Introduction
Obesity, which is caused by an imbalance between
energy intake and expenditure, is associated with various metabolic
diseases, including hypertension, dyslipidemia, diabetes mellitus,
coronary heart disease, congestive heart failure, stroke,
osteoarthritis, sleep apnea and certain types of cancer (e.g.,
colon, breast, endometrial and gall bladder) (1).
Numerous pharmacological approaches for the
prevention and treatment of obesity have been suggested. Obesity
therapies include the suppression of nutrient absorption and the
administration of drugs that control lipid utilization (2). Clinically available anti-obesity
agents include orlistat, which is a lipase inhibitor, and
sibutramine, which is a centrally acting inhibitor of serotonin and
norepinephrine uptake (3,4). However, these agents have been
reported to cause undesirable side-effects, including constipation,
insomnia, vomiting, headaches, stomachaches and heart attacks
(5). Therefore, due to the limited
usage of these pharmacological agents, there is a demand for
alternative therapies, including the use of herbal products, that
have minimal side-effects (6).
Bangpoongtongsungsan (BPT), a traditional herbal medicine composed
of 18 crude drugs, has been reported to be an effective treatment
for obesity, insulin resistance and hypertension (7). In particular, BPT decreases the
weight of white adipose tissues (WATs) and the size of adipocytes
without adverse effects in high-fat diet (HFD)-fed mice (8).
Actinidia polygama Miquel (actinidiaceae)
fructus has been used as a herbal folk medicine for treating pain,
gout, rheumatoid arthritis and inflammation (9). It has been reported that A.
polygama extract (APE) exhibits inhibitory activity against rat
paw edema induced by carrageenans and that it inhibits
lipopolysaccharide-induced nitric oxide (NO) production in RAW
264.7 macrophages (10).
Additionally, APE demonstrates anti-inflammatory and anti-asthmatic
effects in a murine model of asthma (11). However, the anti-obesity effects of
A. polygama have not previously been studied.
In the present study, the activities of a 70%
ethanol extract of A. polygama in changes in body weight,
fat accumulation and serum lipid levels were elucidated in the
mouse model of HFD-induced obesity.
Materials and methods
Preparation of APE
A. polygama fructus was purchased as a dried
herb from Hanherb Co. (Seoul, Korea) and authenticated based on its
microscopic and macroscopic characteristics by the Classification
and Identification Committee of the Korea Institute of Oriental
Medicine (KIOM). The committee was composed of nine experts in the
fields of plant taxonomy, botany, pharmacognosy and herbology. A
voucher specimen (no. JA-85) was deposited at the herbarium of the
Department of Herbal Resources Research in KIOM. Extracts from
dried fructus of A. polygama (300 g) were obtained twice
with 70% ethanol (with 2-h reflux) and the extract was then
concentrated under reduced pressure. The decoction was filtered,
lyophilized and serially stored at 4°C. The yield of the dried
extract from the starting crude materials was ~14.78% (w/w).
Animals and diets
Male 4-week-old C57BL/6J mice were purchased from
Daehan Biolink Co. (Eumsung, Korea) and acclimated for 1 week prior
to the experiments. The mice were housed in an air-conditioned
animal room with a 12-h light/12-h darkness cycle at a temperature
of 22±1°C and humidity of 50±10%. The mice were provided with a
laboratory diet and water ad libitum. All experimental
protocols involving the use of animals were conducted in accordance
with the National Institutes of Health (NIH) guidelines and
approved by the Committee on Animal Care for our institute.
To induce obesity, the mice were fed a HFD (Rodent
diet D12492, Research Diet, New Brunswick, NJ, USA) consisting of
60% energy as fat, 20% as protein and 20% as carbohydrates, in
accordance with previously published studies (12). The control mice were fed a
commercial standard chow diet (Orient Bio, Inc., Seongnam, Korea)
consisting of 14% energy as fat, 21% as protein and 65% as
carbohydrates. Orlistat and BPT were used as positive controls. The
mice were randomly divided into five groups (n=7) and fed a normal
diet (ND), a HFD, a HFD plus APE (HFD+APE), a HFD plus orlistat
(HFD+orlistat) or a HFD plus BPT (HFD+BPT) for 7 weeks. APE or BPT
was dissolved in normal saline and orally administered to the mice
at a dose of 400 mg/kg/day for 7 weeks. Orlistat was administered
to the mice at a dose of 15.9 mg/kg/day. By contrast, the vehicle
(normal saline) was orally administered to the mice in the ND-fed
and HFD-fed control groups. Body weight and food intake were
measured twice a week.
