Introduction
Methamphetamine (METH) possesses a high potential
for abuse and addiction and has become a serious social problem
worldwide (1,2). In Japan, the number of individuals
arrested per year for METH abuse has been recorded as >10,000
for the last several decades (3).
The cardiotoxic effect of METH is particularly dangerous as the
drug accelerates the heart rate and elevates the blood pressure,
which may cause cardiovascular collapse, resulting in not only
acute METH poisoning, but also METH-induced sudden mortality
(4,5). An increasing number of clinical and
autopsy studies associate the use of METH with angina, tachycardia,
hypertension, myocarditis, dilated cardiomyopathy, arrhythmia and
sudden death (5,6). However, stressors also increase the
heart rate and catecholamine release and affect the
hypothalamic-pituitary-adrenal axis (7,8).
Abnormal stress-induced activation of the sympathetic nervous
system exacerbates heart failure (9). Even mental stressors in daily life
are able to more than double the risk of subsequent myocardial
ischemia (10). In addition,
stressors increase drug-seeking or -taking behavior. Exposing
laboratory animals to stress conditions increases the
self-administration of psychostimulants, including amphetamine and
cocaine (11,12). In humans, clinical and laboratory
studies have also indicated that stressors increase drug use
(13–15). Thus, stress-drug interactions would
increase the likelihood of consuming more drugs and play a
significant role in the induction and development of cardiovascular
diseases. Moreover, a recent study on humans has shown that
stressors are able to alter subjective responses, including the
heart rate, to a known drug of abuse (16). Söderpalm et al(17) demonstrated that acute stressors
dampened the subjective responses to a low dose of METH in healthy
volunteers, but that these effects were short-lived. Stratton et
al studied cases of excited delirium leading to sudden
mortality subsequent to a struggle and physical restraint and
suggested the possibility that restraint stress exacerbated the
cardiac damage caused by the stimulant drugs (18). A study by Uemura et al
reported cases of sudden death during restraint that showed cardiac
abnormalities (19). Although the
causal mechanisms remain unknown, subjective responses to an
illicit drug may be further sensitized under acute or chronic
stress conditions.
Abuse of METH ranges from episodes of binge abuse to
chronic abuse over several years. We previously examined the acute
effect of METH on myocardial tissues and showed that METH-stress
interactions affected the induction of heat shock proteins (Hsps),
followed by an increased susceptibility of the hosts to
cardiotoxicity due to the stimulant drug (20). In the present study, the effects of
METH, including the METH-stress interactions, were investigated in
the myocardium and the results of acute and chronic treatments were
compared.
Materials and methods
Chemicals
The methamphetamine (METH) was purchased from
Dainippon Sumitomo Pharma Co., Ltd. (Osaka, Japan). The drug was
dissolved in 0.9% saline immediately prior to use. The chemicals
and other solutions used were all of analytical grade.
Animals
Male C57BL/6J mice (8–9 weeks old) were obtained
from CLEA Japan, Inc. (Tokyo, Japan). In total, 4–5 mice were
housed in each polycarbonate cage and maintained in a controlled
environment at 23±1°C, with a 12-h light/dark cycle. The mice had
free access to commercial rodent mouse feed (MF) pellets (Oriental
Yeast, Tokyo, Japan) and tap water. All the experiments were
approved by the Animal Research Committee (No. 11-030) of the
Kawasaki Medical School, Japan.
Experimental protocol
The animals were divided into the control (C), METH
(M), stress (S) and METH plus stress (MS) groups. The animals in
the S and MS groups were exposed to water-immersion restraint
stress for 6 h (the acute study) or to varied stressors for 4 weeks
(the chronic study). The chronic stress program was as follows:
Monday, a temperature change from 4 to 25°C/1 h for 6 h; Tuesday,
electric foot shock (0.4–0.8 mA for 5 sec at 30 sec intervals for
30 min) followed by a temperature change for 4 h; Wednesday,
temperature change under restraint stress for 6 h; Thursday,
temperature change for 6 h; and Friday, water-immersion restraint
stress for 3 h. Just prior to the stress exposure, the METH was
injected intraperitoneally (i.p.) at a dose of 30 mg/kg for the
acute study or 10 mg/kg 3 times per week (Monday, Wednesday and
Friday) for the chronic study. The animals in the C and M groups
received saline or METH in the same manner. The mice were
sacrificed by cervical dislocation at the end of the treatment and
blood was drawn directly from the heart. The serum obtained was
stored at −80°C until analysis. The hearts were removed for
biochemical estimations.
