Introduction
Osteosarcoma (OS) is one of the most common primary
malignant bone tumors in children and adolescents. In the early
1970s, introduction of doxorubicin and methotrexate with leucovorin
rescue demonstrated promise for the improvement of OS patient
survival. The five-year survival rate for patients treated with
intensive multidrug chemotherapy and aggressive local control has
been reported at 55–80% (1–3).
However, despite the encouraging trend for longer survival, many
patients still have a poor prognosis. Previous studies have
estimated the five-year survival rate of patients with metastatic
diseases to be <20% (4–6). Development of lung metastases is the
main cause of mortality in patients with OS. Therefore,
identification of the molecular mechanisms of metastasis in OS is
likely to have a significant impact on management and prognosis of
the disease.
Fatty acid metabolic pathways have been previously
reported to be associated with carcinogenesis (7). Fatty acid synthase (FASN) is an
important enzyme involved in endogenous lipogenesis in mammals and
is responsible for catalyzing the synthesis of long-chain fatty
acids. FASN has been identified as crucial for sustaining a number
of biological features of cancer cells (8). The enzyme is expressed at high levels
in a variety of human tumors (8–13),
but remains at low levels in normal tissues. Various studies have
reported that inhibition of FASN expression may suppress cancer
cell proliferation in vitro and in vivo(14–18).
In addition, FASN has also been hypothesized to contribute to
cancer cell metastasis (19,20).
However, it is currently unclear whether this molecule is involved
in OS metastasis and the molecular mechanisms associated with FASN
and metastasis remain unknown.
Matrix metalloproteinases (MMPs) are involved in
degradation of the basement membrane and epimatrix, among which
MMP-2 and -9 markedly correlate with tumor invasion and metastasis.
MMP-2 and -9 are overexpressed in OS and promote OS cell migration
and invasion by degrading components of the basement membrane and
epimatrix. A large number of studies indicate that activation of
the nuclear factor-κB (NF-κB) gene, an upstream regulator of MMPs,
is closely associated with tumor invasion and migration (21,22).
In addition, phosphorylation and activation of Akt has been
recognized as an important regulatory factor in NF-κB signaling.
Specifically, activation of Akt has been identified to be essential
for degradation of an inhibitor of NF-κB, inhibitor of κB (IκB) and
NF-κB activation mediated by IκB kinases (IKKs) (23). Previous studies have demonstrated
that fatty acids synthesized by FASN are incorporated into membrane
phospholipids, known modulators of Akt activation (24,25).
Wang et al reported that inhibiting FASN decreases
phosphorylation of Akt in ovarian cancer cells (26,27).
Therefore, we hypothesize that inhibition of FASN may suppress
osteosarcoma cell invasion and migration by downregulation of the
phosphoinositide 3-kinase (PI3K)/Akt/NF-κB signaling pathway.
In the present study, the effect of FASN inhibition
on OS cell invasion and migration was investigated in vitro.
In addition, the effect of inhibition of FASN on the PI3K/Akt/NF-κB
signaling pathway was investigated. Results indicate that
inhibition of FASN may inhibit OS cell invasion and migration via
downregulation of the PI3K/Akt/NF-κB pathway in vitro.
Materials and methods
Contruction of the recombinant plasmid
containing miRNA targeting the FASN gene
The human cDNA sequence encoding FASN protein
(NM_004104.4) was obtained from GenBank and miRNA and control
single-strain DNA oligos were designed and synthesized using the
following primer sequences: forward 5′-TGCTGAACTCCTGCAAGTTCT
CCGACGTTTTGGCCACTGACTGACGTCGGAGATTGC AGGAGTT-3′ and reverse
5′-CCTGAACTCCTGCAATCT CCGACGTCAGTCAGTGGCCAAAACGTCGGAGAACTT
GCAGGAGTTC-3′. Products were cloned into the express vector
pcDNA6.2-GW/EmGFP-miR using the BLOCK-iT™ Pol II miR RNAi
Expression Vector kit with EmGFP (K4936-00; Invitrogen Life
Technologies, Carlsbad, CA, USA). The DNA sequence of the plasmid
was confirmed using the PureLink HiPure Plasmid DNA kit (K2100-03;
Invitrogen Life Technologies).