Biochemical analysis of blood
At the end of the experimental period, the mice were
fasted prior to being sacrificed. The mice were anesthetized with
ether and blood samples were obtained from the inferior vena cava
of each mouse. The blood samples were centrifuged at 2,000 × g for
15 min at 4°C, then the serum was collected and stored at −70°C
prior to analysis.
The serum levels of the triglycerides, total
cholesterol, low-density lipoprotein (LDL)-cholesterol,
high-density lipoprotein (HDL)-cholesterol, aspartate transaminase
(AST), alanine transaminase (ALT), blood urea nitrogen and
creatinine were analyzed with an automated serum analyzer (Hitachi
7080, Hitachi Co., Tokyo, Japan). The concentrations of serum
leptin and adiponectin were measured with mouse leptin and
adiponectin ELISA kits (R&D Systems, Minneapolis, MN, USA),
respectively, according to the manufacturer’s instructions.
Absorbance values were measured using a microplate
spectrophotometer (Bio-Rad, Hercules, CA, USA).
Adipose tissue weight and histological
analysis
Subsequent to blood collection, the WATs
(subcutaneous, epididymal and retroperitoneal) were removed from
the mice and immediately weighed.
To stain the adipocytes, the adipose tissues were
fixed in 10% neutral formalin solution for 1 day and embedded in
paraffin. All tissues were cut to a thickness of 6 μm and stained
with hematoxylin and eosin. To quantify the adipocyte sizes, the
stained sections were analyzed using light microscopy (Olympus
BX51, Olympus Optical Co., Tokyo, Japan) and an image analysis
program (Image pro plus 5.0, Media Cybernetics, Silver Spring, MD,
USA).
Statistical analysis
Differences between the groups were examined using
an unpaired Student’s t-test and analysis of variance (ANOVA)
followed by Duncan’s multiple range test. All data are presented as
mean ± standard error (SE). P<0.05 was considered to indicate a
statistically significant difference.
Results
Effects of APE on body weight and food
intake
The body weights and food intake of the mice in the
five treatment groups are listed in Table I. Subsequent to the 7-week
treatment, the increase in body weight of the HFD group was
significantly greater than that of the ND group (P<0.001).
However, the increase in body weight of the HFD+APE group was
significantly less than that of the HFD group (P<0.01). During
the feeding period, food intake was higher in the ND group than in
the HFD group, but did not differ significantly between the HFD and
HFD+APE groups. The food efficiency ratio (FER) was higher in the
HFD group than in the ND group, whereas the FER was significantly
lower in the HFD+APE mice than in the HFD group (P<0.01).
 | Table IEffects of APE on body weight and food
intake in mice with HFD-induced obesity. |
Table I
Effects of APE on body weight and food
intake in mice with HFD-induced obesity.
| Factor | ND | HFD | HFD+APE | HFD+orlistat | HFD+BPT |
|---|
| Initial body weight
(g) | 19.64±0.21 | 19.64±0.19 | 19.64±0.18 | 19.64±0.20 | 19.64±0.20 |
| Final body weight
(g) | 24.18±0.26 | 29.17±0.48c | 26.97±0.71d | 28.07±0.17 | 28.11±0.98 |
| Body weight
gaina (g/7 weeks) | 4.54±0.16 | 9.63±0.32c | 7.34±0.60e | 8.37±0.25d | 8.49±0.81 |
| Food intake
(g/day) | 4.23±0.13 | 2.33±0.02c | 2.58±0.27 | 2.35±0.03 | 2.29±0.10 |
| FERb | 0.022±0.001 | 0.084±0.002c | 0.060±0.006e | 0.074±0.002d | 0.076±0.006d |
Effects of APE on WAT weights
The WAT weights are listed in Table II. The weights of various WATs,
including subcutaneous, epididymal and retroperitoneal adipose
tissues, were greater in the HFD-fed mice than in the ND-fed mice,
while the weights of epididymal and retroperitoneal adipose tissues
were significantly lower in the HFD+APE group (P<0.05).
