Introduction
Gastric cancer remains the second leading cause of
cancer-related mortality worldwide (1,2).
Even after a curative resection alone or after chemotherapy, more
than 50% of patients are likely to experience local regional
recurrence (3,4). Despite the development of more
diagnostic techniques and new treatment modalities, the five-year
survival rate of gastric cancer has not significantly changed.
Therefore, it is critical to explore more specific and efficient
therapies.
MicroRNAs (miRNAs) are important
post-transcriptional regulators of gene expression. Additionally,
they are a class of highly conserved, small RNA molecules that
regulate key biological processes, including differentiation,
development, proliferation and metabolism (5). Accumulating evidence suggests that
the dysregulation of miRNAs expression contributed to malignant
transformation. Furthermore, miRNAs have been confirmed to act as
oncogenes or tumor suppressors in many types of cancer (6). miRNAs, such as miR-130a, miR-214,
miR-27a and miR-451, have been reported to be associated with
chemotherapy resistance (7–9). In
particular, miR-27a, an oncogene, was found to be widely expressed
in breast cancer, gastric adenocarcinoma, human uveal melanoma and
colon cancer (10–13) and to contribute to drug resistance
in various types of cancer (14–17).
However, the role of miR-27a in gastric cancer remains to be
determined.
Perifosine is an orally bioavailable
alkylphospholipid exhibiting antitumor activity in both preclinical
models and clinical trials (18,19).
Its anticancer activity is known to target the cell membrane,
inhibit Akt activity, and affect cell processes such as growth
arrest, apoptosis and survival. In the preclinical studies,
perifosine combined with UCN-01, the chemotherapeutic agent
etoposide and other antitumor agents shows synergistic antitumor
effects (20,21). In the clinical trials, perifosine
shows substantial antitumor activity in sarcoma and renal cell
carcinoma (22). Combined with
dexamethasone, it also has activity in relapsed/refractory multiple
myeloma (23). However, the growth
inhibitory effect of perifosine on gastric cancer has yet to be
reported. Moreover, no related studies on the effect of combining
miR-27a inhibitors with perifosine on gastric cancer are currently
available.
Therefore, we hypothesized that miR-27a was an
oncogene in gastric cancer and that miR-27a inhibitors or
perifosine were able to suppress gastric cancer cell growth. In the
present study, miR-27a expression was detected in gastric cancer
tissues, their matched non-tumor adjacent tissues and gastric
cancer cell lines. We also investigated the effect of miR-27a
inhibitors or perifosine on human gastric cancer cell growth in
vitro.
Materials and methods
Reagents
Perifosine was supplied by Selleck Chemicals LLC
(Houston, TX, USA). This agent was dissolved in phosphate-buffered
saline (PBS) and stored at −20°C. Stock solution was diluted to the
appropriate concentrations with growth medium immediately before
use.
Human tissue samples
In total, 67 pairs of human gastric tissue samples
were obtained during surgery and used after informed consent was
obtained from all 67 patients. The patients underwent surgical
resection at the First Affiliated Hospital of Nanjing Medical
University between 2009 and 2010 following diagnosis of gastric
cancer based on histopathological evaluation. The matched non-tumor
adjacent tissues were obtained from a segment of the resected
specimens that was at a distance from the tumor (>5 cm). The
samples were snap-frozen in liquid nitrogen and stored at −80°C. No
local or systemic treatment was conducted in these patients prior
to surgery. The tumor histological grade was assessed according to
World Health Organization criteria and was staged using the TNM
staging of the International Union Against Cancer (UICC)/American
Joint Committee on Cancer (AJCC) system (2002). The study was
approved by the Research Ethics Committee of Nanjing Medical
University, China. Sample data, including age, gender, weight,
residence, hypertension, diabetes, smoke, tumor location,
histological grade, depth of tumor invasion, lymph node metastasis
and clinical stage were obtained from the clinical and pathologic
records (Table I).
 | Table ImiR-27a expression and
clinicopathological factors. |
Table I
miR-27a expression and
clinicopathological factors.