Cell culture and transfection
Human OS cell line, U2-OS, (Shanghai Cell Bank,
Chinese Academy of Sciences, Shanghai, China) was cultured in DMEM
with 10% fetal bovine serum (FBS) and incubated at 37°C in 5%
CO2. U2-OS cells were seeded in 6-well plates at 30%
confluence on the day prior to transfection. Transfection with
recombinant plasmid targeting the FASN gene or negative plasmid was
performed using Lipofectamine 2000 reagent. Transfection complexes
were prepared according to the manufacturer’s instructions
(Invitrogen Life Technologies).
Western blot analysis
Total protein from the cells was extracted using
RIPA lysis buffer containing 60 μg/ml PMSF. Cell lysates were then
subjected to sodium dodecyl sulfate polyacrylamide gel
electrophoresis followed by western blot analysis as described
previously (28). Protein
expression levels in cells tranfected by recombinant plasmid were
compared with those transfected by negative plasmid.
Real-time polymerase chain reaction
(RT-PCR)
RT-PCR was used to detect FASN mRNA levels. Total
RNA was extracted from cells using TRIzol reagent (Invitrogen Life
Technologies). Total RNA concentration was determined by
spectrophotometry at 260 nm and the purity was determined by
calculating the 260/280 ratio with a BioPhotometer (Eppendorf,
Hamburg, Germany). RT-PCR and the Two-Step kit (Promega
Corporation, Madison, WI, USA) were used to to obtain cDNA
according to the manufacturer’s instructions, which was then used
as the template for amplification. The following primers were used
to amplify target sequences: FASN forward
5′-CCCACCTACGTACTGGCCTA-3′ and reverse 5′-CTTGGCCTTGGGTGTGTACT-3′,
294 bp; β-actin forward 5′-CGGGAAATCGTGCGTGAC-3′ and reverse
5′-TGGAAGGTGGACAGCGAGG-3′, 443 bp (Sangon, Shanghai, China).
Following amplification, DNA electrophoresis was performed on
standard 1% agarose gels and DNA was labeled and visualised using
ethidium bromide. Images were captured using the Canon Digital IXUS
900Ti. FASN mRNA expression levels in cells transfected with
recombinant plasmid was compared with cells containing the negative
plasmid.
Wound healing assay
Cell migration was assessed by determining the
ability of the cells to move into a cellular space in a
two-dimensional in vitro ‘wound healing assay’. In brief,
cells were grown to confluence in 6-well tissue culture plastic
dishes to a density of ~5×106 cells/well. Cells were
denuded by dragging a rubber policeman (Fisher Scientific, Hampton,
NH, USA) through the center of the plate. Cultures were rinsed with
PBS and replaced with fresh DMEM alone or containing 10% FBS,
following which the cells were incubated at 37°C for 24 h. Images
were captured at 0 and 24 h and the migrated distance was measured
using ImageJ (NIH, Bethesda, MD, USA). Cell migration rate was
calculated using 3 fields/area and presented as the average of 6
independent experiments performed over multiple days. The migration
rate of cells transfected by recombinant plasmid targeting the FASN
gene, was compared with cells transfected by negative plasmid.
Transwell invasion assay
Invasion of U2-OS cells was measured using the BD
BioCoat™ BD Matrigel™ Invasion Chamber (BD Biosciences, Franklin
Lakes, NJ, USA) according to the manufacturer’s instructions.
Medium in the lower chamber contained 5% fetal calf serum as a
source of chemoattractants. Cells were suspended in DMEM and added
to upper chambers at the same time. Cells that passed through the
Matrigel-coated membrane were stained with Diff-Quik (Sysmex, Kobe,
Japan) and images were captured under a microscope (ECLIPSE-TS-100,
Nikon, Japan; magnification, ×400) at 0 and 24 h. Cell counts were
performed using ImageJ. Values for invasion were obtained by
counting 3 fields/membrane and presented as the average of 6
independent experiments performed over multiple days. The number of
invaded cells transfected with recombinant plasmid targeting the
FASN gene, was compared with cells transfected with negative
plasmid.
Statistical analysis
All experiments were repeated 6 times. Data are
expressed as the mean ± SD of ≥3 experiments. Independent-samples
T-test was performed for statistical analysis. P<0.05 was
considered to indicate a statistically significant difference. All
analyses were performed using SPSS version 13.0 (Statistical
Software for Social Sciences, Chicago, IL, USA).
