Introduction
Endoscopy is an important tool in the diagnosis,
follow-up and management of several gastrointestinal diseases,
including inflammatory bowel disease and cancer. The endoscopic
features and results of biopsy examinations are the parameters used
for evaluation in clinical practice. In experimental studies, the
course and progression of gastrointestinal lesions are followed by
sacrificing animals at specific time intervals. The disease is
usually assessed postmortem. This approach has several
disadvantages: i) it requires a large number of experimental
animals; ii) it is not possible to follow the progression and
evaluation of the same lesion and iii) the findings are difficult
to compare with biopsy samples obtained from patients by
endoscopy.
Several studies have reported on attempts to perform
colonoscopy in rats (1–9). However, in these studies, the rats
were either large and old, the bowel was not optimally prepared, a
total colonoscopy was not performed or no biopsies were taken. The
present study tested the feasibility of performing a total
colonoscopy with mucosal biopsies in small, young rats.
Materials and methods
Rats
Eighteen adult male Wistar rats (Hannover GALAS,
Taconic Farms, Laven, Denmark) with a mean average bodyweight of
194 g (range, 166–232 g) and an average age of 6–7 weeks were used.
The rats were housed singly in Macrolon III cages and fed a
standard diet ad libitum (B&K Universal AS, Nittedal,
Norway), consisting of cereal products (88.5%), soy protein (6%),
animal protein (2.5%), soy oil (0.5%), and vitamin, mineral and
amino acid supplements (2.5%). Water was also provided ad
libitum. The rats were maintained at a temperature of 21±1°C, a
relative humidity of 55±5% and under a 12-h light/dark cycle. The
animals were left in cages for a minimum of 7 days in order to
acclimatize prior to colonoscopy. While the rats were fasting, a
grid floor was used so that the feces would fall between the grid
bars, thus preventing the rats from eating them.
Bowel preparation
Three regimens for bowel cleansing were examined: i)
the rats had free access to water, however, food was withdrawn 36 h