| High expression group
(n=42) | Low expression group
(n=25) | |
|---|
|
| |
|---|
| Factors | N | Percentage | N | Percentage | P-value |
|---|
| Age (mean ± SD) | 57.71±11.81 | 65.76±9.62 | |
| Weight (mean ±
SD) | 62.85±10.87 | 65.33±15.21 | |
| Gender |
| Male | 30 | 71.4 | 21 | 84.0 | 0.243 |
| Female | 12 | 28.6 | 4 | 16.0 | |
| Hypertension |
| Absent | 31 | 73.8 | 18 | 72.0 | 0.872 |
| Present | 11 | 26.2 | 7 | 28.0 | |
| Diabetes |
| Absent | 38 | 90.5 | 23 | 92.0 | 0.833 |
| Present | 4 | 9.5 | 2 | 8.0 | |
| Smoke |
| Absent | 26 | 61.9 | 16 | 64.0 | 0.864 |
| Present | 16 | 38.1 | 9 | 36.0 | |
| Location |
| Cardia,
fundus | 23 | 54.8 | 9 | 36.0 | 0.137 |
| Others | 19 | 45.2 | 16 | 64.0 | |
| Histological
grade |
| Well,
moderately | 25 | 59.5 | 21 | 84.0 | 0.037a |
| Poorly,
others | 17 | 40.5 | 4 | 16.0 | |
| Depth of tumor
invasion |
| m, sm, mp | 6 | 14.3 | 4 | 16.0 | 0.849 |
| ss, se, si | 33 | 85.7 | 21 | 84.0 | |
| Lymph node
metastasis |
| Absent | 9 | 21.4 | 5 | 32.0 | 0.905 |
| Present | 33 | 78.6 | 17 | 68.0 | |
| Clinical stage |
| I,II | 10 | 23.8 | 10 | 40.0 | 0.161 |
| III,V | 32 | 76.2 | 15 | 60.0 | |
| Residence |
| Rural | 23 | 54.8 | 9 | 36.0 | 0.137 |
| Urban | 19 | 45.2 | 16 | 64.0 | |
Cell lines and cell culture
The human gastric cancer cell lines (AGS and
MGC-803) were obtained from the Shanghai Institutes for Biological
Sciences, Chinese Academy of Sciences (Shanghai, China). The cells
were cultured in RPMI-1640 medium (Invitrogen Life Technologies
Inc., Carlsbad, CA, USA) supplemented with 10% fetal bovine serum
(Gibco BRL, Grand Island, NY, USA) at 37°C in a humidified
atmosphere consisting of 5% CO2.
miRNA transfection
Cells in the exponential phase of growth were plated
in 60-mm plates at 1×106 cells/plate and cultured for 16
h, and then transfected with miR-27a inhibitors or the negative
control (NC) (100 nM) using Lipofectamine™ 2000 Reagent and
OPTI-MEM I reduced serum medium (Invitrogen Life Technologies
Inc.), following the manufacturer’s protocol. The effects of
miR-27a inhibitors or the NC were examined 48 h following
transfection as described previously (9).
Growth inhibition assay
Cells were seeded in 96-well culture plates and
treated on the second day with perifosine. At the end of a
three-day treatment, the cell number was estimated by the
sulforhodamine B (SRB) assay, as previously described (24).
RNA isolation and quantitative
RT-PCR
Total RNA from specimens and cultured AGS and
MGC-803 cells was extracted using TRIzol reagent (Invitrogen Life
Technologies Inc.) according to the manufacturer’s protocol.
TaqMan microRNA assays (Applied Biosystems Inc., Carlsbad,
CA, USA) were used to quantify miR-27a expression. Small nuclear
RNA, U6B (Applied Biosystems Inc.), was treated as the
normalization control. Real-time amplifications were measured in
triplicate and performed with the ABI Prism® 7300
sequence detection system (Applied Biosystems Inc.). The
fold-change of miR-27a was calculated using the 2−ΔΔCT
method. Therefore, the value of the relative expression ratio
>1.0 was considered as a high expression as compared to the
non-tumor control where a ratio <1.0 was considered as a low
expression of cancer.
Statistical analysis
Data were presented as the mean ± SD from at least
three separate experiments. Statistical analysis was performed with
the Student’s t-test and Pearson’s χ2 test. The
difference between the groups was determined by the one-way
analysis of variance (ANOVA). Differences were considered
statistically significant at P<0.05. The analyses were carried
out with the SPSS 13.0 (SPSS Inc., Chicago, IL, USA) and were based
on two-tailed probability.