Results
Effect of recombinant plasmid targeting
FASN gene on FASN expression in U2-OS cells
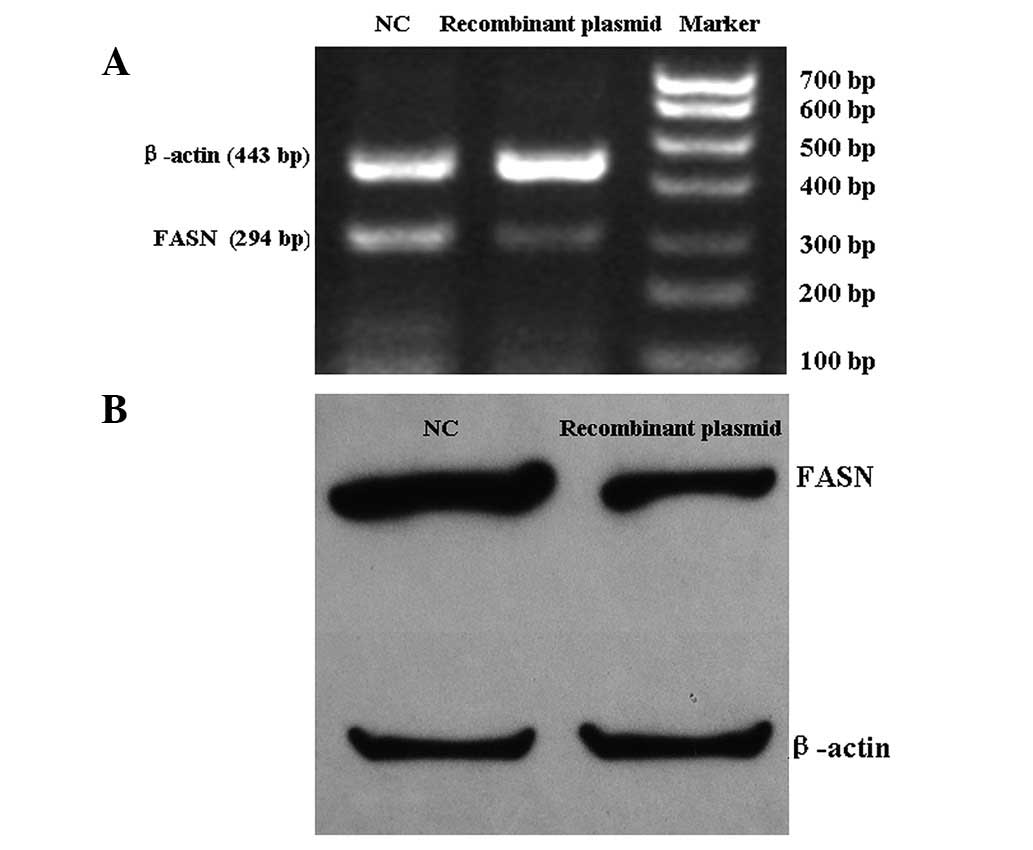
Cultured U2-OS cells were transfected with the
recombinant plasmid for 24 h. FASN mRNA and protein expression
levels in U2-OS cells were detected by RT-PCR and western blot
analysis (Fig. 1). FASN mRNA and
protein expression levels in cells transfected with the recombinant
plasmid were significantly lower than in those transfected with
negative plasmid. These results indicate that recombinant plasmid
miRNA targeting the FASN gene may inhibit FASN expression in U2-OS
cells.