prior to colonoscopy. The rats received a gastric dose of 1 ml of
Picoprep® (Ferring, Saint-Prex, Switzerland) followed by
2 ml of water, 24 h prior to colonoscopy. Picoprep is prepared in
150 ml of water and contains 10 mg of sodium sulfate, 3.5 g of
magnesium oxide and 12 g of citric acid. Picoprep was introduced
via an 8.5 cm long, 2.5 mm round-tip Teflon feeding gauge (AgnTho’s
AB, Lidingö, Sweden); ii) the rats were fasted for 24 h and then
received a gastric dose of 1 ml of Picoprep followed by 2 ml of
water, 24 h prior to colonoscopy and iii) the rats were fasted for
24 h and received gastric doses of 1 and 2 ml of Picoprep at 24 and
12 h prior to colonoscopy, respectively. Following this, 2 ml of
water was provided to the rats in both groups.
Colonoscopy and biopsy
The rats were anesthetized by inhalation of
isoflurane (Merk Pharmaceutical co., Inc., West Point, PA, USA)
prior to and during colonoscopy. They were then placed into a
supine position and secured onto an acrylic surgical table (World
Precision Instruments, Sarasota, FL, USA). The rats were placed on
a warming pad (Gaymar T/Pad, Gaymar Industries, Orchard Park, NY,
USA) using a heat therapy pump (TP500 t/Pump, Gaymar Industries) to
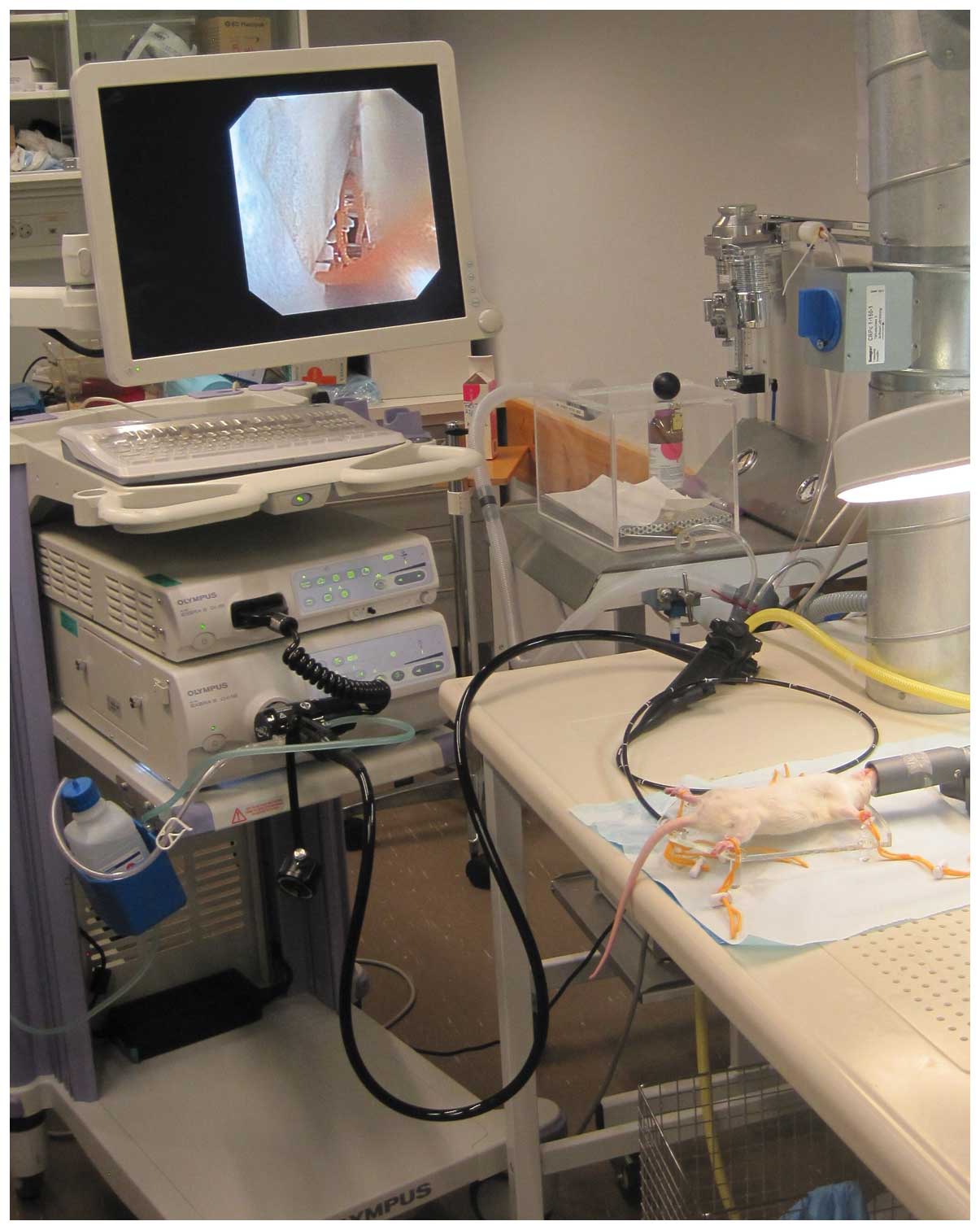
maintain normothermia during the procedure. A video gastroscope
with a 4.9 mm outer diameter, 210/120° up/down tip deflection, 103
cm working length, 140° view field and a 2 mm working channel
(GIF-N180, Olympus Medical System, Tokyo, Japan) was used. In
addition to the gastroscope, a light source (CLV-180, 230V S EVIS
EXERA II Xenon, Olympus Medical System), Olympus CV-180 possessor
(EVIS EXERA II video center, Olympus Medical System) and a monitor
(21 inch, Advan, Fremont, CA, USA) were used (Fig. 1). Biopsy samples were obtained by
disposable biopsy forceps (EndoJaw FB-231K, Olympus Medical System)
with a working length of 1550 mm and a minimum channel diameter of
2 mm. These forceps have a jaw length of 3 mm and a fenestrated jaw
swing diameter of 1.9 mm. The top of the endoscope was lubricated
with 2% lidocaine (Xylocaine®, Astra Zeneca
Pharmaceutics, Wilmington, UK) and introduced gently into the
anus.