Results
Expression of miR-27a in gastric cancer
tissues and its correlation with clinicopathological
characteristics of gastric cancer
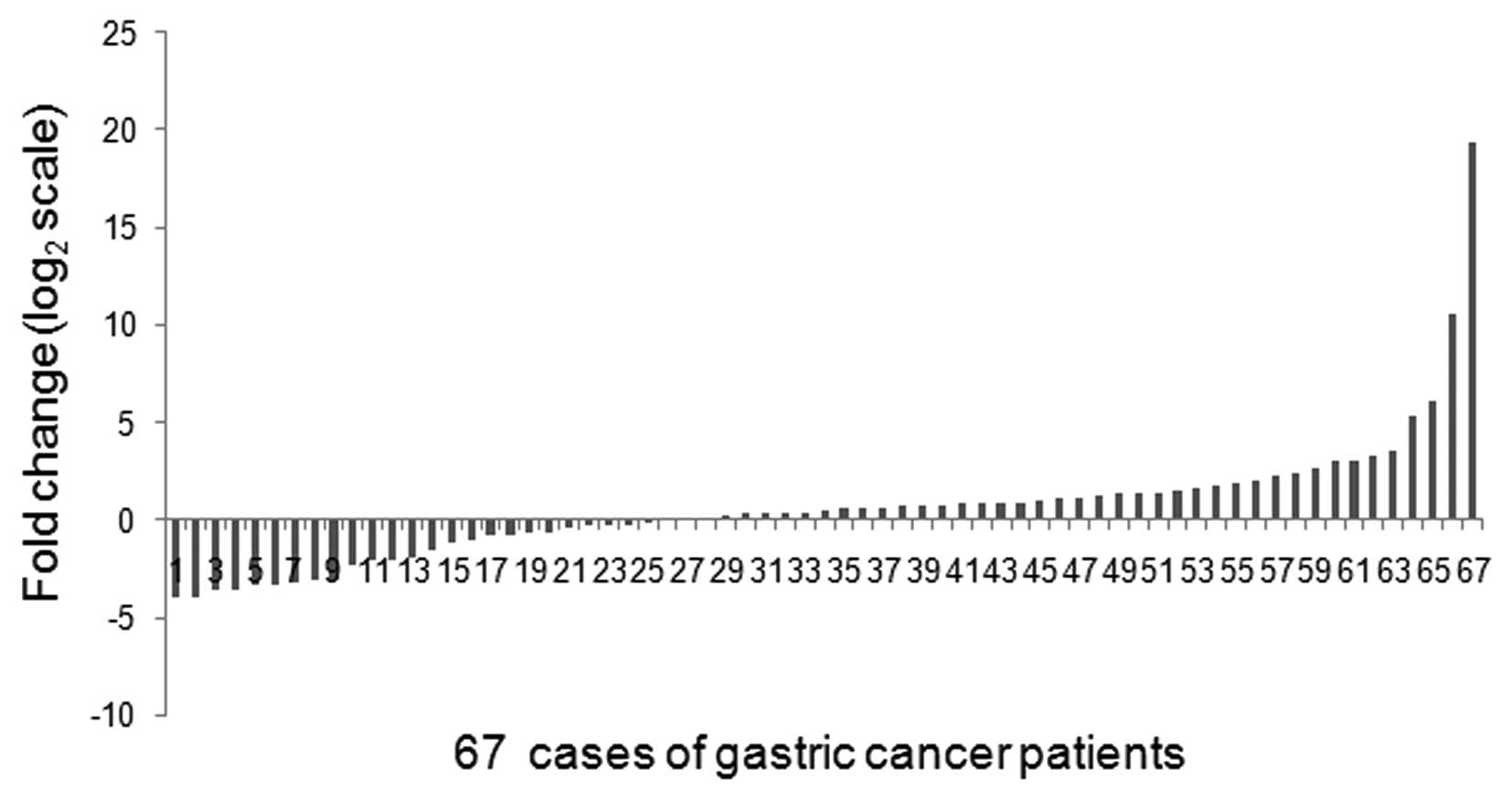
Using a qRT-PCR method, miR-27a was detected in all
67 (100%) pairs of gastric cancer tissues and their matched
non-tumor adjacent tissues. Of the 67 patients with gastric cancer,
42 (62.69%) cases revealed >50% increase in the miR-27a level as
compared to their matched non-tumor adjacent tissues (Fig. 1).
The correlation between miR-27a expression and
clinicopathological characteristics of gastric cancer was examined.
The Pearson’s χ2 test revealed that the expression
levels of miR-27a were associated with tumor histological grade
(P=0.037) in gastric cancer patients (Table I). The patients with a high miR-27a
expression tended to have a poor tumor histological grade.