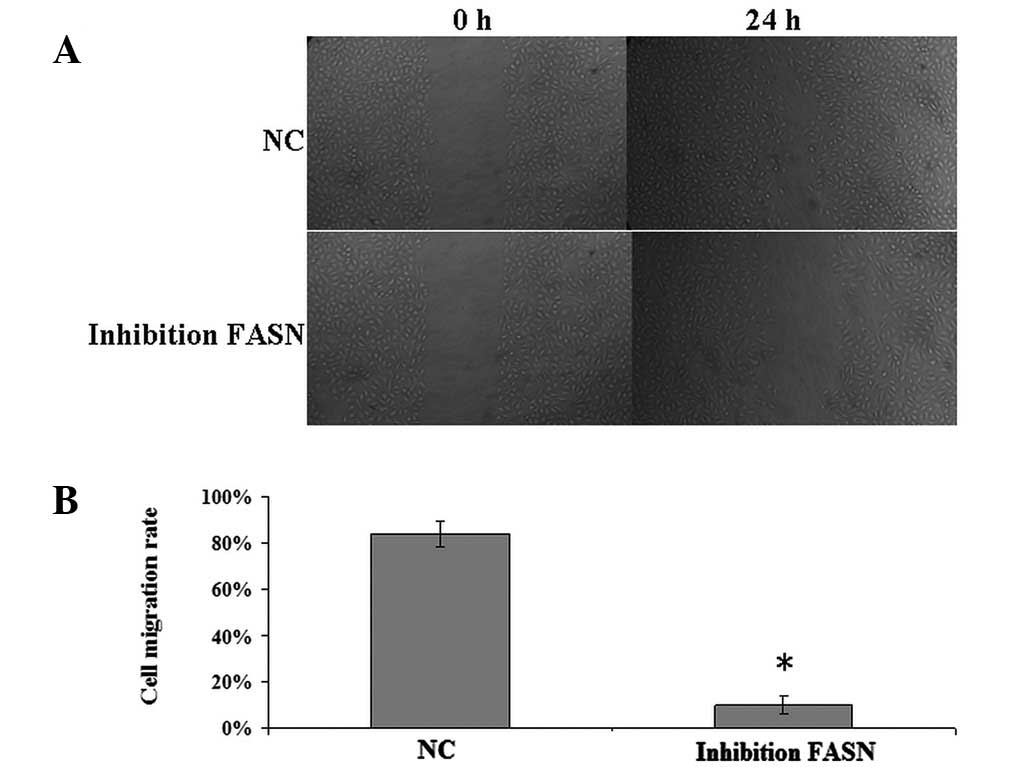
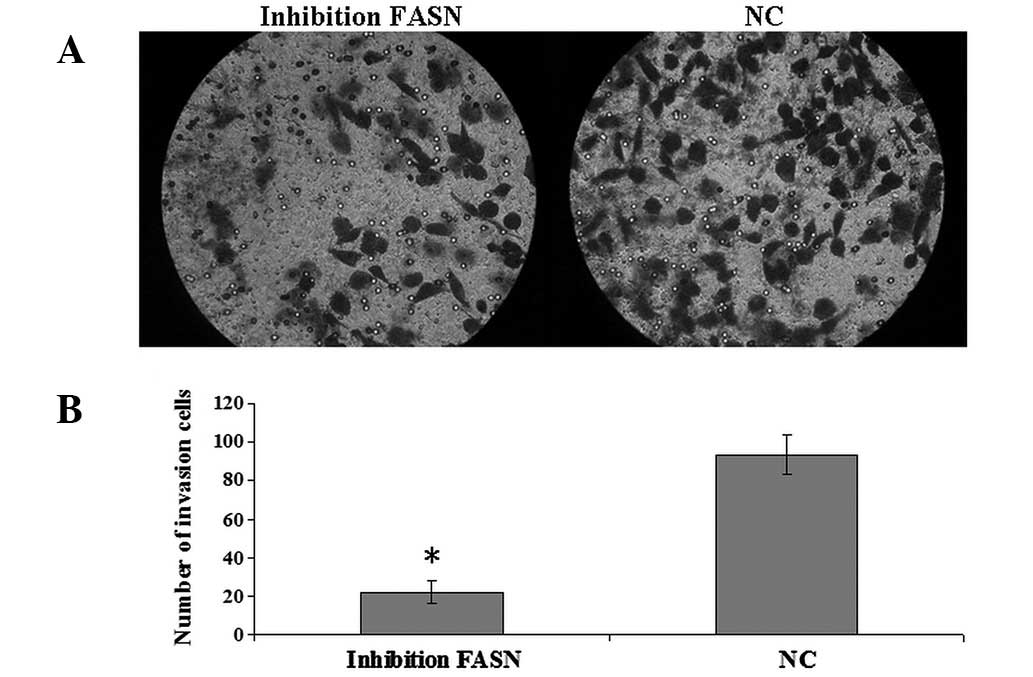
Effect of FASN inhibition on U2-OS cell
invasion and migration in vitro
The recombinant plasmid was transfected into U2-OS
cells. Wound healing and Transwell invasion assays were performed
to measure the migration and invasion of U2-OS cells and revealed
that the number of transmembrane cells (22.2±5.6 cells/membrane)
and migration rate (10±4%) in cells transfected by the recombinant
plasmid were identified to be significantly reduced compared with
cells transfected with negative plasmid (invasion, 93.7±10.3
cells/membrane; migration; 84±5.4%; P<0.05; Figs. 2 and 3). The results indicate that FASN
inhibition may suppress U2-OS cell invasion and migration in
vitro.