Surveillance and necropsy
Following the procedure, the animals were allowed to
recover from anesthesia and were monitored for ~1 h. They were then
sacrificed by inhalation of CO2 and a postmortem
laparotomy was performed. The colon was dissected and its length
from the anus to the cecum was measured. Tissue samples from the
colon were collected for histological examination.
Histological examination
The biopsy samples obtained during colonoscopy and
from the colon tissue during postmortem laparotomy were fixed in 4%
buffered paraformaldehyde overnight, embedded in paraffin and then
cut into 5-μm sections. The sections were stained with hematoxylin
and eosin. The depth of the colonic wall was measured in samples
taken postmortem from rats by computer image analysis using Cell^D
software (Olympus Medical System).
The study was carried out in accordance with the
Directive for the Protection of Vertebrate Animals used for
Experimental and other Scientific Purposes of the European Union
(86/609/EEC), in compliance with the Helsinki Declaration. The
local ethical committee for experimental animals approved the
protocols of the study.
Results
Bowel preparation
The colon of rats that were fasted for 36 h and had
received 1 ml of Picoprep, 24 h prior to colonoscopy, were filled
with solid feces, therefore hindering the observation of the lining
mucosa, even following flushing with water. The colon of rats that
were fasted for 24 h and had received 1 ml of Picoprep, 24 h prior
to colonoscopy, were considerably cleaner, however, solid feces
were occasionally encountered. The colon of rats that were fasted
for 24 h and received 1 and 2 ml Picoprep at 24 and 12 h prior to
colonoscopy, respectively, were completely clean of feces.
Colonoscopy, biopsy and histological
examination
To facilitate the insertion of the endoscope and
relaxation of the anal sphincter, a 2.5-mm round-tip Teflon tube
was inserted into the anus prior to insertion of the endoscope. The
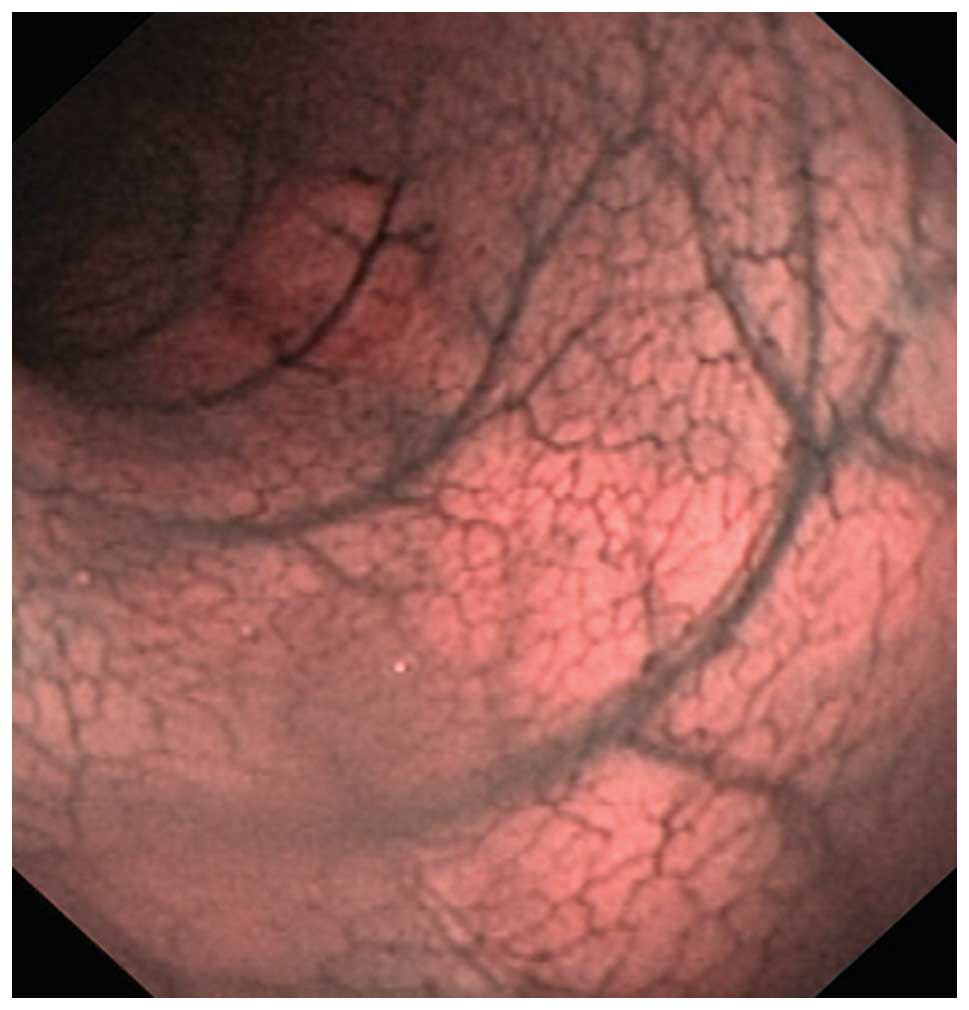
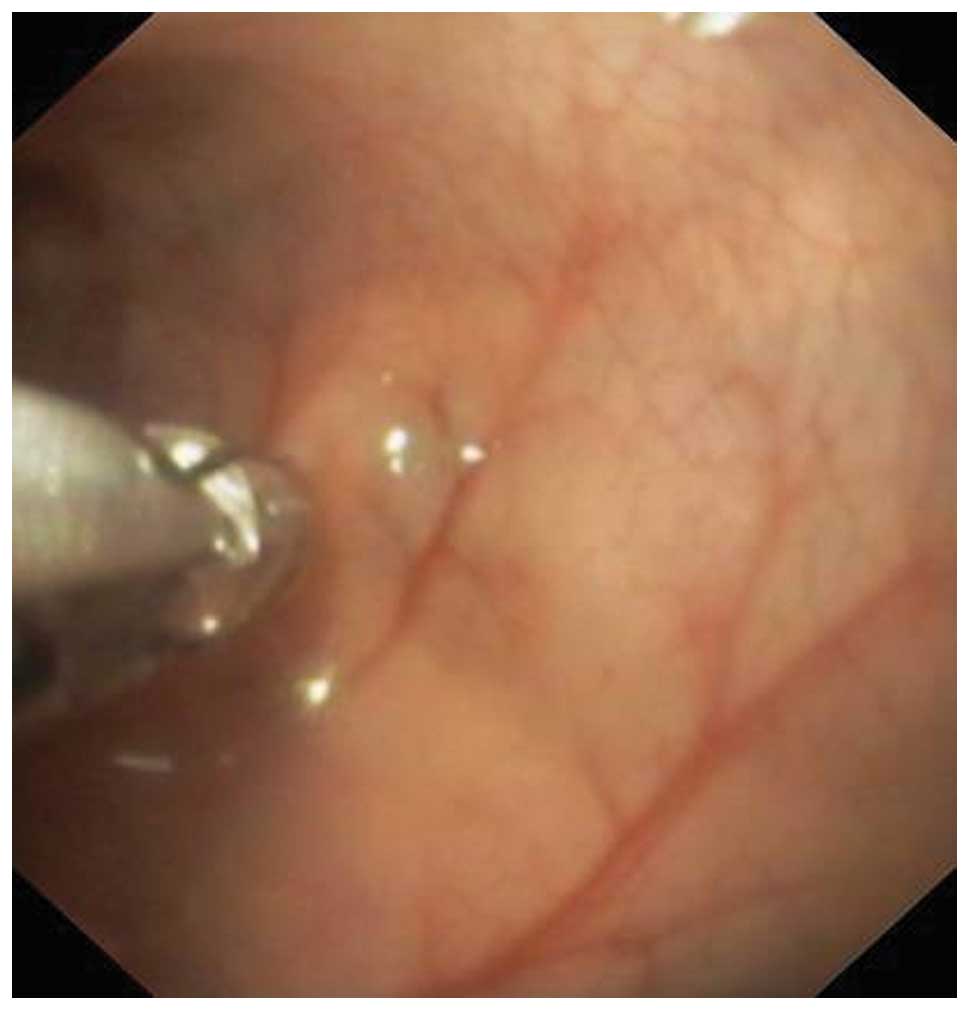