Effect of downregulation of miR-27a on
cell growth in vitro
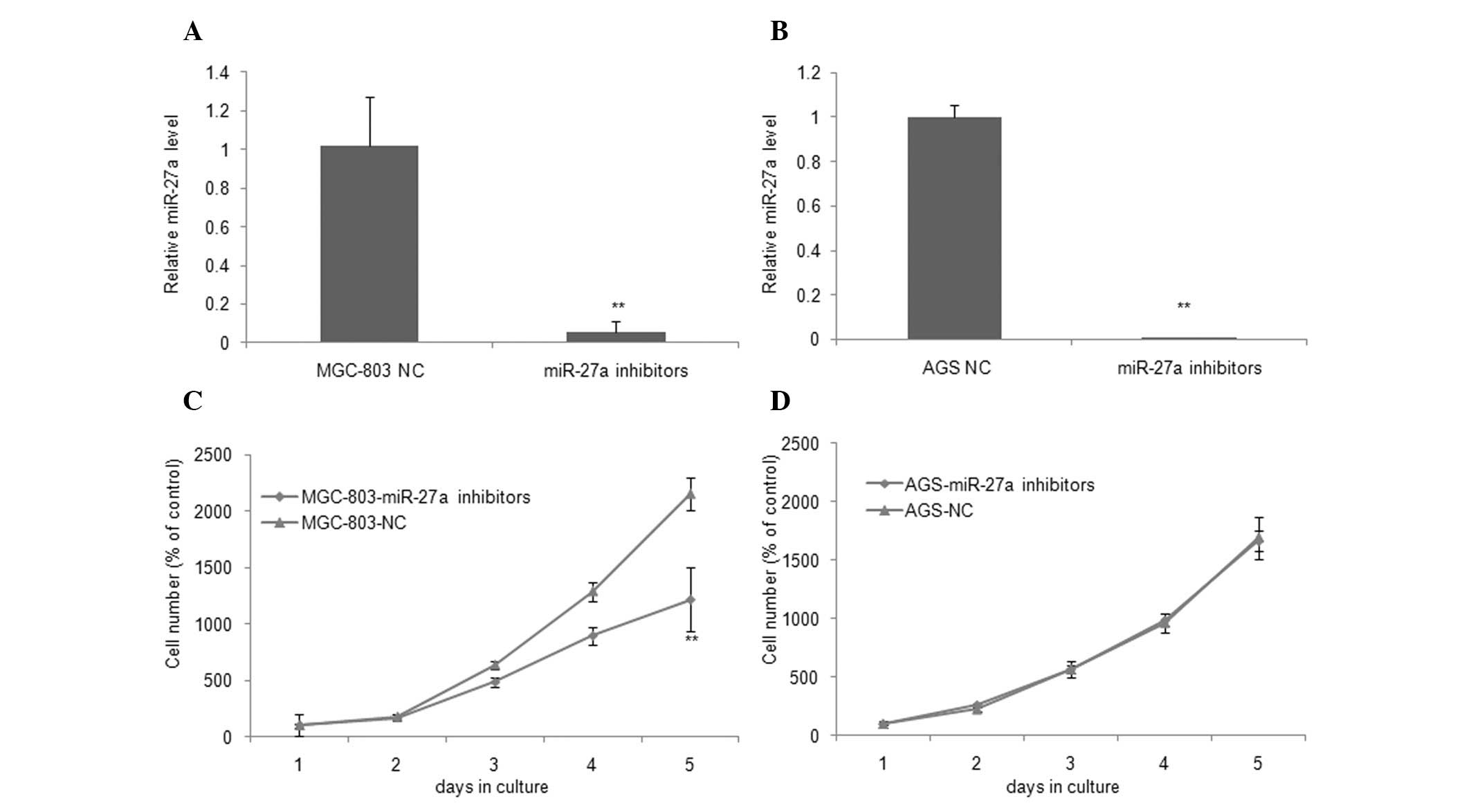
The potential involvement of miR-27a in
tumorigenesis was determined following the significant increase of
miR-27a expression in gastric cancer samples. As an initial step,
we examined the expression of miR-27a by qRT-PCR 48 h following
transfection with miR-27a inhibitors or their respective NCs in
MGC-803 and AGS cell lines. As shown in Fig. 2A and B, miR-27a inhibitors
significantly inhibited the expression of miR-27a by 94.22 and
99.78% as compared with NCs in MGC-803 and AGS cells, respectively.
According to the results of the SRB assay and growth curves, the
MGC-803 cell line, which was transiently transfected with miR-27a
inhibitors, was found to have a significant growth inhibition as
compared with NC (P=0.005, Fig.
2C). On day 5, the cell number decreased to 0.57-fold in cells
expressing the downregulation of miR-27a. However, this decrease in
cell number did not suppress the growth of AGS cells (P=0.766,
Fig. 2D). These findings suggest
that the downregulation of miR-27a selectively inhibited cell
growth in MGC-803 cells.
