Introduction
As a potential method, cell transplantation provides
a novel strategy for the therapy of incurable human diseases
(1–3). Endothelial progenitor cells (EPCs)
isolated from peripheral blood play a significant role in the
treatment of injured blood vessels and ischemic tissues. Numerous
studies have shown that EPCs exist in the peripheral blood and
migrate into the damaged endothelium and neovascularization sites.
They may play a role in the therapeutic procedure of repairing the
endothelium and in angiogenesis (4,5).
Furthermore, EPCs are capable of migrating into the tumor and
participating in tumor angiogenesis (6,7).
In order to better understand the mechanism of EPC
therapy, the in vivo monitoring of the cellular dynamics of
transplanted EPCs has been proposed. A non-invasive in vivo
technique that permits an evaluation of the potential migration of
the transplanted EPCs would prove to be an essential tool for the
treatment procedure. Many studies have indicated that magnetic
resonance imaging (MRI) is effective in tracking the distribution
of transplanted EPCs in vivo by labeling the cells with
superparamagnetic iron oxide (SPIO) nanoparticles (8).
However, it is not very clear whether the SPIO
labeling technique in EPCs is effective and safe. Additionally, the
effect of SPIO upon EPCs remains unclear on a cellular level
(9). The objective of the present
study was to investigate whether and to what extent the labeling of
EPCs with SPIO affects the main biological characteristics of
EPCs.
Materials and methods
EPC culture and characterization
The present study was approved by the Animal Use and
Care Committee of our institution. EPCs were generated from the
peripheral blood of 5 adult New Zealand white rabbits weighing
2–2.5 kg. Blood (20 ml) was obtained from the central ear artery of
the rabbits. The fresh blood was heparinized and then diluted with
phosphate-buffered saline (PBS), and the layer of peripheral blood
mononuclear cells was selected with density centrifugation and was
then resuspended in microvascular growth medium-2 (EGM-2 MV;
Cambrex, Walkersville, MD, USA) supplemented with 10% fetal bovine
serum (SAFC Biosciences, St. Louis, MO, USA). Before being planted
in a 25-cm3 culture flask, the cells were gently blown
in the culture solution, adjusted to 1×106/ml of the
concentration, and then grown in standard culture medium at 37°C
with 5% CO2. The growth and morphology of EPCs in the
culture was observed every day with an inverted phase-contrast
microscope (Axioscop; Zeiss Co. Ltd., Oberkochen, Germany).
In order to characterize the EPCs, the expression of
membranous antigen on the cells cultured after 7 days was detected
by cytofluorimetric analysis with a flow cytometer
(Becton-Dickinson, San Jose, CA, USA). The primary anti-human
antibodies (cross-reaction with rabbit), anti-CD31, anti-CD34 and
anti-CD133 (BioLegend, San Diego, CA, USA), were employed.
EPC labeling and identification of the
labeled EPCs
The SPIO (Fe3O4) nanoparticles
were presented by S.P. Q. (Molecular Imaging Platform,
Zhejiang-California International Nanosystems Institute, Zhejiang
University, Hangzhou, China). The EPCs were grown in
25-cm3 flasks. After 21 days, the grown cells were
transferred to the culture medium containing SPIO nanoparticles for
labeling. The concentration of 20 μg/ml iron was used for culture.
The EPCs were incubated continuously for 24 h at 37°C in a 95%
air/5% CO2 incubator.
The EPCs were collected by removing the free SPIO
nanoparticles and washed with PBS 3 times. For the purpose of
Prussian blue staining to identify the EPC profile and
intracellular iron nanoparticles, the cells were continuously
incubated for 20 min with 2% potassium ferrocyanide in 6%
hydrochloric acid, and then counterstained with nuclear fast red
for 3 min.
To detect the iron concentration within the EPCs,
the EPC suspension was dissolved in 37% hydrochloric acid followed
by analysis with a polarized atomic absorption spectrometer
(Shengyang Huaguang HG-9602A, Shengyang, China). The analysis
process was repeated 3 times and the mean value was obtained. The
distribution of the SPIO nanoparticles within the EPCs was shown
under an electron microscope. The harvested labeled EPCs were fixed
at 4°C in 2.5% buffered glutaraldehyde for 1 h, followed by 1%
osmium tetroxide for 2 h. The samples of these EPCs were examined
with a transmission electron microscope (H600; Hitachi, Tokyo,
Japan).