 | Table IIEffects of APE on organ weights in
mice with HFD-induced obesity (g/100 g body weight). |
Table II
Effects of APE on organ weights in
mice with HFD-induced obesity (g/100 g body weight).
| WAT weights |
|---|
|
|
|---|
| Type of tissue | ND | HFD | HFD+APE | HFD+orlistat | HFD+BPT |
|---|
| Subcutaneous WAT | 0.94±0.04 |
2.85±0.54a | 2.17±0.14 | 2.43±0.15 | 2.92±0.42 |
| Epididymal WAT | 1.13±0.14 |
5.29±0.58b |
3.44±0.35c | 4.19±0.15 | 4.78±0.81 |
| Retroperitoneal
WAT | 0.36±0.11 |
2.22±0.39b |
1.19±0.11c | 1.56±0.13 | 1.68±0.30 |
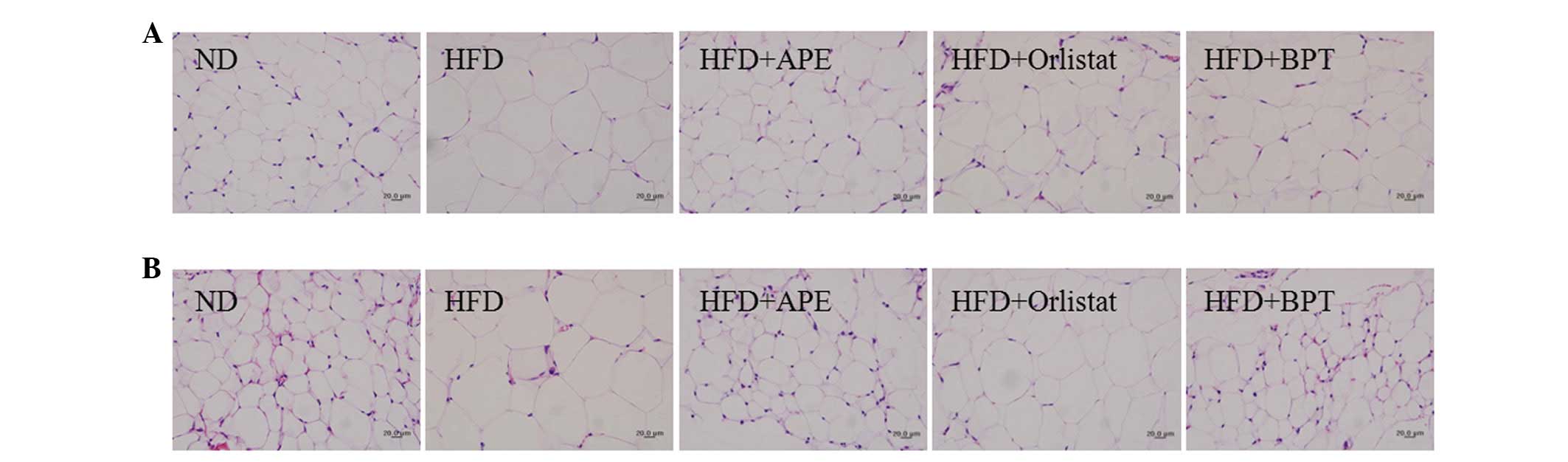
Histological analysis of WAT
Adipose tissues were further examined histologically
(Fig. 1). The sizes of the
retroperitoneal and epididymal adipocytes were larger in the HFD
group than in the ND group. APE treatment decreased the sizes of
the adipocytes in the obese mice relative to the adipocytes in the
HFD-fed mice.