colonoscopy was straightforward as this is a relatively simple
procedure for a physician with training in flexible endoscopy
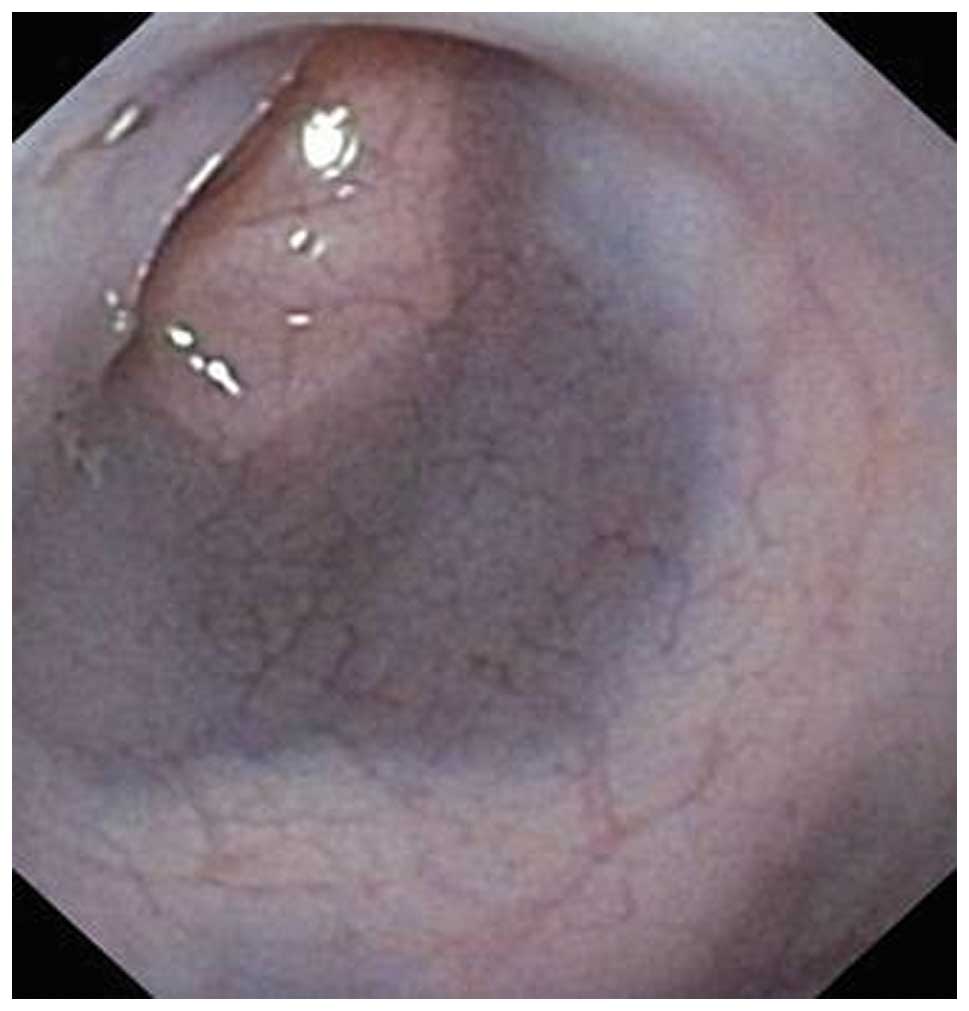
(Fig. 2). Rats have no splenic
flexure and therefore the only difficulty arose when maneuvering
the endoscope past the hepatic flexure (Fig. 3). When using a two-way endoscope
(up and down), this can only be achieved by manual rotation of the
endoscope shift to the right and left of a two-way endoscope.
Abdominal manipulation increases the risk of perforation. The cecum
was identified by the sudden increase in bowel diameter,
trans-illumination of the abdominal wall and the presence of a
small amount of solid feces in an otherwise clean colon. Inflation
with air should be avoided, however, if necessary, this should be
performed using air under low pressure. The lumen was visualized by
flushing with water to avoid abdominal distension. The time
required to perform a total colonoscopy and to obtain three colonic
biopsies varied between 6 and 10 min.