Labeled EPC viability and
proliferation
Cell viability and proliferative activity of
SPIO-labeled and -unlabeled EPCs were evaluated and compared. All
procedures were performed 3 times.
EPC viability was evaluated by trypan blue staining.
The proliferative activity of the EPCs was observed under a light
microscope (Axioscop; Zeiss). Additionally, tetrazolium salt (MTT)
assay was performed to evaluate the toxicity and the effect of SPIO
labeling upon EPC proliferation. The EPCs of passage
(P)1 were grown in 96-well plates at 1×104
cells/well. SPIO solution at a final iron concentration of 20 μg/ml
was added into 40 wells, and the remaining 40 wells to which SPIO
was not added served as the control. The absorbance values of the
unlabeled EPCs and SPIO-labeled EPCs were measured from days 1 to 5
of the culture process (8 wells/day). For the assay, 20 μl of MTT
(5 mg/ml; Fluka Co., St. Gallen, Switzerland) were added into each
well and incubated at 37°C in 5% CO2 for 4 h. Dimethyl
sulfoxide (DMSO; 150 μl; Sigma-Aldrich, St. Louis, MO, USA) was
added and the medium was stirred for 10 min. When the indigo
crystals (formazan crystals) were dissolved evenly in the medium,
the light absorption value of each well was measured with a
spectrophotometer (Model 680; Bio-Rad Laboratories, Inc., Hercules,
CA, USA) using a 490-nm wavelength.
Statistical analyses