Histological analysis
Blocks of ventricular tissue were fixed in 10%
neutral-buffered formalin immediately subsequent to removal,
processed using routine histology methods, paraffin-embedded,
sliced into 5-μm sections and stained with Azan Mallory stain. An
independent observer who was blinded to the treatment examined the
sections.
Assays of serum interleukin-6 and
corticosterone
The interleukin-6 (IL-6) and corticosterone levels
were determined using commercial ELISA (Invitrogen, Carlsbad, CA,
USA) and EIA kits (Yanaihara, Shizuoka, Japan), respectively,
according to the manufacturers’ instructions.
Quantitative analysis of the mRNA
The total RNAs were isolated from the ventricular
tissues stabilized with RNA using an RNeasy mini kit (Qiagen,
Hilden, Germany) according to the manufacturer’s instructions. The
residual DNA was removed by DNase digestion. The concentration and
purity of the total RNA that was obtained were determined by
absorbance at 260/280 nm using a UV spectrophotometer (Beckman
DU640). Reverse transcription was performed using 1 μg total RNA
and an Advantage RT-for-PCR kit (Clontech, Palo Alto, CA, USA) with
an oligo (dT)18 primer.
Real-time PCR was conducted on the cDNAs from each
individual animal using a SYBR-Green QPCR Master Mix (Stratagene,
Agilent Technologies, Santa Clara, CA, USA) and synthetic
gene-specific primer sets that were designed based on the sequences
deposited in the NCBI GenBank database. β-actin was used as an
endogenous control. A standard curve was employed to quantify the
results of the real-time PCR.
Statistical analysis
Statistical analyses were carried out using JMP
software (version 8.0). The results were expressed as the mean ± SD
and analyzed using a one-way analysis of variance, followed by
Tukey’s HSD test for multiple comparisons. P<0.05 or P<0.01
were considered to indicate statistically significant
differences.
Results
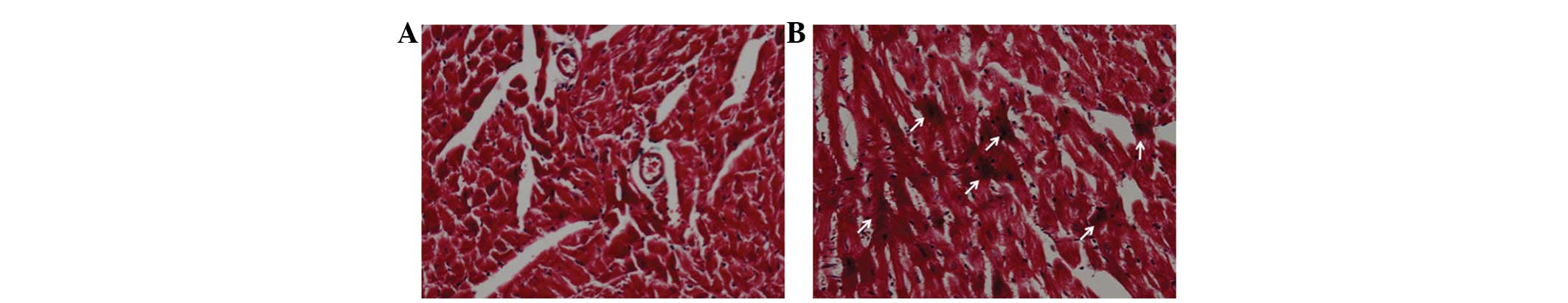
Histological examination of chronically
treated cardiac muscle
Although there was no increase in the collagenous
fiber levels, hypercontraction, severe coagulation necrosis and
ischemia were observed in the mice of the chronically treated MS
group. By contrast, the damage was restricted to cytoplasmic
eosinophilia, early ischemia and limited hypercontraction in the
mice of the M and S groups. Fig. 1
shows the hypercontraction of the tissues of a chronically treated
mouse. These results were in agreement with those obtained in the
acute study (20), although the
severity was increased by repeated treatment.