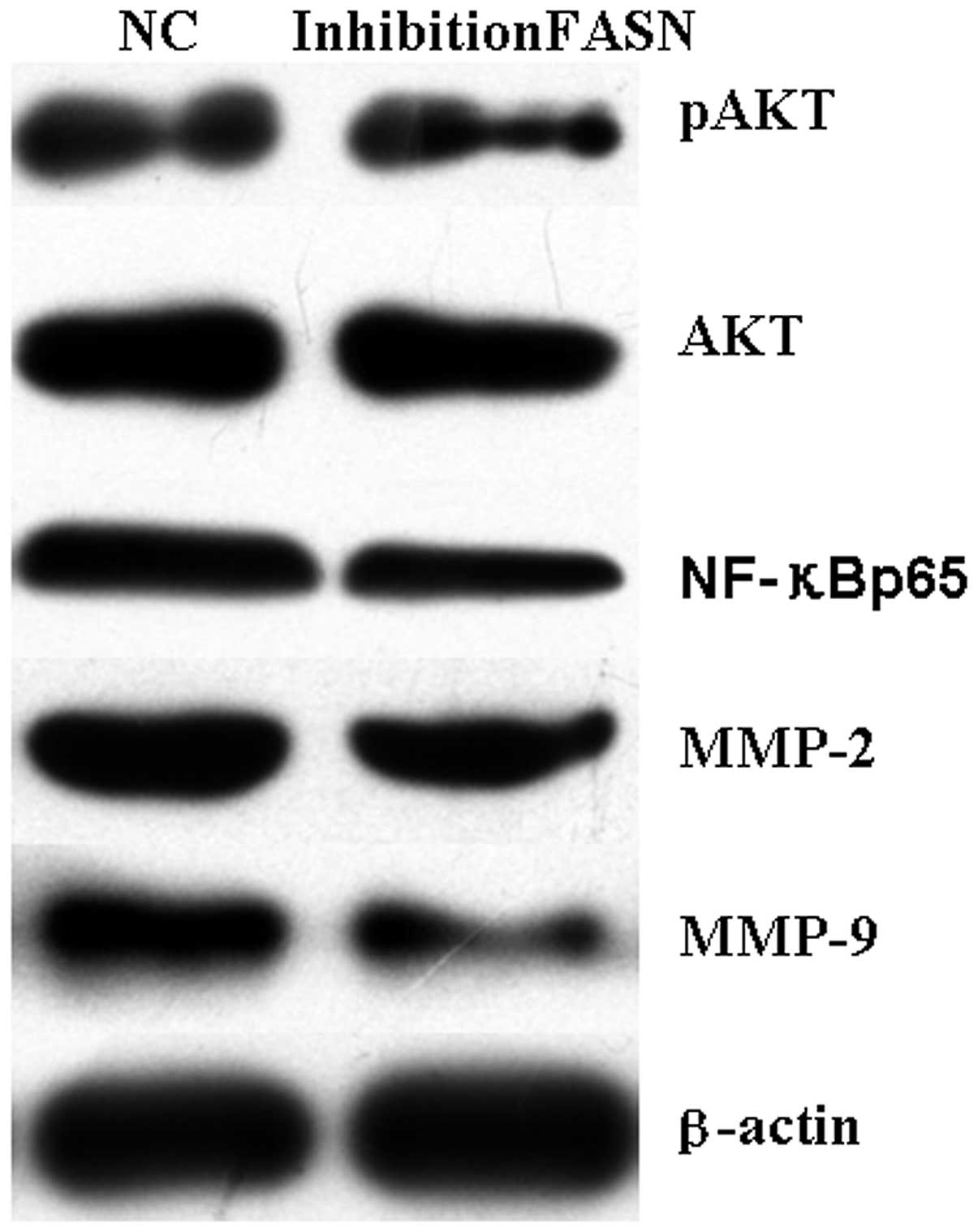
Effect of FASN inhibition on the
PI3K/Akt/NF-κB signaling pathway
To investigate the effect of inhibition of FASN on
phosphorylation of Akt, expression levels of pAkt protein in U2-OS
cells were measured using western blot analysis. Results indicate
that pAkt protein expression levels in cells transfected with
recombinant plasmid were significantly lower than cells with
negative plasmid (Fig. 4). This
observation indicated that inhibition of FASN may decrease
phosphorylation of Akt. In addition, protein expression levels of
NF-κB (p65), MMP-2 and -9 were detected. NF-κB (p65), MMP-2 and -9
protein were decreased significantly in cells transfected with the
recombinant plasmid compared with cells transfected with the
negative plasmid. These observations demonstrate that inhibition of
the FASN gene inhibits MMP-2 and -9 protein expression and nuclear
transfer of NF-κB in U2-OS cells (Fig.