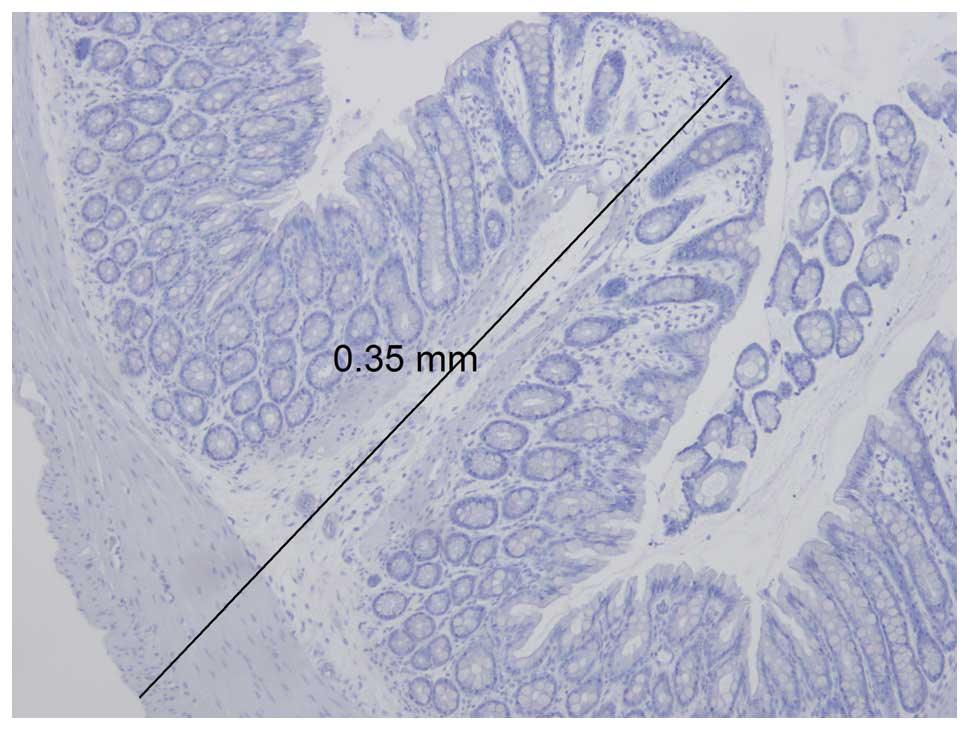
The maximum thickness of the colon wall was 0.35 mm
(Fig. 4) and the dimensions of the
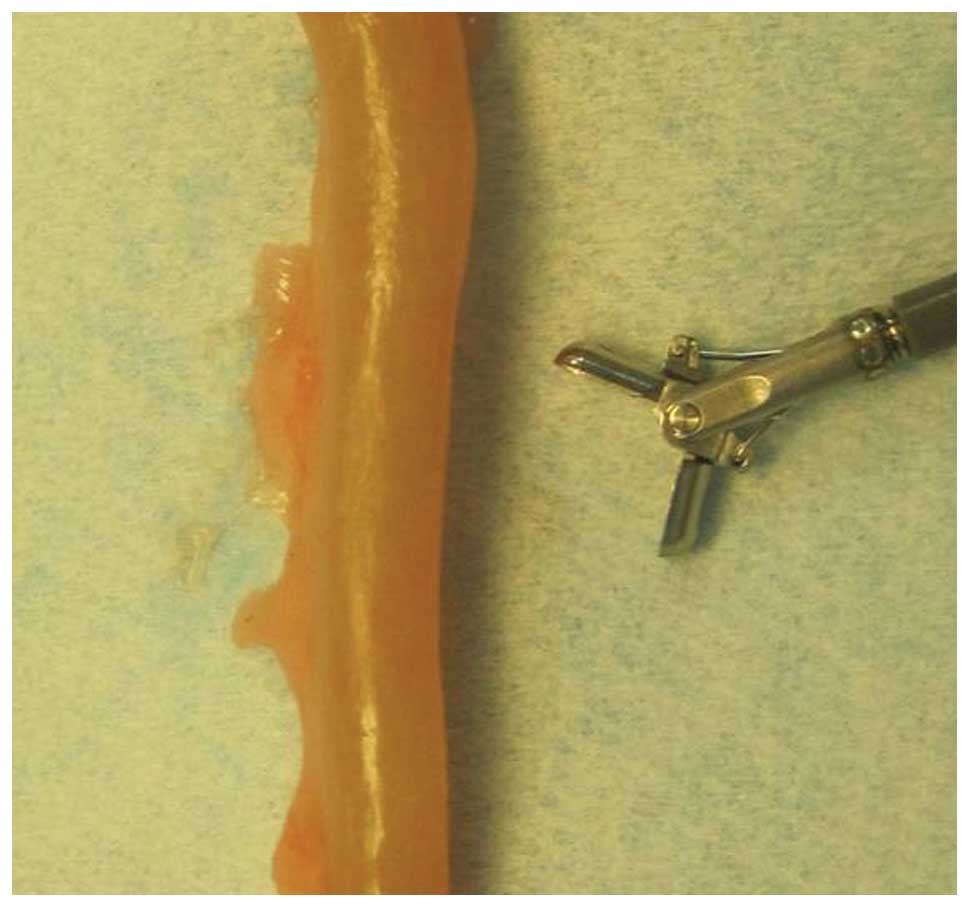
biopsy forceps were almost as large as the circumference of the rat
