
In addition to rat PMNs, we also examined the
effects of A. okamotoanum sap on canine PMNs to determine
whether A. okamotoanum sap had antimicrobial activity in
other species. We tested various doses (0.01, 0.1, 1, 10 and 100
ng/ml) of the sap and found that the effects on phagocytic activity
(Fig. 3) were different from that
of rat PMNs. The sap increased phagocytic capacity at a dose of 100
ng/ml in canine PMNs while significant changes were not observed in
rat PMNs. The effect on OBA in canine PMNs was similar to that
observed in rats; this activity was significantly augmented at a
high dose of the sap (100 ng/ml). Canine PMN F-actin content,
following treatment with the sap at 0.01, 1 and 100 ng/ml doses,
was not significantly altered. These results were similar to those
revealed in the rat PMNs (Fig.
3C).
Discussion
In the present study, we examined the effects of
A. okamotoanum sap on the phagocytic response of canine and
mouse neutrophils. First, we monitored changes in phagocytic
activity, OBA and F-actin contents in the absence and presence of
DEX in vivo. Our results showed that DEX reduced OBA and
that this effect was reversed in the presence of the sap in mice.
The effect of glucocorticoids on the phagocytic responses of
phagocytes, including neutrophils, monocytes and macrophages, has
been extensively studied, and contradictory results have been
reported. Glucocorticoids were found to have an inhibitory effect
on ROS production by human monocytes (14), rat peritoneal phagocytes (15) and canine peripheral blood PMNs
(13). By contrast, in one study
glucocorticoids were reported not to have any effect on the release
of reactive oxygen intermediates in cultures of macrophages derived
from human blood (16). Another
study demonstrated that glucocorticoids increased the phagocytic
capacity of human monocytes (14).
To confirm the results of our in vivo study,
we performed experiments using rat and canine PMNs. The OBA of rat
and canine PMNs was increased by addition of the sap. However, the
effects of the sap on phagocytic activity differed between the
species. In the canine PMNs, the sap enhanced phagocytic activity
at a high dose while it did not have any significant effect on rat
PMNs.
Oxidative elimination of microbes is essential for
the defense mechanism of neutrophils in the innate immune system
(17). This process is
accomplished through the generation of ROS by phagocyte
nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, and
its significance is highlighted in cases of chronic granulomatous
disease (18). This disease is
characterized by oxidative burst-deficient neutrophils with
dysfunctional NADPH oxidase (19).
ROS formation, respiratory burst or neutrophil
phagocytosis remove pathogens and thereby offer a defense against
these microorganisms. However, the production of free radicals,
such as ROS, by activated neutrophils at the site of inflammation
also inflicts damage on host tissues. Therefore, regulation of
neutrophilic OBA is important for maintaining a balance between
host tissue injury and the immune defense during the inflammatory
process.
The effects of plant extracts on neutrophil function
have previously been reported. For example, Nepeta ucrainica
L., a herbal tea, was shown to have a positive effect on
respiratory bursts in neutrophils (20). By contrast, other plant extracts,
including those from Harpagophytum procumbens, Liriope
spicata var. prolifera and apples, are believed to
inhibit ROS production, immune responses and inflammation (21–23).
In summary, we determined that treatment with A.
okamotoanum sap stimulated the activity of neutrophils from
mice, rat and canines by increasing phagocytic activity and OBA
in vivo and in vitro. These findings suggest that
this sap may have potential antimicrobial effects on the PMNs of
patients with infection.
Acknowledgements
This study was supported by the National Research
Foundation of Korea (NRF) grant funded by the Korea government
(MEST; no. 2010-0011433).
References
|
1
|
Lee GS, Byun HS, Kim MH, et al: The
beneficial effect of the sap of Acer mono in an animal with
low-calcium diet-induced osteoporosis-like symptoms. Br J Nutr.
100:1011–1018. 2008.PubMed/NCBI
|
|
2
|
Jin W, Thuong PT, Su ND, et al:
Antioxidant activity of cleomiscosins A and C isolated from Acer
okamotoanum. Arch Pharm Res. 30:275–281. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kunkel G: Plants for Human Consumption: An
Annotated Checklist of the Edible Phanerogams and Ferns. 1st
edition. Koeltz Scientific Books; Koenigstein: 1984
|
|
4
|
Segal AW: How neutrophils kill microbes.