Statistical analyses were performed using the
SPSS® statistical package, version 11.0 (SPSS Inc.,
Chicago, IL, USA) for Windows®. Data are presented as
the means ± standard deviation (SD). To compare the differences
between the labeled and unlabeled EPCs in the various experiments,
the Kruskal-Wallis rank sum test was used to calculate the
difference in absorbance of MTT. A p-value (two-tailed) <0.05
denoted a statistically significant difference.
Results
EPC morphology and characterization
In the present study, EPCs were obtained from rabbit
peripheral blood and purified by density centrifugation.
The morphology of the obtained EPCs was similar to
that described in the literature (10). During culture, inverse microscopy
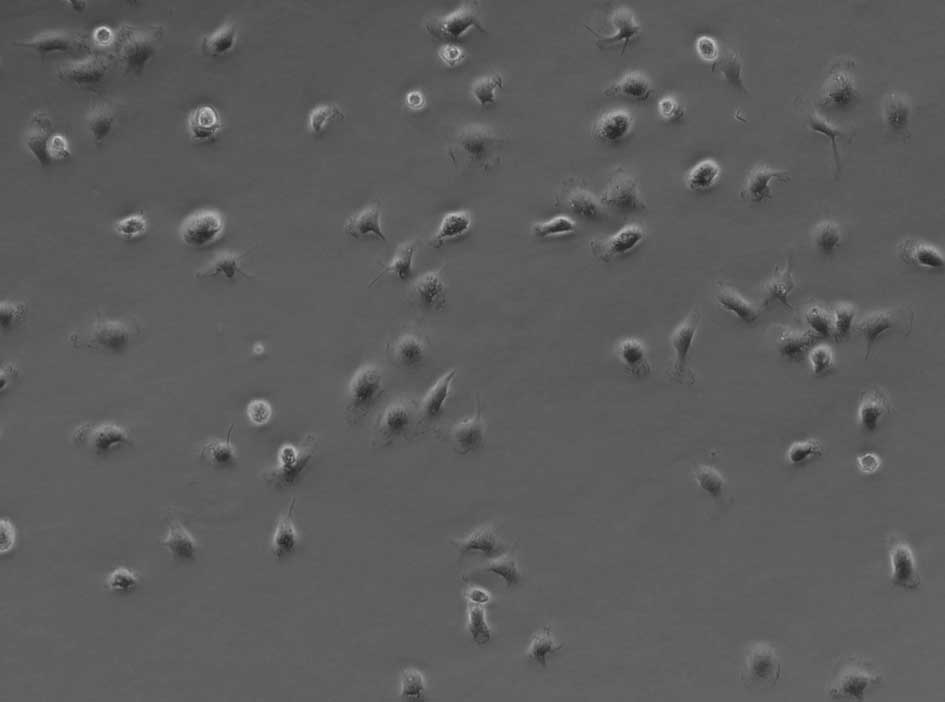
of EPCs showed that isolated EPCs had a round shape with variable
sizes (Fig. 1). After 1 week in
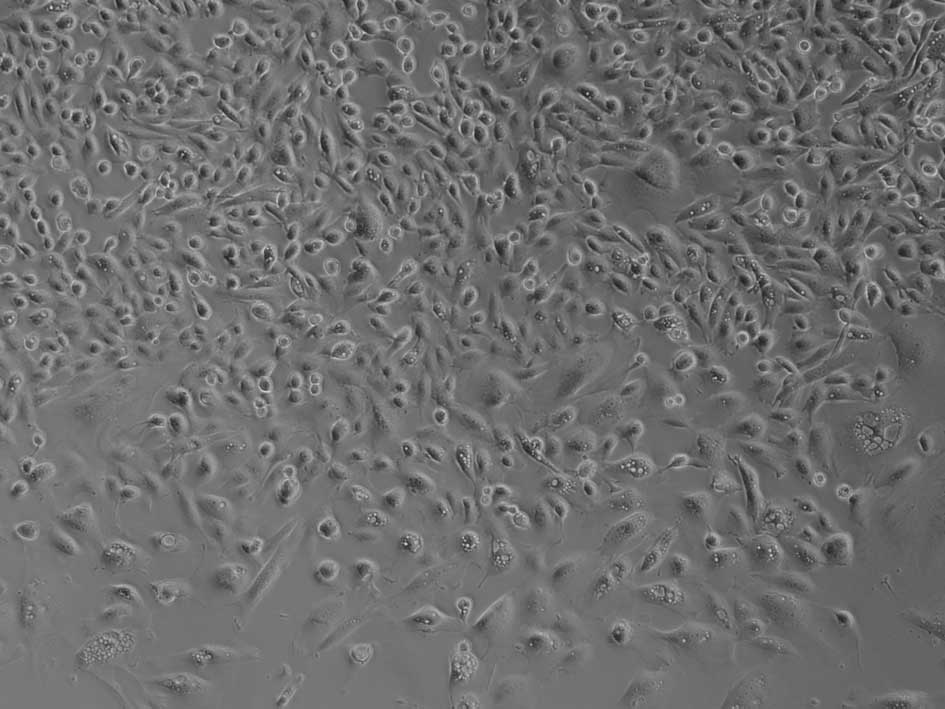
culture, the cells became spindle-shaped, with a centrally located
nucleus, and sometimes formed cluster-like colonies (Fig. 2). The cells grew and divided
rapidly, and tended to touch each other. After 3 weeks, the
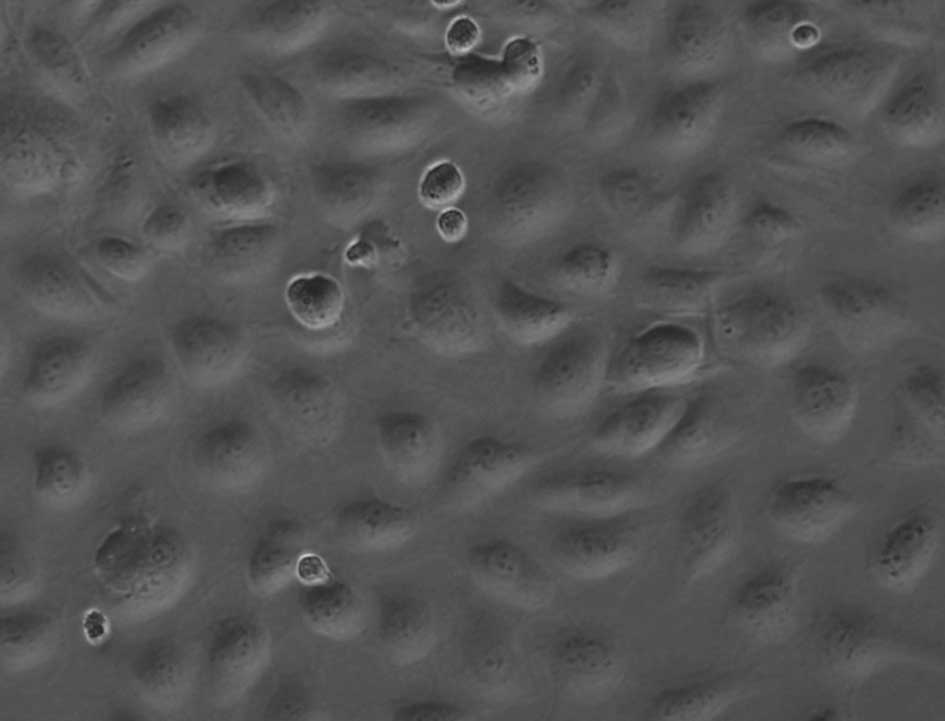
cultured EPCs showed the typical ‘cobblestone’ morphology of
endothelial cells when they grow in colonies (Fig. 3). When they were passed to
P6, the homogeneity of the cells reached approximately
99%.