colon (Fig. 5). Obtaining biopsies
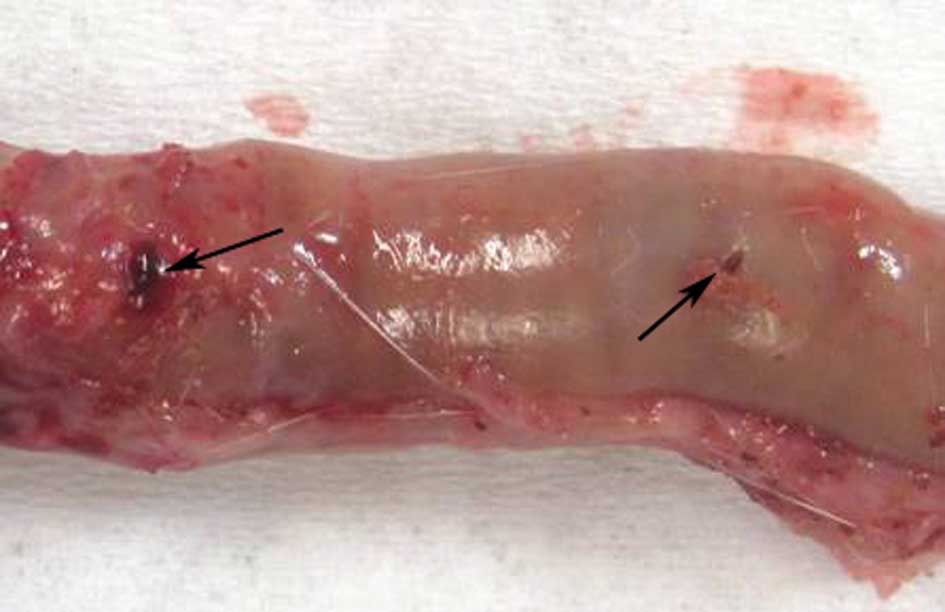
with the forceps full jaw length and swing resulted in perforation
(Fig. 6). Perforation was avoided
by obtaining the biopsies with the outer tip of the biopsy forceps
(Fig. 7).