Expression of miR-27a in gastric cancer
cell lines and the effect of perifosine
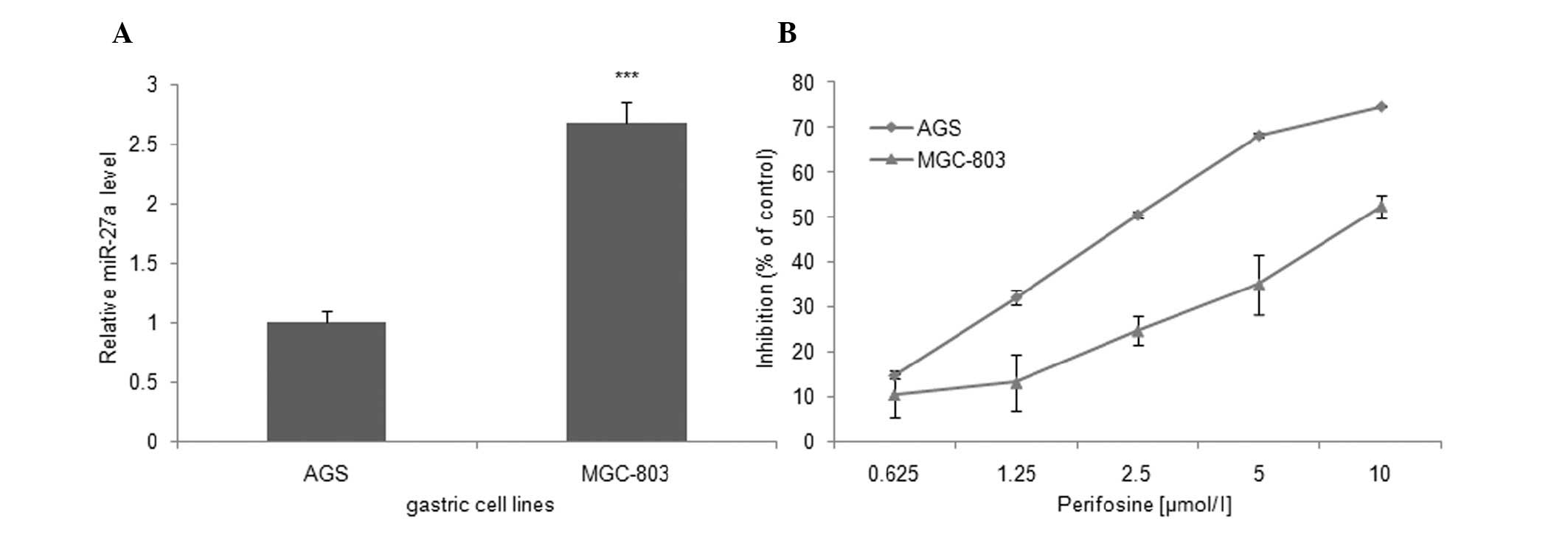
To explain the above finding, we detected the
expression level of miR-27a in MGC-803 and AGS gastric cancer cell
lines. Compared with AGS cells, the expression level of miR-27a was
increased in MGC-803 cells (2.67±0.18-fold, Fig. 3A). These data suggest that the
selective sensitivity of MGC-803 cells to miR-27a inhibitors is
likely to be correlated with the high miR-27a expression
levels.
In this study, the effect of perifosine as a single
agent on the growth of the MGC-803 and AGS cells was investigated
for the first time. Subsequent to treatment with varying
concentrations of perifosine for 72 h, the results of SRB assay
showed that perifosine decreased MGC-803 and AGS cell growth by
52.44±2.50 and 74.72±0.29% in 10 μM/l group compared to control
cells, respectively (Fig. 3B).
Additionally, the 50% inhibitory concentration (IC50)
values of perifosine in MGC-803 and AGS cells were 9.84 and 2.76
μM/l, respectively, for the same intervals. These results suggest
that perifosine inhibited the growth of gastric cancer cells in a
dose-dependent manner, but AGS cells were more sensitive to
perifosine compared to MGC-803 cells. We hypothesized that the
insensitivity of MGC-803 cells to perifosine may also be correlated
with the high miR-27a expression levels and that the downregulation
of miR-27a may enhance the effects of perifosine on MGC-803
cells.
Growth inhibitory effect of perifosine
following transfection with miR-27a inhibitors
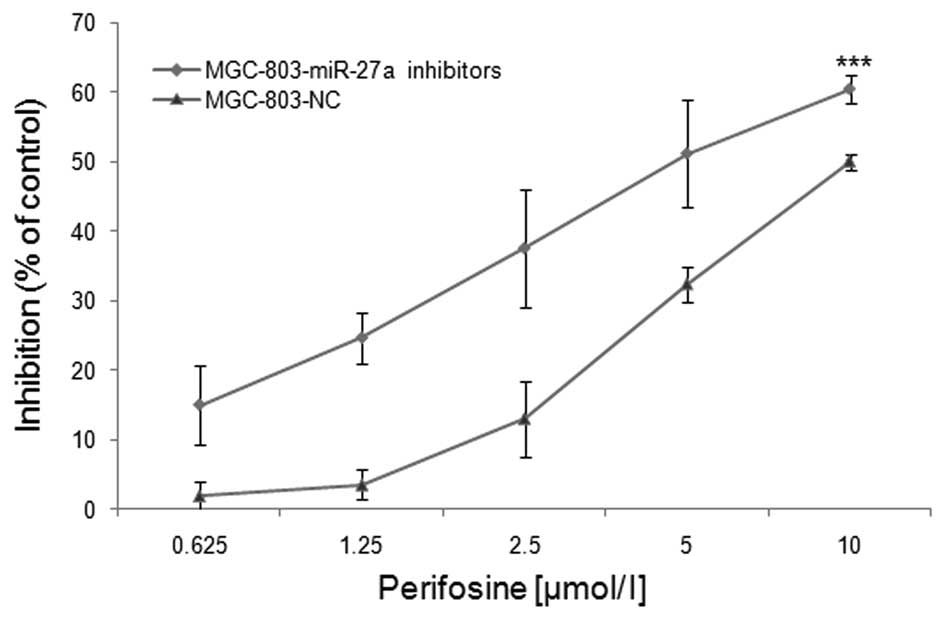
To confirm that perifosine insensitivity is
correlated with miR-27a expression in MGC-803 cells, cells were
transfected with either the miR-27a inhibitors or the NC and
treated with varying concentrations of perifosine. Cell viability
was then measured using the SRB assay. As shown in Fig. 4, at the maximum concentration of 10
μM/l perifosine, the cell viability of the MGC-803 cells with
miR-27a transfection was reduced by 60.53±1.99%, whereas the
NC-transfected cell viability was reduced by 50.04±1.17%. The
IC50 of perifosine was 4.93 and 9.21 μM/l in MGC-803
cells with miR-27a inhibitors and the NC-transfected cells,
respectively (Fig. 4). The
IC50 of perifosine was significantly decreased after
miR-27a inhibitor transfection (P<0.001). These data suggest the
downregulation of miR-27a enhanced the inhibitory effects of
perifosine on gastric cancer MGC-803 cells.