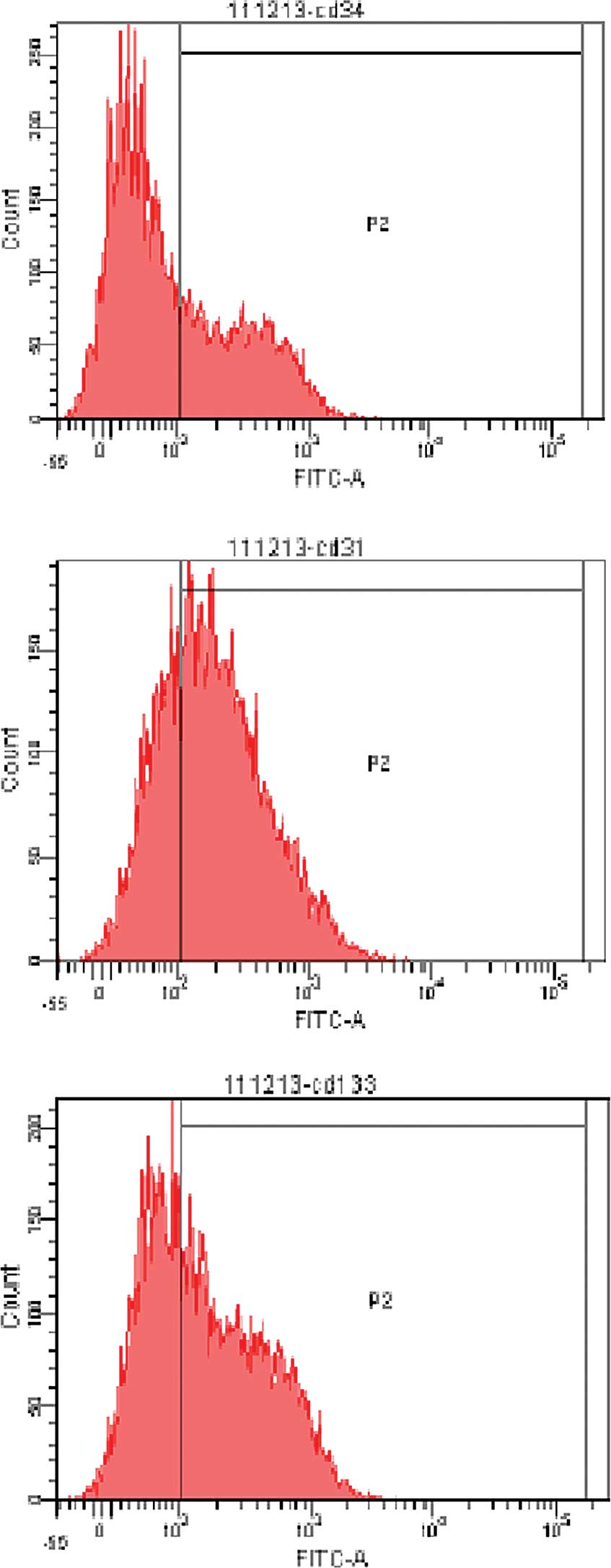
The expression of surface markers, including CD34,
CD31 and CD133, is typically found on EPCs as a demonstration of
their immature character. The flowcytometry analyses revealed that
36.3±1.2, 68.4±2.3 and 56.0±2.5% of the cultured cells were
positive for CD34, CD31 and CD133, respectively, after being
cultured for 7 days (Fig. 4).