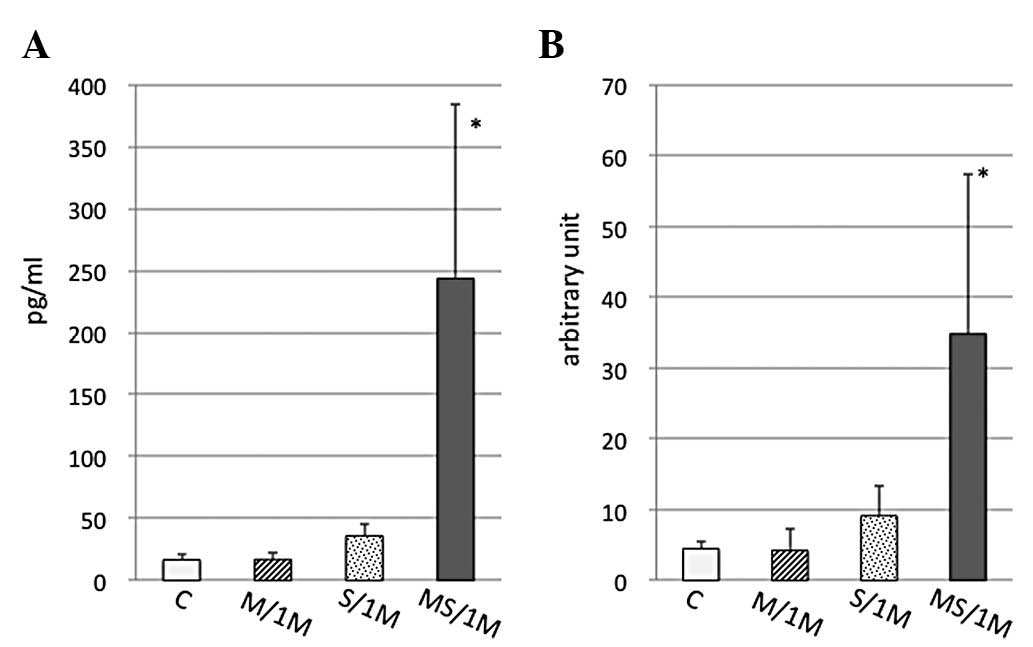
IL-6 and corticosterone levels of
chronically treated mice
IL-6 levels in the serum of the chronically treated
mice were then determined. The IL-6 levels were significantly
increased under MS conditions, but not in the M or S group
(Fig. 2A). This result was the
same as the change observed in the mice subjected to the acute
treatment; the IL-6 level in the MS group was higher than that in
the M and S groups (20).
Moreover, in the chronic study, a significant increase was obtained
in the IL-6 RNA expression in the myocardial tissues of the MS
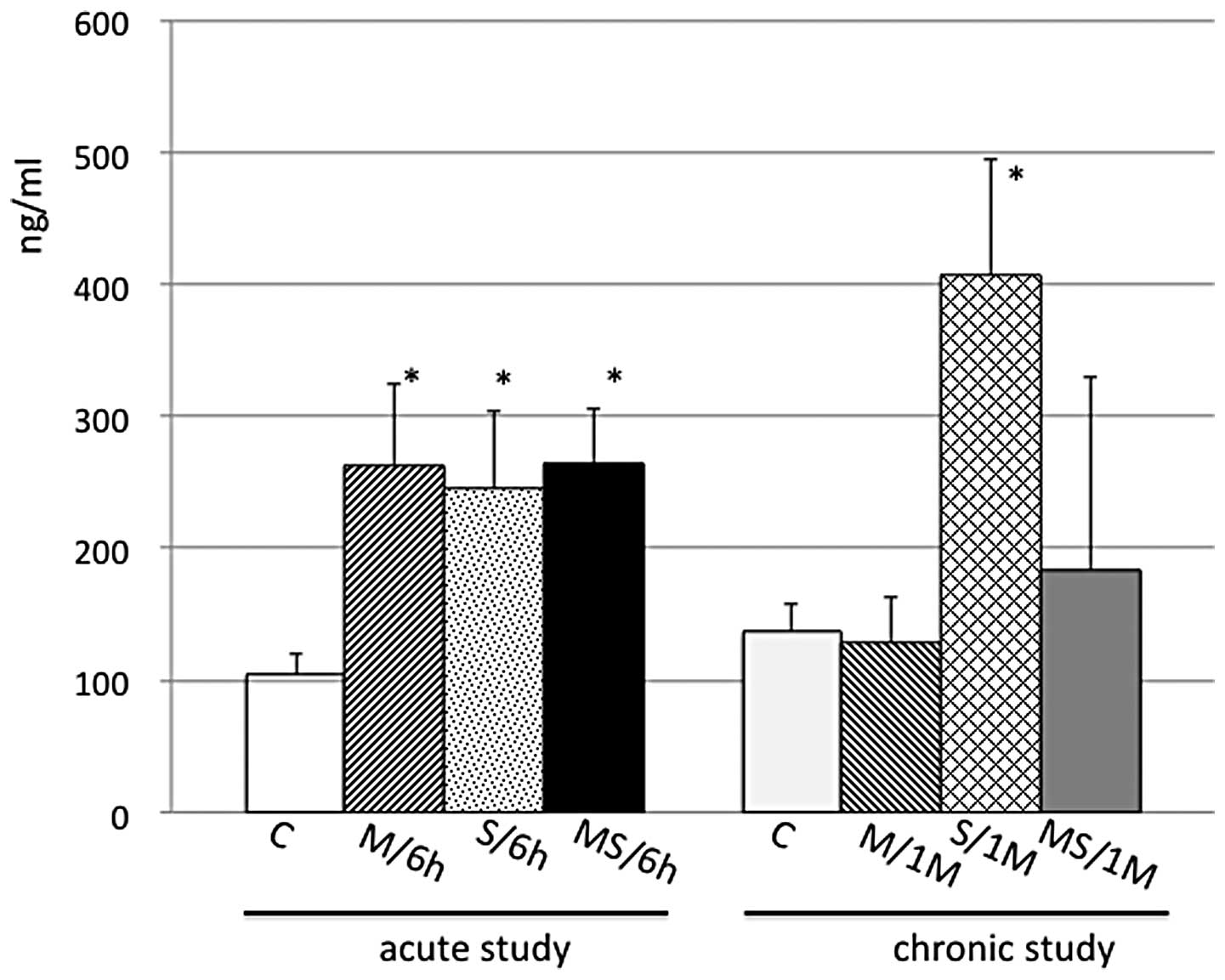
group, but not in the M or S groups (Fig. 2B). However, the serum
corticosterone levels differed in the acute and chronic treatments.
The levels in the acute study were increased equally by M, S or MS
conditions, whereas the levels in the chronic study were increased
only by exposure to stressors, but not by the repeated injection of
METH in the M and MS group (Fig.
3).
RNA expression
Table I summarizes
the RNA expression at 3 h subsequent to the injection of 30 mg/kg
METH (the acute study) and the expression subsequent to one-month
of treatment with 10 mg/kg METH (the chronic study), with or
without the stress conditions. As described previously (20), the increase in the expression of
the heat shock proteins (Hsps), particularly Hsp70, in the M group
was marked in the acute study, while the stimulation was lower
during the one-month treatment. The RNA expression of the Hsps,
including Hsp70, in the M group showed a tendency to increase
subsequent to the one-month treatment, but the upregulation was not
comparable to the increase due to the acute treatment. The RNA
expression for methallothionein (MT) also differed in the acute and
chronic treatments. The expression increased markedly in the M
group of the acute study, whereas in the chronic study, the
increase was more marked in the S and MS groups. The RNA expression
of the other anti-oxidant enzymes, including superoxide dismutase 2
(Sod2), catalase, glutathione peroxidase 1 (Gpx1) and glutathione
S-transferase (GST), was examined. The expression of these enzymes
was significantly increased in the M, S and/or MS groups by the
chronic treatment, but not by the acute treatment.
 | Table IComparison of RNA expression. |
Table I
Comparison of RNA expression.