Effects of APE on serum biochemical
levels
The serum biochemical parameters are listed in
Table III. The serum
triglyceride, total cholesterol and leptin levels were
significantly increased in the HFD group compared with the ND
group. APE treatment of the HFD-fed mice significantly reduced the
triglyceride and leptin levels (P<0.05). However, the total
cholesterol levels did not differ between the HFD and HFD+APE
groups. The HDL-cholesterol, LDL-cholesterol and adiponectin levels
did not change among the five groups.
 | Table IIIEffects of APE on biochemical
parameters in serum of mice with HFD-induced obesity. |
Table III
Effects of APE on biochemical
parameters in serum of mice with HFD-induced obesity.
| Parameter | ND | HFD | HFD+APE | HFD+orlistat | HFD+BPT |
|---|
| Total cholesterol
(mg/dl) | 121±2.6 |
161±2.7b | 157±12.1 |
147±4.1c | 151±7.8 |
| Triglyceride
(mg/dl) | 68±23.3 |
83±4.5a |
57±7.7c | 76±9.8 |
51±4.9d |
| LDL-cholesterol
(mg/dl) | 4.2±0.5 | 4.8±0.3 | 6.6±1.3 | 4.7±0.2 | 5.8±0.6 |
| HDL-cholesterol
(mg/dl) | 64.4±1.9 | 66.6±1.0 | 65.3±5.0 | 68.2±2.3 | 65.9±3.0 |
| Adiponectin
(μg/ml) | 12.27±0.54 | 13.43±0.44 | 13.20±0.19 | 11.51±0.9 | 12.85±0.5 |
| Leptin (ng/ml) | 4.09±0.69 |
19.40±3.20b |
9.44±0.97c |
9.17±0.9c | 10.87±2.8 |
Assessment of potential toxicological
effects
The effects of APE on the toxicological parameters
are listed in Table IV. To
evaluate the potential toxic effects of APE ingestion, serum
toxicological markers, which indicate liver and kidney injury, were
measured at the end of the experimental period. The AST levels were
significantly lower in the HFD+APE mice than in the HFD mice
(P<0.05). The serum concentrations of ALT, blood nitrogen urea
and creatinine were not significantly changed in the HFD+APE group
compared with the HFD group. AST, ALT, blood nitrogen urea and
creatinine levels were within previously published normal ranges
for all groups (13,14). Additionally, HFD+APE mice did not
exhibit significant changes in the liver and spleen weights (data
not shown). These data indicate that administration of 400
mg/kg/day APE for 7 weeks induced no detectable adverse toxic
effects in the mice.
 | Table IVEffects of APE on toxicological
parameters in serum of mice with HFD-induced obesity. |
Table IV
Effects of APE on toxicological
parameters in serum of mice with HFD-induced obesity.
| Parameter | ND | HFD | HFD+APE | HFD+orlistat | HFD+BPT |
|---|
| AST (U/l) | 90.6±13.8 | 124.8±22.4 | 62.3±10.0b | 80.0±11.9 | 78.5±23.6 |
| ALT (U/l) | 46.4±2.5 | 35.0±9.9 | 20.9±1.7 | 25.4±1.9 | 24.7±0.5 |
| Blood urea nitrogen
(mg/dl) | 31.6±1.1 |
21.4±1.3c | 20.1±2.0 | 20.1±1.1 | 21.0±1.2 |
| Creatinine
(mg/dl) | 0.45±0.02 | 0.41±0.22 | 0.38±0.03 | 0.43±0.03 | 0.48±0.02 |
Discussion
The results of the present study demonstrated that
APE treatment inhibited body weight gain, visceral (epididymal and
retroperitoneal) adipose tissue mass, adipocyte size and serum
triglyceride levels in mice with HFD-induced obesity. APE treatment
in the HFD-fed mice did not change the food intake levels but
decreased the FER, implying that APE-treated, HFD-fed mice were
less efficient at converting nutrients into their own body mass.
These results show that APE has potent hypolipidemic and
anti-obesity effects. In addition, the 7-week APE treatment induced
no detectable adverse toxic effects in the mice. These data suggest
that the decreased fat mass observed in APE-treated mice was not
due to the toxicity of APE but rather due to a direct
pharmacological action on the adipose tissues.
APE treatment in HFD-fed mice reduced the serum
triglyceride and leptin concentrations with decreased fat masses.