EPC labeling and identification of the
labeled EPCs
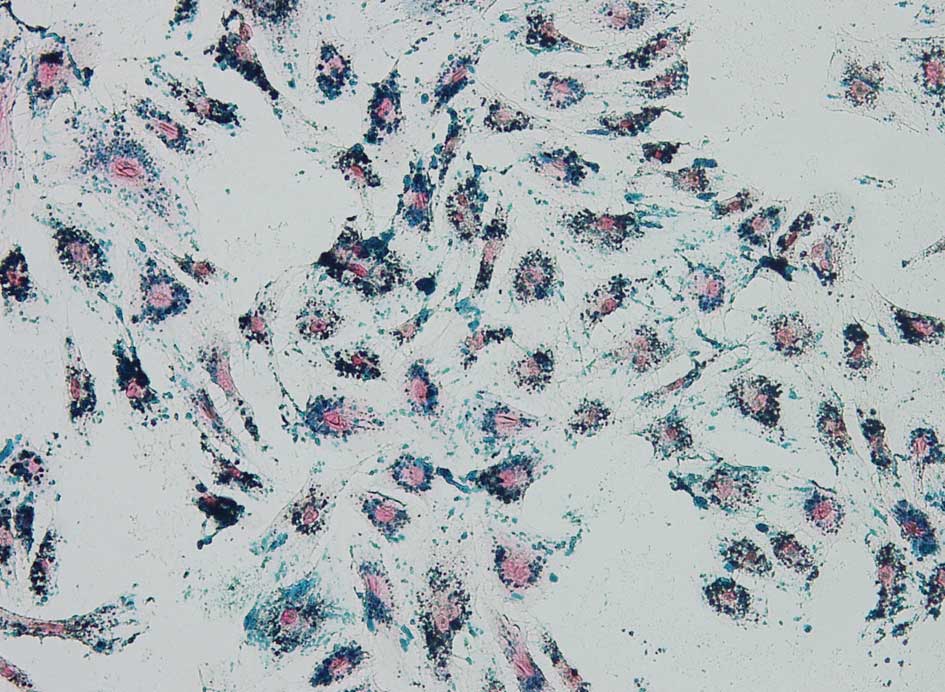
The prevalence of iron content in the labeled EPCs
was revealed by Prussian blue staining and transmission electron
microscopy. Cells were stained with Prussian blue, and blue iron
particles were found within the labeled EPCs (Fig. 5), while no blue iron particles were
found in unlabeled EPCs. Microscopic cell counting post Prussian
blue staining showed that the SPIO labeling rate was >95% among
all the EPCs.
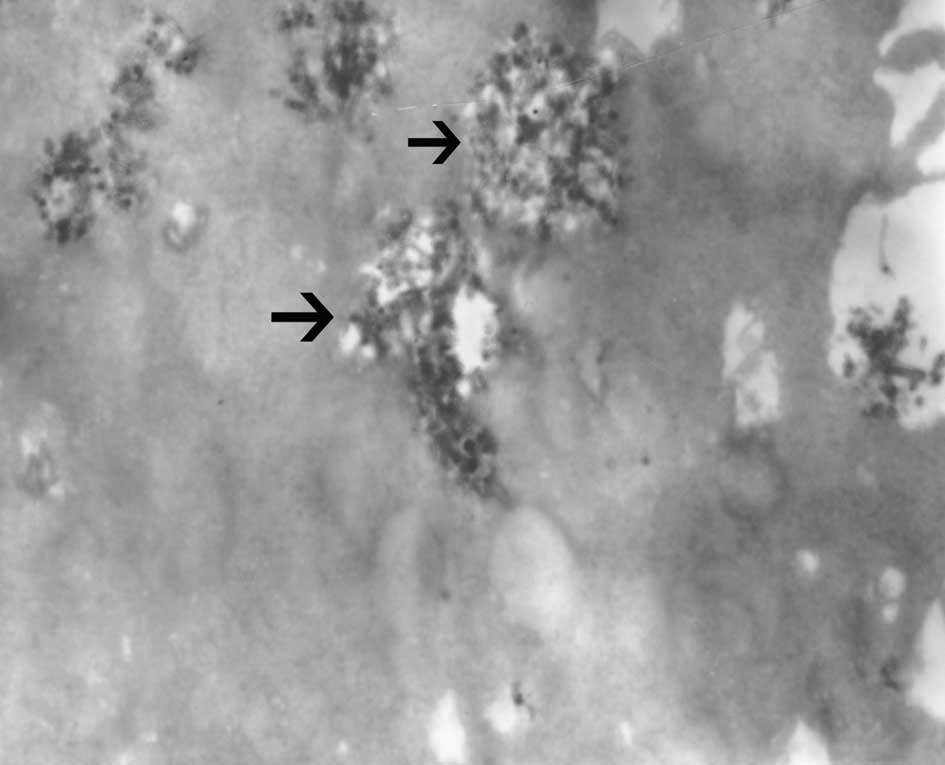
The iron quantification per cell measured by an
atomic absorption spectrometer was 13.6±1.8 pg. Transmission
electron microscopy showed SPIO nanoparticles located in the
endosomal vesicles in the cytoplasm of labeled EPCs (Fig. 6).