| One dose of METH (30
mg/kg) | One-month treatment
with METH (10 mg/kg) |
|---|
|
|
|
|---|
| Gene | M | S | MS | M | S | MS |
|---|
| Hsp60 | 181.48±56.36a | 93.23±11.18 | 90.89±11.94 | 131.58±19.15 | 85.75±13.45 | 94.01±11.81 |
| Hsp70 |
2422.86±996.80a | 189.35±164.71 | 191.1±96.42 | 254.42±132.96b | 111.02±8.25 | 127.21±45.72 |
| Hsp90 | 402.78±332.74a | 129.51±26.56 | 101.54±19.84 | 138.08±35.05 | 99.48±16.48 | 96.46±7.15 |
| MT1 | 269.94±92.49a | 130.57±28.55 | 122.55±41.11 | 125.73±31.85 | 159.84±32.98 | 186.6±66.16b |
| MT2 | 668.12±290.67a |
407.16±146.6b | 302.3±127.02 | 115.39±44.86 |
312.85±61.88b |
427.21±212.13a |
| Prdx1 | 98.21±30.03 | 81.69±32.39 | 68.43±20.2 | 121.7±32.11 | 75.98±14.24 | 96.14±21.4 |
| Gcs | 123.3±29.48 | 101.32±28.41 | 87.26±18.59 | 127.06±24.11 | 105.88±16.64 | 102.94±17.85 |
| Sod2 | 108.6±23.66 | 98.3±14.94 | 110.38±40.92 |
167.85±20.88a | 127.98±22.84 |
150.58±12.76a |
| Catalase | 121.27±29.99 | 91.93±32.62 | 87.88±21.58 |
136.41±11.37a | 94.37±12.81 | 114.92±11.79 |
| Gpx1 | 96.89±27.25 | 127.56±40.2 | 94.59±44.62 | 115.41±10.97 | 104.92±19.12 |
139.34±17.25a |
| GST | 86.51±35.11 | 102.26±40.07 | 95.37±38 |
298.75±47.06a |
240.00±50.12a |
323.44±74.19a |
| iNOS | 48.92±19.25a | 54.53±8.04a | 61.13±8.31a | 98.36±18.08 | 57.4±18.02a | 50.45±12.55a |
| ACE | 104.22±26.11 | 59.85±23.42b | 92.56±46.19 | 150.52±16.23 | 131.83±23.57 |
172.49±46.38b |
| PGI2 synthase | 119.89±23.79 | 92.06±14.68 | 101.28±28.59 | 170.4±37.37 | 194.4±42.76b | 201±43.13b |
| IP | 96.65±34.42 | 51.43±10.02a | 61.49±24.24b |
176.97±17.88a | 90.91±22.68 | 112.12±3.5 |
| Bcl2 | 76.29±24.99 | 73.58±23.46 | 72.25±18.85 | 140±25.56b | 108.57±23.9 | 75±7.14 |
| Pgk1 | 97.03±21.6 | 86.1±12.24 | 80.27±21.42 |
183.83±24.52a | 137.87±25.1 | 129.79±22.38 |
| PLTP | 78.07±26.5 | 65.87±13.3b | 73.95±18.81 | 117.5±6.85 | 100±15.31 |
140.63±18.75a |
The RNA expression for inducible nitric oxide
synthase (iNOS) decreased in the M, S and MS groups of the acute
study, while, for the one-month treatment, a significant decrease
was observed in the S and MS groups, but not in the M group. The
level in the M group was the same as the control level. Moreover,
the angiotensin-converting enzyme (ACE) RNA expression increased
subsequent to the chronic treatment and showed a significant
increase under the METH plus stress conditions.
The RNA expression for prostacyclin (PGI2) synthase
increased in the chronic treatment group, but not in the acute
study group. The RNA expression for the prostacyclin receptor, IP,
however, showed a significant increase only in the M group
subjected to chronic treatment. Moreover, a significant increase
was obtained in the RNA expression of the anti-apoptosis factor,
B-cell lymphoma-2 (Bcl2), and the ATP-generating enzyme of
glycolysis, phosphoglycerate kinase 1 (Pgk1), but only in the M
group treated for one month. In addition, the RNA expression of the
plasma phospholipid transfer protein (PLTP), which is related to
the development of atherosclerosis, significantly increased in the
MS group following the chronic treatment.