4) and indicate that silencing FASN may downregulate the
PI3K/Akt/NF-κB pathway.
Discussion
OS is the most common childhood and adolescent
primary malignant tumor. Metastasis to the lungs is one of the main
causes of mortality in patients with OS. Therefore, study of the
molecular mechanisms of metastasis of OS is important to improve
survival of patients with metastatic disease.
Human FASN is a 270-kDa cytosolic dimeric enzyme,
responsible for fatty acid synthesis. Endogenous fatty acid
synthesis from the small carbon precursors acetyl-CoA and
malonyl-CoA is dependent on the activity of FASN. In the majority
of the cells, FASN is downregulated by dietary fatty acids, with
the exception of lipogenic tissues, including the liver, lactating
breast, fetal lung and adipose tissue. Previous studies have
identified that neoplastic lipogenesis is essential for cancer cell
survival (29). In addition,
downregulation of FASN has been revealed to decrease invasion and
migration in a variety of human tumors (19,20).
Previously, we reported that cerulenin, an inhibitor of FASN
inhibits OS cell proliferation in vivo and in
vitro(30). However, it is
currently unknown whether silencing FASN suppresses OS cell
invasion and migration and the molecular mechanisms associated with
this process have yet to be defined. In the present study, a
recombinant plasmid, containing miRNA designed to target the FASN
gene, was constructed for inhibition of FASN in OS cells. The
inhibitory effect was investigated by RT-PCR and western blot
analysis and the results demonstrated that FASN expression levels
were significantly inhibited by the recombinant plasmid (Fig. 1). In addition, wound healing and
Transwell invasion assays were performed to detect the migration
and invasion of U2-OS cells. The migration rate of cells
transfected by the recombinant plasmid was identified to be
significantly lower than the negative plasmid cells (Fig. 2). Invasion was also inhibited
(Fig. 3). Results indicate that
silencing the FASN gene may inhibit OS cell invasion and migration
in vitro.
The molecular mechanisms associated with FASN
silencing and inhibition of OS cell migration and invasion were
also analyzed. The role of the PI3K/Akt/NF-κB signaling pathway in
OS invasion and migration was confirmed. Upregulation of FASN
expression in cancer cells has been previously associated with the
PI3K/Akt signaling pathway (31,32).
In addition, FASN inhibition leads to downregulation of activated
Akt and its downstream targets (33–35).
Akt is essential for NF-κB activation by stimulation of the IKK
complex, which phosphorylates and inactivates IκB, an inhibitor of
NF-κB. NF-κB is composed of DNA-binding subunits (p50 and p52) and
subunits with transcriptional activity (p65 and RelB or c-Rel),
which dimerize in various combinations. The primary form of NF-κB
is a heterodimer of the p50 and p65 subunits and is localized
mainly to the cytoplasm in an inactive form bound to IκB.
Previously, NF-κB was demonstrated to upregulate MMP-9 (36). In addition, inhibition of NF-κB was
identified to downregulate MMP-2 (37). During the development of
metastases, cancer cells must degrade the components of the
extracellular matrix. MMPs, particularly MMP-2 and -9, are markedly
associated with this process due to their capacity to degrade the
extracellular matrix, promoting tumor invasion.
In the present study, pAkt protein expression levels
were detected by western blot analysis to investigate whether
silencing FASN led to downregulation of the PI3K/Akt/NF-κB
signaling pathway. Expression of pAkt protein was decreased in
FASN-inhibited compared with negative control cells (Fig. 4), indicating that FASN inhibition
downregulates phosphorylation of Akt. In addition, western blot
analysis was performed to investigate expression levels of NF-κB
(p65) and MMP-2 and -9 protein. Again, protein expression levels
were reduced in FASN-inhibited compared with negative control cells
(Fig. 4), indicating that FASN
inhibition reduces nuclear translocation of NF-κB and attenuates
activation of MMP-2 and -9 protein.
The present study demonstrates that inhibition of
FASN may suppress OS cell invasion and migration via downregulation
of the PI3K/Akt/NF-κB pathway in vitro. Results indicate
that targeting FASN and the PI3K/Akt/NF-κB pathway may be a
potential treatment strategy for treating OS metastases.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China (No. 81260400) and the
Natural Science Fundation of Jiangxi Province (No.