Labeled EPC viability and proliferative
capability
Microscopic cell counting after trypan blue
exclusion testing revealed a mean viability of 97.5±2.1% for the
SPIO-labeled EPCs. The mean viability of the unlabeled EPCs was
96.3±2.9%. There were no significant differences in viability
between the labeled and unlabeled EPCs (p>0.05). The
characteristics of the labeled EPCs, including figure, shape and
nucleolus structure, did not differ from those of the unlabeled
EPCs under a light microscope.
From days 1 to 5, the absorbence of the labeled EPCs
was 0.196±0.005, 0.205±0.011, 0.244±0.013, 0.309±0.014 and
0.363±0.022, respectively. The absorbence of the unlabeled EPCs was
0.190±0.007, 0.202±0.010, 0.249±0.015, 0.315±0.016 and 0.377±0.019,
respectively. There were no significant differences in MTT
absorbance values between the labeled and unlabeled EPCs at each
time-point (p>0.05).
Discussion
The transplantation of stem cells is a potential
strategy for the treatment of many types of human diseases due to
its capability of regenerating tissues and organs. Cell
transplantation has the advantages of lower cost and risk compared
to organ transplantation (11,12).
Furthermore, autologous cell transplantation has no risk of
immunological rejection. Among different types of cells, ESCs have
been previously demonstrated to have better characteristics in
terms of applicability for transplantation; studies have indicated
that ESCs are capable of multi-directional differentiation
(13,14). EPCs were first isolated from human
peripheral blood by Asahara et al (15). Over the past decade, the plasticity
of EPCs has been intensively investigated. Many studies have
demonstrated the great therapeutic potential of EPCs in tissue
repair and wound healing (16–18).
There are also less ethical and social controversies associated
with the isolation of EPCs from bone marrow and peripheral blood,
and the use of EPCs isolated from peripheral blood offers several
advantages, such as easy collection and rapid in vivo and
in vitro repopulation. It has been concluded by many studies
that EPCs promote tissue vascular regeneration in vivo and
provide potential treatments for ischemia, wound healing, vascular
insufficiency and tumor inhibition (19). Moreover, EPCs play an essential
role in post-natal neovascularization and maintaining angiogenesis
(18,20). Additionally, certain evidence
suggests that EPCs have a potentially protective role in
endothelial dysfunction in early atherosclerosis formation
(21,22).
For a better understanding of the destiny of
transplanted cells following transplantation, it is essential to
monitor their migration and differentiation. In order to monitor
the transplanted cells, several non-invasive in vivo
tracking imaging techniques, such as MRI, nuclear medicine and
optical imaging, have been investigated (23–25).
The MRI technique holds obvious advantages, as it has a wide
variety of imaging sequences, high resolution and better
soft-tissue contrast without radiation damage. The labeled cells
are easily detected by MRI using a cell labeling technique with
specific agents. Certain evidence implies that SPIO nanoparticles
have strong penetrating capabilities among the MRI tracing agents,
which makes it possible to cause signal change in MRI at a
low-tracer concentration (26). A
number of studies have already revealed the feasibility of in
vivo tracking of the transplanted stem cells by labeling them
with SPIO nanoparticles (27–29).