Discussion
In a previous study focusing on Hsps, acute stress
depressed the induction of the Hsps due to the METH and was
followed by enhanced METH-induced myocardial damage (20). In the present study, M, S or
MS-induced cardiotoxicity were examined and compared subsequent to
acute and chronic treatments. The histological results from the
myocardium subsequent to the chronic treatment showed more severe
damage in the mice of the MS group than the M or S group. In
addition, the serum IL-6 level was markedly increased in the MS
group (Fig. 2A). Elevated serum
IL-6 levels suggest that IL-6 may play a significant role in the
pathogenesis of heart disease, as described in a previous study
(21). Although IL-6 is secreted
by various types of cells (22,23),
an increase in the RNA expression in the myocardial tissues of the
present study (Fig. 2B) indicated
that an increase in the IL-6 in the heart may in part account for
the elevated serum levels. The histological findings and the
increase in IL-6 observed in the chronically treated MS group
indicated that more severe injuries may occur in the myocardial
tissues under MS conditions. These results obtained from the
chronic study were almost the same as those obtained from the acute
experiment (20). By contrast, the
corticosterone levels subsequent to the one-month treatment
differed from those of the acute study. The levels in the M, S and
MS groups were equally increased in the acute study, whereas the
levels in the chronic study were increased only by exposure to
stressors and not by repeated injections of METH (Fig. 3). Thus, METH may have had some
effect on the corticosterone release during the one-month
treatment.
The expression of numerous genes is supposed to be
increased or decreased in the cardiac myocytes as a result of METH
injections administered with or without stress, but the dynamic
phase of these genes has not been elucidated. The effect on the
Hsps was prominent in the acute treatment in the present study.
Upregulation, particularly of Hsp70, was markedly elicited by METH
in the acute study, whereas its expression was limited to only a
mild tendency to increase in the chronic study. MT also showed
differing effects in the two regimens; stimulation in the M group
following the acute treatment, but in the S and MS groups following
the chronic treatment. MT exists in the majority of organs,
including animal and human hearts, and is inducible to a high level
by various oxidative or pathogenic stresses (24). The induction of cardiac MT by
various agents was previously shown to significantly prevent
oxidative damage in hearts (25).
Other investigators have suggested that oxidation of the myocardial
proteins contributes to the heart’s dysfunction (26). In the present study, anti-oxidant
enzymes, including Sod2, catalase, Gpx1 and GST, were significantly
increased by the chronic, but not by the acute treatment. These
findings suggested that oxidative stress may play a significant
role in the cardiac damage caused by the chronic treatment.
One NOS isoform, iNOS, is expressed in a wide
variety of cell types, including cardiac myocytes and cardiac
endothelial cells, in response to certain stimuli, including
hypoxia (27). The inhibition of
iNOS raises the peroxidative and apoptotic level in the hypoxic
heart, indicating that this isoform may protect the organ from
hypoxia/reoxygenation injuries (28). In the present study, only the
chronically treated M group retained the basal iNOS level, while
the expression levels in the remaining groups were downregulated.
ACE has angiotensin II-dependent and -independent effects on
cardiovascular function and is a logical target for the regulation
of the renin-angiotensin system. Specifically, ACE inhibition
reduces blood pressure, left ventricular hypertrophy and cardiac
inflammation in spontaneously hypertensive rats (29). Clinical studies have shown that
various ACE inhibitors are effective in the treatment of congestive
heart failure, acute myocardial infarction, coronary artery disease
and hypertension (30). As shown
in Table I, the present study
suggested that the upregulation of ACE in the chronic treatment
caused serious cardiac damage, particularly when under METH plus
stress conditions. Prostacyclin also has vasodilatory and
anti-thrombotic properties, showing multiple cardiovascular
protective actions by the activation of its G protein-coupled
receptor, IP (31). Although the
RNA expression for PGI2 synthase was increased by the chronic
treatment and not by the acute treatment, the expression for its
receptor, IP, was significantly increased only in the chronically
treated M group. This suggested that the PGI2/IP pathway was
effective only in the mice of the M group that were treated for one
month.