Discussion
It is well known that surgical resection is the most
effective treatment for gastric cancer and is effective in
prolonging the survival of patients with early gastric cancer,
whereas the prognosis for individuals with advanced gastric cancer
remains poor (25). Although
numerous chemotherapy drugs have been studied, there is no
internationally recognized standard of care for chemotherapy
strategy (26). Therefore, new
therapeutic approaches should be developed.
MiRNAs are important post-transcriptional regulators
of gene expression. Moreover, they are a class of highly conserved,
small RNA molecules that regulate key biological processes, while
the dysregulation of miRNA expression contributes to malignant
transformation (5,6). Recent evidence shows that the
dysregulation of miRNAs may be important in cancer initiation,
progression and prognosis (27,28).
Therefore, the study of their clinical potential in gastric cancer
is imperative.
MiR-27a, which is considered to be a carcinogenesis,
has been found to be widely expressed in many tumors (10–13).
Liu et al(12) have shown
that miR-27a was upregulated in human gastric adenocarcinoma and
MGC-803 gastric cancer cells. In this study, we found a significant
high expression of miR-27a in gastric cancer tissues compared with
their matched non-tumor adjacent tissues, which was consistent with
previous reports (12). Taken
together, we have further suggested that miR-27a may be involved in
carcinogenesis as an oncogene in human gastric cancer. Furthermore,
miR-27a expression was associated with tumor histological grade
(P=0.037) in gastric cancer patients. The patients with a high
miR-27a expression tended to have a poor tumor histological grade,
which was an independent prognostic factor in patients with cancer.
Additionally, miR-27a may be involved in gastric cancer progression
and serve as a potential prognostic marker for gastric cancer
patients.
The correlation of cell growth and the expression of
miR-27a was detected. In present experiments, we transfected
MGC-803 and AGS cells with the miR-27a inhibitors or the NCs and
yielded a high transfection efficiency. Moreover, results of the
SRB assay revealed that the downregulation of miR-27a significantly
inhibited cell growth in the MGC-803 cell line, but did not
suppress the growth of AGS cells. The reason for the downregulation
of miR-27a inhibiting cell growth of MGC-803 versus AGS cells was
also investigated, and the results suggest varying miR-27a
expression levels. Our findings also confirmed that the expression
of miR-27a was higher in the MGC-803 cell line as compared to the
AGS cell line. Thus, miR-27a acts as an oncogene in gastric cancer
and downregulation of its expression significantly inhibited
gastric cancer cell growth. However, the lack of a significant
effect on AGS cells may be due to the low miR-27a expression
levels.
Perifosine is a synthetic, orally bioavailable
alkylphospholipid analogue that acts primarily at the cell membrane
targeting signal transduction pathways. Perifosine has been shown
to exert anticancer activity, which is partly associated with its
ability to inhibit Akt activity and the mTOR signaling pathway, and
to affect multiple cell processes, including proliferation,
survival and apoptosis (19,29,30).
Perifosine has recently received wide attention due to its
potential health beneficial effects (31). Therefore, to improve our
understanding of the antitumor activity of perifosine, we measured
its effects on human gastric cancer cells. Results of the present
study have shown for the first time that perifosine inhibited human
gastric cancer cell growth in vitro in a
concentration-dependent manner. However, perifosine exerted its
activity selectively on AGS cells, which is a miR-27a low
expression gastric cancer cell line. These data therefore suggest
that the insensitivity of perifosine is correlated with the high
expression levels of miR-27a in gastric cancer cell lines.