Studies have also reported that mononuclear cells isolated from
peripheral blood were tracked with MRI using colloidal
superparamagnetic nanoparticles (30,31).
Evidently, the low efficiency of loading these particles into the
cells and the cytotoxicity of these particles limit their usage as
the tracing probe (32). It has
been concluded by a number of studies that the SPIO nanoparticles
have little toxicity and few side-effects for cell biological
characteristics (32,33). However, few studies have
investigated the biological effect of SPIO upon labeled EPCs on a
cellular level. Therefore, it is important to discover an efficient
labeling method without deleterious effects on EPC viability and
proliferative capability.
In the present study, we show that EPCs from
peripheral blood of rabbits can be effectively labeled by
home-synthesized SPIO nanoparticles. Our results demonstrated that
there were no differences in cell viability and proliferative
capability between the SPIO-labeled EPCs and unlabeled EPCs.
Therefore, labeled home-synthesized SPIO nanoparticles have little
influence on the main biological properties of EPCs. Additionally,
these results are important for the application of MRI to localize
and monitor the transplanted magnetically labeled EPCs by in
vivo techniques.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (no. 30901446), the Program for
Innovative Research Team of Science and Technology of Zhejiang
Province (no. 2009R50038), the Foundation for Innovative Research
Groups of the National Natural Science Foundation of China (no.
81121002), the Medical Health Fund of Zhejiang Province (no.
2008A053), and the Program of Chinese Medical Science of Zhejiang
Province (no. 2009CB040).
References
|
1
|
Orlic D, Kajstura J, Chimenti S, et al:
Mobilized bone marrow cells repair the infarcted heart, improving
function and survival. Proc Natl Acad Sci USA. 98:10344–10349.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sato Y, Araki H, Kato J, et al: Human
mesenchymal stem cells xenografted directly to rat liver are
differentiated into human hepatocytes without fusion. Blood.
106:756–763. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Qian H, Yang H, Xu W, et al: Bone marrow
mesenchymal stem cells ameliorate rat acute renal failure by
differentiation into renal tubular epithelial-like cells. Int J Mol
Med. 22:325–332. 2008.PubMed/NCBI
|
|
4
|
Murasawa S and Asahara T: Endothelial
progenitor cells for vasculogenesis. Physiology. 20:36–42. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schmidt-Lucke C, Rössig L, Fichtlscherer
S, et al: Reduced number of circulating endothelial progenitor
cells predicts future cardiovascular events: proof of concept for
the clinical importance of endogenous vascular repair. Circulation.
111:2981–2987. 2005. View Article : Google Scholar
|
|
6
|
Liang PH, Tian F, Lu Y, et al: Vascular
endothelial growth inhibitor (VEGI; TNFSF15) inhibits bone
marrow-derived endothelial progenitor cell incorporation into Lewis
lung carcinoma tumors. Angiogenesis. 14:61–68. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
George AL, Bangalore-Prakash P, Rajoria S,
et al: Endothelial progenitor cell biology in disease and tissue
regeneration. J Hematol Oncol. 24:242011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gazeau F and Wilhelm C: Magnetic labeling,
imaging and manipulation of endothelial progenitor cells using iron
oxide nanoparticles. Future Med Chem. 2:397–408. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang JX, Tang WL and Wang XX:
Superparamagnetic iron oxide nanoparticles may affect endothelial
progenitor cell migration ability and adhesion capacity.
Cytotherapy. 12:251–259. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wu H, Riha GM, Yang H, et al:
Differentiation and proliferation of endothelial progenitor cells
from canine peripheral blood mononuclear cells. J Surg Res.