Injections of METH for one month increased the RNA
expression of Bcl2, a well-known anti-apoptosis factor. The
upregulation of Bcl-2 significantly inhibited the extent of the
apoptosis of the cardiomyocytes induced by ischemia/reperfusion
(32), suggesting that Bcl-2 was
able to protect the cardiomyocytes. In addition, a significant
increase was observed in the Pgk1 expression in the M group
subjected to the chronic treatment. This result supports an
adaptive response to hypoxia that underlies the cellular and
systemic oxygen homeostasis in the mice of this group (33). By contrast, PLTP is a significant
modulator of the phospholipid transfer and exchange among the
proteins and also plays a role in inflammation and oxidative stress
(34). The PLTP activity is likely
a novel marker for the systolic dysfunction of the left ventricle
in patients with known or suspected coronary artery disease
(35). Upregulation of the PLTP
expression was observed in the chronically treated MS group,
suggesting that the mice in this group were at risk of developing
atherosclerosis. Taken together, these RNA expression results
indicated that METH intake under stress conditions enhanced
cardiotoxicity in short- and long-term abuse.
In conclusion, METH induced more deleterious effects
in the myocardial tissues of the acute and chronic studies when
subjected to stress conditions, even when the level of stress
hormone was lowered to the basal level. The Hsps play a critical
role in the acute phase, while a number of genes, including
anti-oxidant, anti-apoptotic and physiological functional genes,
are involved in the chronic phase. Stressors increase drug cravings
in humans and METH-induced myocardial toxicity would be a severely
deleterious event.
Acknowledgements
The authors would like to thank Mr. N. Iwachidou and
Ms. E. Ohtsuki for their excellent technical assistance in
preparing the tissue sections. This study was supported by
Grants-in-Aid for Scientific Research (KAKENHI; No. 22590644).
References
|
1
|
Brecht ML, von Mayrhauser C and Anglin MD:
Predictors of relapse after treatment for methamphetamine use. J
Psychoactive Drugs. 32:211–220. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Comer SD, Hart CL, Ward AS, Haney M,
Foltin RW and Fischman MW: Effects of repeated oral methamphetamine
administration in humans. Psychopharmacology (Berl). 155:397–404.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wada K: The history and current state of
drug abuse in Japan. Ann NY Acad Sci. 1216:62–72. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Saito T, Takeichi S, Nakajima Y, Yukawa N
and Osawa M: Fatal methamphetamine poisoning in police custody. J
Clin Forensic Med. 3:183–185. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Inoue H, Ikeda N, Kudo K, Ishida T, Terada
M and Matoba R: Methamphetamine-related sudden death with a
concentration which was of a ‘toxic level’. Leg Med (Tokyo).
8:150–155. 2006.
|
|
6
|
Wijetunga M, Seto T, Lindsay J and Schatz
I: Crystal methamphetamine-associated cardiomyopathy: tip of the
iceberg? J Toxicol Clin Toxicol. 41:981–986. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tsigos C and Chrousos GP:
Hypothalamic-pituitary-adrenal axis, neuroendocrine factors and
stress. J Psychosom Res. 53:865–871. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Miller GE, Chen E and Zhou ES: If it goes
up, must it come down? Chronic stress and the
hypothalamic-pituitary-adrenocortical axis in humans. Psychol Bull.
133:25–45. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kishi T: Heart failure as an autonomic
nervous system dysfunction. J Cardiology. 59:117–122. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pickering TG: Does psychological stress
contribute to the development of hypertension and coronary heart
disease? Eur J Clin Pharmacol. 39(Suppl 1): S1–S7. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tidey JW and Miczek KA: Acquisition of
cocaine self-administration after social stress: role of accumbens
dopamine. Psychopharmacology (Berl). 130:203–212. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Piazza PV and Le Moal M: The role of
stress in drug self-administration. Trends Pharmacol Sci. 19:67–74.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Perkins KA and Grobe JE: Increased desire
to smoke during acute stress. Br J Addict. 87:1037–1040. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sinha R: How does stress increase risk of
drug abuse and relapse? Psychopharmacology (Berl). 158:343–359.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sinha R, Catapano D and O’Malley S:
Stress-induced craving and stress response in cocaine dependent
individuals. Psycho- pharmacology (Berl). 142:343–351. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhao LY, Shi J, Zhang XL and Lu L:
Psychosocial stress enhances non-drug-related positive memory
retrieval in male abstinent heroin addicts. Neurosci Lett.