In their study, Zhu et al(9) reported that miR-27a and miR-451 were
involved in activating the multidrug resistant gene 1 (MDR1)
P-glycoprotein expression, which was associated with cancer cell
resistance to a series of chemotherapeutics. Zhang et
al(15) also reported that the
downregulation of miR-27a has the potential to reverse MDR of
esophageal squamous cell carcinoma. Additionally, the mechanism is
likely to decrease the expression of P-glycoprotein, Bcl-2, and the
transcription of MDR1, but upregulate the expression of Bax.
Notably, the growth inhibitory effect of perifosine was enhanced
when combined with miR-27a inhibitors. The result of SRB assay
indicated that the downregulation of miR-27a promoted perifosine
sensitivity of the MGC-803 cell line, in which miR-27a expression
was higher than that in AGS cell line. This finding may be partly
due to the fact that miR-27a was associated with chemotherapy
resistance and that downregulation of miR-27a modulated MDR in
cancer cells. Taken together, miR-27a is able to potentially
participate in the establishment of a drug-resistant network of
perifosine in gastric cancer. Co-targeting the miR-27a might
provide a new therapy to increase chemotherapy sensitivity, with
the possible mechanism being that the downregulation of miR-27a was
capable of significantly decreasing the expression of
P-glycoprotein, which functioned as an ATP-dependent drug-efflux
pump (15).
In conclusion, miR-27a is an oncogene in gastric
cancer whose downregulation has the potential to inhibit the growth
of MGC-803 cells in vitro. Moreover, a high miR-27a
expression has been associated with poor tumor histological grade
in gastric cancer patients. Furthermore, our study has demonstrated
for the first time that perifosine exerts growth inhibitory
activities in gastric cancer cells in vitro, and that the
downregulation of miR-27a may significantly increase the growth
inhibitory effect of perifosine. Therefore, miR-27a may be a
therapeutic target and potential prognostic biological marker in
gastric cancer. MiR-27a inhibitors alone or in combination with
perifosine may be a novel therapeutic approach against gastric
cancer. However, more investigations should be conduced to clarify
the mechanism underlying the correlation between the miR-27a and
tumor chemoprevention of perifosine.
Acknowledgements
This study was supported by the following grants:
‘Medical ZhongDianRenCai Project’ of Jiangsu (RC2011059), ‘333
Project’, ‘Six RenCai Gaofeng’ Funding for the Young Academic
Leader of Jiangsu to LY National Natural Science Foundation of
China (nos. 30873099 and 81001444), and the Science &
Technology Development Project of Nanjing (nos. 201201055,
2012YX001).
References
|
1
|
Pisani P, Parkin DM, Bray F and Ferlay J:
Erratum: estimates of the worldwide mortality from 25 cancers in
1990. Int J Cancer. 83:870–873. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Danaei G, Vander Hoorn S, Lopez AD, Murray
CJ and Ezzati M: Causes of cancer in the world: comparative risk
assessment of nine behavioural and environmental risk factors.
Lancet. 366:1784–1793. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Orditura M, De Vita F, Muto P, et al:
Adjuvant chemoradiotherapy in patients with stage III or IV
radically resected gastric cancer: a pilot study. Arch Surg.
145:233–238. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Milne AN, Carneiro F, O’Morain C and
Offerhaus GJ: Nature meets nurture: molecular genetics of gastric
cancer. Hum Genet. 126:615–628. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dykxhoorn DM: MicroRNAs and metastasis:
little RNAs go a long way. Cancer Res. 70:6401–6406. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Croce CM: Causes and consequences of
microRNA dysregulation in cancer. Nat Rev Genet. 10:704–714. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang H, Kong W, He L, et al: MicroRNA
expression profiling in human ovarian cancer: miR-214 induces cell
survival and cisplatin resistance by targeting PTEN. Cancer Res.
68:425–433. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sorrentino A, Liu CG, Addario A, Peschle
C, Scambia G and Ferlini C: Role of microRNAs in drug-resistant
ovarian cancer cells. Gynecol Oncol. 111:478–486. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhu H, Wu H, Liu X, et al: Role of
MicroRNA miR-27a and miR-451 in the regulation of
MDR1/P-glycoprotein expression in human cancer cells. Biochem
Pharmacol. 76:582–588. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mertens-Talcott SU, Chintharlapalli S, Li
X and Safe S: The oncogenic microRNA-27a targets genes that
regulate specificity protein transcription factors and the G2-M
checkpoint in MDA-MB-231 breast cancer cells. Cancer Res.