20114BAB205093).
References
|
1
|
Meyers PA, Schwartz CL, Krailo M, et al:
Osteosarcoma: a randomized, prospective trial of the addition of
ifosfamide and/or muramyl tripeptide to cisplatin, doxorubicin, and
high-dose methotrexate. J Clin Oncol. 23:2004–2011. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bacci G, Forni C, Longhi A, et al: Local
recurrence and local control of non-metastatic osteosarcoma of the
extremities: a 27-year experience in a single institution. J Surg
Oncol. 96:118–123. 2007.PubMed/NCBI
|
|
3
|
Jawad MU, Cheung MC, Clarke J, et al:
Osteosarcoma: improvement in survival limited to high-grade
patients only. J Cancer Res Clin Oncol. 137:597–607. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mialou V, Philip T, Kalifa C, et al:
Metastatic osteosarcoma at diagnosis: prognostic factors and
long-term outcome - the French pediatric experience. Cancer.
104:1100–1109. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hegyi M, Semsei AF, Jakab Z, et al: Good
prognosis of localized osteosarcoma in young patients treated with
limb-salvage surgery and chemotherapy. Pediatr Blood Cancer.
57:415–422. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Stokkel MP, Linthorst MF, Borm JJ, et al:
A reassessment of bone scintigraphy and commonly tested
pretreatment biochemical parameters in newly diagnosed
osteosarcoma. J Cancer Res Clin Oncol. 128:393–399. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yeh CS, Wang JY, Cheng TL, et al: Fatty
acid metabolism pathway play an important role in carcinogenesis of
human colorectal cancers by microarray-bioinformatics analysis.
Cancer Lett. 233:297–308. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hess D and Igal RA: Genistein
downregulates de novo lipid synthesis and impairs cell
proliferation in human lung cancer cells. Exp Biol Med (Maywood).
236:707–713. 2011.PubMed/NCBI
|
|
9
|
Alo PL, Amini M, Piro F, et al:
Immunohistochemical expression and prognostic significance of fatty
acid synthase in pancreatic carcinoma. Anticancer Res.
27:2523–2527. 2007.PubMed/NCBI
|
|
10
|
Walter K, Hong SM, Nyhan S, et al: Serum
fatty acid synthase as a marker of pancreatic neoplasia. Cancer
Epidemiol Biomarkers Prev. 18:2380–2385. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Okawa Y, Hideshima T, Ikeda H, et al:
Fatty acid synthase is a novel therapeutic target in multiple
myeloma. Br J Haematol. 141:659–671. 2008. View Article : Google Scholar
|
|
12
|
Migita T, Ruiz S, Fornari A, et al: Fatty
acid synthase: a metabolic enzyme and candidate oncogene in
prostate cancer. J Natl Cancer Inst. 101:519–532. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Silva SD, Cunha IW, Younes RN, et al: ErbB
receptors and fatty acid synthase expression in aggressive head and
neck squamous cell carcinomas. Oral Dis. 16:774–780. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Orita H, Coulter J, Tully E, et al:
Inhibiting fatty acid synthase for chemoprevention of chemically
induced lung tumors. Clin Cancer Res. 14:2458–2464. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Saati GE and Archer MC: Inhibition of
fatty acid synthase and Sp1 expression by 3,3′-diindolylmethane in
human breast cancer cells. Nutr Cancer. 63:790–794. 2011.
|
|
16
|
Notarnicola M, Pisanti S, Tutino V, et al:
Effects of olive oil polyphenols on fatty acid synthase gene
expression and activity in human colorectal cancer cells. Genes
Nutr. 6:63–69. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Notarnicola M, Messa C, Refolo MG, et al:
Polyunsaturated fatty acids reduce fatty acid synthase and
hydroxy-methyl-glutaryl CoA-reductase gene expression and promote
apoptosis in HepG2 cell line. Lipids Health Dis. 10:102011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zecchin KG, Rossato FA, Raposo HF, et al:
Inhibition of fatty acid synthase in melanoma cells activates the
intrinsic pathway of apoptosis. Lab Invest. 91:232–240. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Carvalho MA, Zecchin KG, Seguin F, et al:
Fatty acid synthase inhibition with Orlistat promotes apoptosis and
reduces cell growth and lymph node metastasis in a mouse melanoma
model. Int J Cancer. 123:2557–2565. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Murata S, Yanagisawa K, Fukunaga K, et al:
Fatty acid synthase inhibitor cerulenin suppresses liver metastasis
of colon cancer in mice. Cancer Sci. 101:1861–1865. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang XX, Fu Z, Zhang Z, et al:
Microcystin-LR promotes melanoma cell invasion and enhances matrix
metalloproteinase-2/-9 expression mediated by nf-kappab activation.