485:16–20. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Söderpalm AH, Nikolayev L and de Wit H:
Effects of stress on responses to methamphetamine in humans.
Psychopharmacology (Berl). 170:188–199. 2003.PubMed/NCBI
|
|
18
|
Stratton SJ, Rogers C, Brickett K and
Gruzinski G: Factors associated with sudden death of individuals
requiring restraint for excited delirium. Am J Emerg Med.
19:187–191. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Uemura K, Ueyama T, Shintani-Ishida K,
Unuma K and Yoshida K: An autopsy report on four sudden cardiac
death cases by immobilization. Int Med J. 15:301–305. 2008.
|
|
20
|
Tomita M, Katsuyama H, Watanabe Y, et al:
Water-restraint stress enhances methamphetamine-induced
cardiotoxicity. Chem Biol Interact. 190:54–61. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ikeda U, Ohkawa F, Seino Y, et al: Serum
interleukin 6 levels become elevated in acute myocardial
infarction. J Mol Cell Cardiol. 24:579–84. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jirik FR, Podor TJ, Hirano T, et al:
Bacterial lipopolysaccharide and inflammatory mediators augment
IL-6 secretion by human endothelial cells. J Immunol. 142:144–147.
1989.PubMed/NCBI
|
|
23
|
Yamauchi-Takihara K, Ihara Y, Ogata A,
Yoshizaki K, Azuma J and Kishimoto T: Hypoxic stress induces
cardiac myocyte-derived interleukin-6. Circulation. 91:1520–1524.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kang YJ: The antioxidant function of
metallothionein in the heart. Proc Soc Exp Biol Med. 222:263–273.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nath R, Kumar D, Li T and Singal PK:
Metallothioneins, oxidative stress and the cardiovascular system.
Toxicology. 155:17–26. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Canton M, Menazza S, Sheeran FL, Polverino
de Laureto P, Di Lisa F and Pepe SP: Oxidation of myofibrillar
proteins in human heart failure. J Am Coll Cardiol. 57:300–309.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jung F, Palmer LA, Zhou N and Johns RA:
Hypoxic regulation of inducible nitric oxide synthase via hypoxia
inducible factor-1 in cardiac myocytes. Circ Res. 86:319–325. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rus A, del Moral ML, Molina F and Peinado
MA: Does inducible NOS have a protective role against
hypoxia/reoxygenation injury in rat heart ? Cardiovasc Pathol.
20:e17–e25. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Miguel-Carrasco JL, Zambrano S, Blanca AJ,
et al: Captopril reduces cardiac inflammatory markers in
spontaneously hypertensive rats by inactivation of NF-kB. J Inflamm
(Lond). 7:212010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Katragadda S and Arora RR: Role of
angiotensin-converting enzyme inhibitors in vascular modulation:
beyond the hypertensive effects. Am J Ther. 17:e11–e23. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chan EC, Dusting GJ, Guo N, et al:
Prostacyclin receptor suppresses cardiac fibrosis: role of CREB
phosphorylation. J Mol Cell Cardiol. 49:176–185. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Maulik N, Engelman RM, Rousou JA, Flack JE
III, Deaton D and Das DK: Ischemic preconditioning reduces
apoptosis by upregulating anti-death gene Bcl-2. Circulation.
100(19 Suppl): II369–II375. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wu S, Storey JM and Storey KB:
Phosphoglycerate kinase 1 expression responds to freezing, anoxia,
and dehydration stresses in the freeze tolerant wood frog, Rana
sylvatica. J Exp Zool A Ecol Genet Physiol. 311:57–67. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jiang XC, Tall AR, Qin S, et al:
Phospholipid transfer protein deficiency protects circulating
lipoproteins from oxidation due to the enhanced accumulation of
vitamin E. J Biol Chem. 277:31850–31856. 2002. View Article : Google Scholar
|
|
35
|
Cavusoglu E, Marmur JD, Chhabraa S,
Choprab V, Eng C and Jiangc XC: Relation of baseline plasma
phospholipid transfer protein (PLTP) activity to left ventricular
systolic dysfunction in patients referred for coronary angiography.
Atherosclerosis. 207:261–265. 2009. View Article : Google Scholar
|