Annu Rev Immunol. 23:197–223. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gabrilovich DI: The Neutrophils: New
Outlook for Old Cells. 1st edition. Imperial College Press; London:
1999, View Article : Google Scholar
|
|
6
|
May RC and Machesky LM: Phagocytosis and
the actin cytoskeleton. J Cell Sci. 114:1061–1077. 2001.PubMed/NCBI
|
|
7
|
Fialkow L, Wang Y and Downey GP: Reactive
oxygen and nitrogen species as signaling molecules regulating
neutrophil function. Free Radic Biol Med. 42:153–164. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Long F, Wang YX, Liu L, Zhou J, Cui RY and
Jiang CL: Rapid nongenomic inhibitory effects of glucocorticoids on
phagocytosis and superoxide anion production by macrophages.
Steroids. 70:55–61. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Löwenberg M, Tuynman J, Bilderbeek J, et
al: Rapid immunosuppressive effects of glucocorticoids mediated
through Lck and Fyn. Blood. 106:1703–1710. 2005.PubMed/NCBI
|
|
10
|
Lee SC, Ju SA, Pack HN, et al: 4-1BB
(CD137) is required for rapid clearance of Listeria
monocytogenes infection. Infect Immun. 73:5144–5151. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hasegawa H, Suzuki K, Nakaji S and
Sugawara K: Analysis and assessment of the capacity of neutrophils
to produce reactive oxygen species in a 96-well microplate format
using lucigenin- and luminol-dependent chemiluminescence. J Immunol
Methods. 210:1–10. 1997. View Article : Google Scholar
|
|
12
|
Kang JH and Yang MP: In vitro evaluation
of the effect of trans-10, cis-12 conjugated linoleic acid on
phagocytosis by canine peripheral blood polymorphonuclear
neutrophilic leukocytes exposed to methylprednisolone sodium
succinate. Am J Vet Res. 69:494–500. 2008. View Article : Google Scholar
|
|
13
|
Kang JH and Yang MP: Effect of a
short-term infusion with soybean oil-based lipid emulsion on
phagocytic responses of canine peripheral blood polymorphonuclear
neutrophilic leukocytes. J Vet Intern Med. 22:1166–1173. 2008.
View Article : Google Scholar
|
|
14
|
Ehrchen J, Steinmüller L, Barczyk K, et
al: Glucocorticoids induce differentiation of a specifically
activated, anti-inflammatory subtype of human monocytes. Blood.
109:1265–1274. 2007. View Article : Google Scholar
|
|
15
|
Røshol H, Skrede KK, AErø CE and Wiik P:
Dexamethasone and methylprednisolone affect rat peritoneal
phagocyte chemiluminescence after administration in vivo. Eur J
Pharmacol. 286:9–17. 1995.PubMed/NCBI
|
|
16
|
Schaffner A and Schaffner T:
Glucocorticoid-induced impairment of macrophage antimicrobial
activity: mechanisms and dependence on the state of activation. Rev
Infect Dis. 9(Suppl 5): S620–S629. 1987. View Article : Google Scholar
|
|
17
|
Roos D, van Bruggen R and Meischl C:
Oxidative killing of microbes by neutrophils. Microbes Infect.
5:1307–1315. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Curnutte JT, Whitten DM and Babior BM:
Defective superoxide production by granulocytes from patients with
chronic granulomatous disease. N Engl J Med. 290:593–597. 1974.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Heyworth PG, Cross AR and Curnutte JT:
Chronic granulomatous disease. Curr Opin Immunol. 15:578–584. 2003.
View Article : Google Scholar
|
|
20
|
Akbay P, Calis I, Undeger U, Basaran N and
Basaran AA: In vitro immunomodulatory activity of verbascoside from
Nepeta ucrainica L. Phytother Res. 16:593–595. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pastene E, Speisky H, Troncoso M, Alarcón
J and Figueroa G: In vitro inhibitory effect of apple peel extract
on the growth of Helicobacter pylori and respiratory burst
induced on human neutrophils. J Agric Food Chem. 57:7743–7749.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Qi J, Li N, Zhou JH, Yu BY and Qiu SX:
Isolation and anti-inflammatory activity evaluation of
triterpenoids and a monoterpenoid glycoside from Harpagophytum
procumbens. Planta Med. 76:1892–1896. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hu ZF, Chen LL, Qi J, Wang YH, Zhang H and
Yu BY: Two new benzofuran derivatives with anti-inflammatory
activity from Liriope spicata var. prolifera. Fitoterapia.
82:190–192. 2011. View Article : Google Scholar : PubMed/NCBI
|