Furthermore, APE treatment in 3T3-L1 preadipocytes inhibited
adipocyte differentiation and lipid (triglyceride) accumulation
(data not shown). Moreover, histological examination revealed a
decrease in the adipocyte size in the HFD+APE group compared with
that of the HFD group. Adipose tissue is known for its capacity to
store excess dietary energy in the form of triglycerides within the
lipid droplets of adipocytes (15). Obesity is characterized by
increased adipose tissue mass that results from an increased fat
cell number (hyperplasia) and an increased fat cell size
(hypertrophy) (16). Specifically,
visceral obesity due to adipocyte hypertrophy is more closely
associated with various metabolic and circulatory diseases
(17).
Visceral fat accumulation induces the production of
a variety of proteins known as adipokines, including visfatin,
tumor necrosis factor-α, interleukin-6, leptin and adiponectin
(18). The secretion levels of
leptin, a key fat-derived regulator of food intake and energy
expenditure, are positively correlated with adiposity and body
weight changes in humans and rodents (19). In the present study, the serum
leptin levels were decreased in the HFD+APE group compared with the
levels in the HFD group. Adiponectin is known to contribute to
insulin sensitivity and fatty acid oxidation and adiponectin levels
are decreased in obesity, type 2 diabetes and atherosclerosis
(20,21). However, the levels of adiponectin
in the present study were not significantly changed in the HFD+APE
mice compared with levels in the HFD-fed mice. This result suggests
that the decreased serum leptin levels from the APE treatment in
the HFD-fed mice may be attributable to a decreased lipid
accumulation in the adipose tissues.
In conclusion, the APE treatment reduced body weight
gain, adipose tissue accumulation, adipocyte size and serum
triglyceride and leptin levels without toxic effects in mice with
HFD-induced obesity. The potent effect of APE on adipocyte
composition and lipidemia without apparent toxic side-effects makes
APE a promising candidate in the development of anti-obesity
pharmacotherapy.
Acknowledgements
This study was supported by the Discovery of Herbal
Medicine for the ‘Prevention of Prehypertension Project’ (K12202)
and the ‘Construction of the Basis for Practical Application of
Herbal Resources’ (K12020) funded by the Ministry of Education,
Science and Technology (MEST) of Korea to the Korea Institute of
Oriental Medicine (KIOM).
Abbreviations:
|
ALT
|
alanine transaminase
|
|
APE
|
Actinidia polygama extract
|
|
AST
|
aspartate transaminase
|
|
BPT
|
Bangpoongtongsungsan
|
|
FER
|
food efficiency ratio
|
|
HDL
|
high-density lipoprotein
|
|
HFD
|
high-fat diet
|
|
LDL
|
low-density lipoprotein
|
|
ND
|
normal diet
|
|
WAT
|
white adipose tissue
|
References
|
1
|
Devlin MJ, Yanovski SZ and Wilson GT:
Obesity: what mental health professionals need to know. Am J
Psychiatry. 157:854–866. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lee YS, Cha BY, Saito K, Choi SS, Wang XX,
Choi BK, Yonezawa T, Teruya T, Nagai K and Woo JT: Effects of a
Citrus depressa Hayata (shiikuwavsa) extract on obesity in
high-fat diet-induced obese mice. Phytomedicine. 18:648–654.
2011.
|
|
3
|
Hofbauer KG, Nicholson JR and Boss O: The
obesity epidemic: current and future pharmacological treatments.
Annu Rev Pharmacol Toxicol. 47:565–592. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sharma B and Henderson DC: Sibutramine:
current status as an anti-obesity drug and its future perspectives.
Expert Opin Pharmacother. 9:2161–2173. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bray GA: Drug treatment of obesity. Rev
Endocr Metab Disord. 2:403–418. 2001. View Article : Google Scholar
|
|
6
|
Song MY, Lv N, Kim EK, Kwon KS, Yoo YB,
Kim JH, Lee SW, Song JH, Lee JH, Lee SK, Shin BC, Ryu DG, Park BH
and Kwon KB: Antiobesity activity of aqueous extracts of Rhizoma
Dioscoreae Tokoronis on high-fat diet-induced obesity in mice.