126:193–198. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Stutchfield BM, Forbes SJ and Wigmore SJ:
Prospects for stem cell transplantation in the treatment of hepatic
disease. Liver Transpl. 16:827–836. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shabbir A, Zisa D, Suzuki G, et al: Heart
failure therapy mediated by the trophic activities of bone marrow
mesenchymal stem cells: a noninvasive therapeutic regimen. Am J
Physiol Heart Circ Physiol. 296:H1888–H1897. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zeng L, Hu Q, Wang X, et al: Bioenergetic
and functional consequences of bone marrow-derived multipotent
progenitor cell transplantation in hearts with postinfarction left
ventricular remodeling. Circulation. 115:1866–1875. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jickling G, Salam A, Mohammad A, et al:
Circulating endothelial progenitor cells and age-related white
matter changes. Stroke. 40:3191–3196. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Asahara T, Murohara J, Sullivan A, et al:
Isolation of putative progenitor endothelial cells for
angiogenesis. Science. 275:964–967. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ding DC, Shyu WC, Lin SZ, et al: The role
of endothelial progenitor cells in ischemic cerebral and heart
diseases. Cell Transplant. 16:273–284. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rotmans JI, Heyligers JM, Stroes ES, et
al: Endothelial progenitor cell-seeded grafts: rash and risky. Can
J Cardiol. 22:1117–1119. 2006. View Article : Google Scholar
|
|
18
|
Werner N, Kosiol S, Schieql T, et al:
Circulating endothelial progenitor cells and cardiovascular
outcomes. N Engl J Med. 353:999–1007. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Moore MAS: Putting the neo into
neoangiogenesis. J Clin Invest. 109:313–315. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mihail H, Wolfgang E and Peter CW:
Endothelial progenitor cells: mobilization, differentiation, and
homing. Arterioscler Thromb Vasc Biol. 23:1185–1189. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Roberts N, Jahangiri M and Xu Q:
Progenitor cells in vascular disease. J Cell Mol Med. 9:583–591.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Werner N and Nickenig G: Clinical and
therapeutical implications of EPC biology in atherosclerosis. J
Cell Mol Med. 10:318–332. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Modo M, Cash D, Mellodew K, et al:
Tracking transplanted stem cell migration using bifunctional,
contrast agent-enhanced, magnetic resonance imaging. Neuro Image.
17:803–811. 2002.PubMed/NCBI
|
|
24
|
Hung SC, Deng WP, Yang WK, et al:
Mesenchymal stem cell targeting of microscopic tumors and tumor
stroma development monitored by noninvasive in vivo positron
emission tomography imaging. Clin Cancer Res. 11:7749–7756. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shichinohe H, Kuroda S, Lee JB, et al: In
vivo tracking of bone marrow stromal cells transplanted into mice
cerebral infarct by fluorescence optical imaging. Brain Res Brain
Res Protoc. 13:166–175. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Weissleder R: Molecular imaging: exploring
the next frontier. Radiology. 212:609–614. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Daldrup-Link HE, Rudelius M, Oostendorp
RA, et al: targeting of hematopoietic progenitor cells with MR
contrast agents. Radiology. 228:760–767. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Togel F, Hu Z, Weiss K, et al:
Administered mesenchymal stem cells protect against ischemic acute
renal failure through differentiation-independent mechanisms. Am J
Physiol Renal Physiol. 289:F31–F42. 2005. View Article : Google Scholar
|
|
29
|
Yocum GT, Wilson LB, Ashari P, Jordan EK,
Frank JA and Arbab AS: Effect of human stem cells labeled with
ferumoxides-poly-L-lysine on hematologic and biochemical
measurements in rats. Radiology. 235:547–552. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jendelová P, Herynek V, Urdziková L, et
al: Magnetic resonance tracking of human CD34+
progenitor cells separated by means of immunomagnetic selection and
transplanted into injured rat brain. Cell Transplant. 14:173–182.
2005.
|
|
31
|
Weber A, Pedrosa I, Kawamoto A, et al:
Magnetic resonance mapping of transplanted endothelial progenitor
cells for therapeutic neovascularization in ischemic heart disease.
Eur J Cardiothorac Surg. 26:137–143. 2004. View Article : Google Scholar
|
|
32
|
Crabbe A, Vandeputte C, Dresselaers T, et
al: Effects of MRI contrast agents on the stem cell phenotype. Cell
Transplant. 19:919–936. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Suh JS, Lee JY, Choi YS, et al: Efficient
labeling of mesenchymal stem cells using cell permeable magnetic
nanoparticles. Biochem Biophys Res Commun. 379:669–675. 2009.
View Article : Google Scholar : PubMed/NCBI
|