Environ Sci Technol. 46:11319–11326. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kao SJ, Su JL, Chen CK, et al: Osthole
inhibits the invasive ability of human lung adenocarcinoma cells
via suppression of NF-kappaB-mediated matrix metalloproteinase-9
expression. Toxicol Appl Pharmacol. 261:105–115. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kuan YH, Huang FM, Li YC, et al:
Proinflammatory activation of macrophages by bisphenol
A-glycidyl-methacrylate involved NFkappaB activation via PI3K/Akt
pathway. Food Chem Toxicol. 50:4003–4009. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Swinnen JV, Van Veldhoven PP, Timmermans
L, et al: Fatty acid synthase drives the synthesis of phospholipids
partitioning into detergent-resistant membrane microdomains.
Biochem Biophys Res Commun. 302:898–903. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Uddin S, Siraj AK, Al-Rasheed M, et al:
Fatty acid synthase and AKT pathway signaling in a subset of
papillary thyroid cancers. J Clin Endocrinol Metab. 93:4088–4097.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang HQ, Altomare DA, Skele KL, et al:
Positive feedback regulation between AKT activation and fatty acid
synthase expression in ovarian carcinoma cells. Oncogene.
24:3574–3582. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Menendez JA, Vellon L, Mehmi I, et al:
Inhibition of fatty acid synthase (FAS) suppresses HER2/neu
(erbB-2) oncogene overexpression in cancer cells. Proc Natl Acad
Sci USA. 101:10715–10720. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bai X, Ma D, Liu A, et al: Rheb activates
mTOR by antagonizing its endogenous inhibitor, FKBP38. Science.
318:977–980. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li W, Tai Y, Zhou J, et al: Repression of
endometrial tumor growth by targeting SREBP1 and lipogenesis. Cell
Cycle. 11:2348–2358. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu ZL, Wang G, Shu Y, et al: Enhanced
antitumor activity of epirubicin combined with cerulenin in
osteosarcoma. Mol Med Report. 5:326–330. 2012.PubMed/NCBI
|
|
31
|
Bandyopadhyay S, Pai SK, Watabe M, et al:
FAS expression inversely correlates with PTEN level in prostate
cancer and a PI 3-kinase inhibitor synergizes with FAS siRNA to
induce apoptosis. Oncogene. 24:5389–5395. 2005. View Article : Google Scholar
|
|
32
|
Porstmann T, Griffiths B, Chung YL, et al:
PKB/Akt induces transcription of enzymes involved in cholesterol
and fatty acid biosynthesis via activation of SREBP. Oncogene.
24:6465–6481. 2005.PubMed/NCBI
|
|
33
|
Tomek K, Wagner R, Varga F, et al:
Blockade of fatty acid synthase induces ubiquitination and
degradation of phosphoinositide-3-kinase signaling proteins in
ovarian cancer. Mol Cancer Res. 9:1767–1779. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zaytseva YY, Rychahou PG, Gulhati P, et
al: Inhibition of fatty acid synthase attenuates CD44-associated
signaling and reduces metastasis in colorectal cancer. Cancer Res.
72:1504–1517. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Uddin S, Jehan Z, Ahmed M, et al:
Overexpression of fatty acid synthase in Middle Eastern epithelial
ovarian carcinoma activates AKT and Its inhibition potentiates
cisplatin-induced apoptosis. Mol Med. 17:635–645. 2011. View Article : Google Scholar
|
|
36
|
Andela VB, Gordon AH, Zotalis G, et al:
NFkappaB: a pivotal transcription factor in prostate cancer
metastasis to bone. Clin Orthop Relat Res. 415(Suppl): S75–S85.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Felx M, Guyot MC, Isler M, et al:
Endothelin-1 (ET-1) promotes MMP-2 and MMP-9 induction involving
the transcription factor NF-kappaB in human osteosarcoma. Clin Sci
(Lond). 110:645–654. 2006. View Article : Google Scholar : PubMed/NCBI
|