J Med Food. 12:304–309. 2009.PubMed/NCBI
|
|
7
|
Shimada T, Kudo T, Akase T and Aburada M:
Preventive effects of Bofutsushosan on obesity and various
metabolic disorders. Biol Pharm Bull. 31:1362–1367. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Akagiri S, Naito Y, Ichikawa H, Mizushima
K, Takagi T, Handa O, Kokura S and Yoshikawa T: Bofutsushosan, an
oriental herbal medicine, attenuates the weight gain of white
adipose tissue and the increased size of adipocytes associated with
the increase in their expression of uncoupling protein 1 in
high-fat diet-fed male KK/Ta mice. J Clin Biochem Nutr. 42:158–166.
2008. View Article : Google Scholar
|
|
9
|
Bang MH, Chae IG, Lee EJ, Baek NI, Baek
YS, Lee DY, Lee IS, Lee SP and Yang SA: Inhibitory effects of
actinidiamide from Actinidia polygama on allergy and
inflammation. Biosci Biotechnol Biochem. 76:289–293. 2012.
View Article : Google Scholar
|
|
10
|
Kim YK, Kang HJ, Lee KT, Choi JG and Chung
SH: Anti-inflammation activity of Actinidia polygama. Arch
Pharm Res. 26:1061–1066. 2003. View Article : Google Scholar
|
|
11
|
Lee YC, Kim SH, Seo YB, Roh SS and Lee JC:
Inhibitory effects of Actinidia polygama extract and
cyclosporine A on OVA-induced eosinophilia and bronchial
hyperresponsiveness in a murine model of asthma. Int
Immunopharmacol. 6:703–713. 2006.
|
|
12
|
Choi H, Eo H, Park K, Jin M, Park EJ, Kim
SH, Park JE and Kim S: A water-soluble extract from Cucurbita
moschata shows anti-obesity effects by controlling lipid
metabolism in a high fat diet-induced obesity mouse model. Biochem
Biophys Res Commun. 359:419–425. 2007.
|
|
13
|
Han SS, Han HS, Cho KH, Kim YB, Seo JW and
Song CW: Studies on the basic data of Ktc: C57BL/6 mice with age:
body weight, organ weight, hematology, serum chemistry and
urinalysis. Korea J Lab Anim Sci. 10:197–209. 1994.
|
|
14
|
Schnell MA, Hardy C, Hawley M, Propert KJ
and Wilson JM: Effect of blood collection technique in mice on
clinical pathology parameters. Hum Gene Ther. 13:155–161. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vázquez-Vela ME, Torres N and Tovar AR:
White adipose tissue as endocrine organ and its role in obesity.
Arch Med Res. 39:715–728. 2008.PubMed/NCBI
|
|
16
|
Shin SS, Jung YS, Yoon KH, Choi S, Hong Y,
Park D, Lee H, Seo BI, Lee HY and Yoon M: The Korean traditional
medicine gyeongshingangjeehwan inhibits adipocyte hypertrophy and
visceral adipose tissue accumulation by activating PPARalpha
actions in rat white adipose tissues. J Ethnopharmacol. 127:47–54.
2010. View Article : Google Scholar
|
|
17
|
Tchernof A: Visceral adipocytes and the
metabolic syndrome. Nutr Rev. 65:S24–S29. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fasshauer M and Paschke R: Regulation of
adipocytokines and insulin resistance. Diabetologia. 46:1594–1603.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Maffei M, Halaas J, Ravussin E, Pratley
RE, Lee GH, Zhang Y, Fei H, Kim S, Lallone R, Ranganathan S, et al:
Leptin levels in human and rodent: measurement of plasma leptin and
ob RNA in obese and weight-reduced subjects. Nat Med. 1:1155–1161.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rosen ED and Spiegelman BM: Adipocytes as
regulators of energy balance and glucose homeostasis. Nature.
444:847–853. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Arita Y, Kihara S, Ouchi N, Takahashi M,
Maeda K, Miyagawa J, Hotta K, Shimomura I, Nakamura T, Miyaoka K,
Kuriyama H, Nishida M, Yamashita S, Okubo K, Matsubara K, Muraguchi
M, Ohmoto Y, Funahashi T and Matsuzawa Y: Paradoxical decrease of
an adipose-specific protein, adiponectin, in obesity. Biochem
Biophys Res Commun. 257:79–83. 1999. View Article : Google Scholar : PubMed/NCBI
|