67:11001–11011. 2007. View Article : Google Scholar
|
|
11
|
Pathi SS, Jutooru I, Chadalapaka G, et al:
GT-094, a NO-NSAID, inhibits colon cancer cell growth by activation
of a reactive oxygen species-microRNA-27a: ZBTB10-specificity
protein pathway. Mol Cancer Res. 9:195–202. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu T, Tang H, Lang Y, Liu M and Li X:
MicroRNA-27a functions as an oncogene in gastric adenocarcinoma by
targeting prohibitin. Cancer Lett. 273:233–242. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sun Q, Cong R, Yan H, et al: Genistein
inhibits growth of human uveal melanoma cells and affects
microRNA-27a and target gene expression. Oncol Rep. 22:563–567.
2009.PubMed/NCBI
|
|
14
|
Zhao X, Yang L and Hu J: Down-regulation
of miR-27a might inhibit proliferation and drug resistance of
gastric cancer cells. J Exp Clin Cancer Res. 30:552011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang H, Li M, Han Y, et al:
Down-regulation of miR-27a might reverse multidrug resistance of
esophageal squamous cell carcinoma. Dig Dis Sci. 55:2545–2551.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li Z, Hu S, Wang J, et al: MiR-27a
modulates MDR1/P-glycoprotein expression by targeting HIPK2 in
human ovarian cancer cells. Gynecol Oncol. 119:125–130. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Feng DD, Zhang H, Zhang P, et al:
Down-regulated miR-331–5p and miR-27a are associated with
chemotherapy resistance and relapse in leukemia. J Cell Mol Med.
15:2164–2175. 2011.
|
|
18
|
Vink SR, Schellens JH, van Blitterswijk WJ
and Verheij M: Tumor and normal tissue pharmacokinetics of
perifosine, an oral anti-cancer alkylphospholipid. Invest New
Drugs. 23:279–286. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hilgard P, Klenner T, Stekar J, Nossner G,
Kutscher B and Engel J: D-21266, a new heterocyclic
alkylphospholipid with antitumour activity. Eur J Cancer.
33:442–446. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nyakern M, Cappellini A, Mantovani I and
Martelli AM: Synergistic induction of apoptosis in human leukemia T
cells by the Akt inhibitor perifosine and etoposide through
activation of intrinsic and Fas-mediated extrinsic cell death
pathways. Mol Cancer Ther. 5:1559–1570. 2006. View Article : Google Scholar
|
|
21
|
Dasmahapatra GP, Didolkar P, Alley MC,
Ghosh S, Sausville EA and Roy KK: In vitro combination treatment
with perifosine and UCN-01 demonstrates synergism against prostate
(PC-3) and lung (A549) epithelial adenocarcinoma cell lines. Clin
Cancer Res. 10:5242–5252. 2004. View Article : Google Scholar
|
|
22
|
Van Ummersen L, Binger K, Volkman J, et
al: A phase I trial of perifosine (NSC 639966) on a loading
dose/maintenance dose schedule in patients with advanced cancer.
Clin Cancer Res. 10:7450–7456. 2004.PubMed/NCBI
|
|
23
|
Richardson PG, Wolf J, Jakubowiak A, et
al: Perifosine plus bortezomib and dexamethasone in patients with
relapsed/refractory multiple myeloma previously treated with
bortezomib: results of a multicenter phase I/II trial. J Clin
Oncol. 29:4243–4249. 2011. View Article : Google Scholar
|
|
24
|
Papazisis KT, Geromichalos GD, Dimitriadis
KA and Kortsaris AH: Optimization of the sulforhodamine B
colorimetric assay. J Immunol Methods. 208:151–158. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ueda T, Volinia S, Okumura H, et al:
Relation between microRNA expression and progression and prognosis
of gastric cancer: a microRNA expression analysis. Lancet Oncol.
11:136–146. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
De Vita F, Giuliani F, Silvestris N,
Catalano G, Ciardiello F and Orditura M: Human epidermal growth
factor receptor 2 (HER2) in gastric cancer: a new therapeutic
target. Cancer Treat Rev. 36(Suppl 3): S11–S15. 2010.
|
|
27
|
Ruan K, Fang X and Ouyang G: MicroRNAs:
novel regulators in the hallmarks of human cancer. Cancer Lett.
285:116–126. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mirnezami AH, Pickard K, Zhang L, Primrose
JN and Packham G: MicroRNAs: key players in carcinogenesis and
novel therapeutic targets. Eur J Surg Oncol. 35:339–347. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Vink SR, Lagerwerf S, Mesman E, et al:
Radiosensitization of squamous cell carcinoma by the
alkylphospholipid perifosine in cell culture and xenografts. Clin
Cancer Res. 12:1615–1622. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Momota H, Nerio E and Holland EC:
Perifosine inhibits multiple signaling pathways in glial
progenitors and cooperates with temozolomide to arrest cell
proliferation in gliomas in vivo. Cancer Res. 65:7429–7435. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gills JJ and Dennis PA: Perifosine: update
on a novel Akt inhibitor. Curr Oncol Rep. 11:102–110. 2009.
View Article : Google Scholar : PubMed/NCBI
|