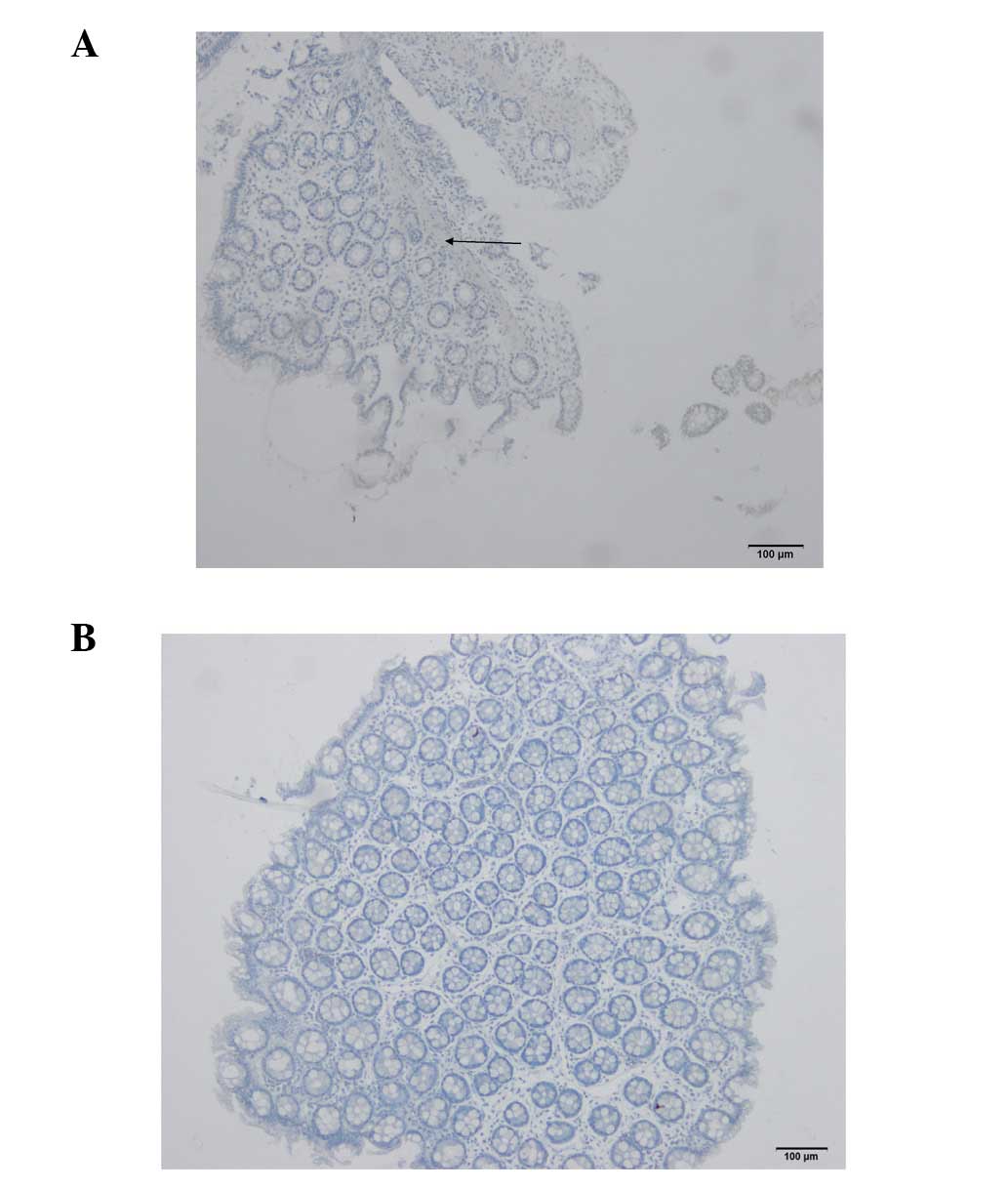
Histological examination of the biopsies collected
from perforated rats revealed that they included the epithelium,
lamina propria and muscularis mucosa (Fig. 8A). By contrast, those obtained with
the tip of the biopsy forceps included only the epithelium and
lamina propria (Fig. 8B), which is
comparable with biopsies collected during a standard colonoscopy in
humans.
Surveillance and necropsy
One rat died of aspiration caused by the gastric
tube during bowel preparation and two rats died due to colonic
perforation. A total colonoscopy was performed successfully in the
remaining rats, which recovered well thereafter. Postmortem
macroscopic examination of the abdomen and colon did not reveal any
complications. The length of the colon from the anus to the cecum
varied between 14 and 17 cm.
Discussion
The results of the present study demonstrate that
colonoscopy with mucosal biopsies is feasible in small, young rats.
However, several aspects should be taken into consideration when
using equipment designed for endoscopy in humans and when performed
by an endoscopist only trained in conducting endoscopy in human
patients.
Several studies examining colonoscopies were
conducted in large and old rats weighing up to 780 g (2,3,5,8).
Although this simplified the procedure, it has several
disadvantages, including the introduction of age as a limiting
factor in the experimental procedure and limitiation of long-term
follow-up. In addition, only partial colonoscopies have been
performed in rats of ~200 g bodyweight (1,4). In
the present study, total colonoscopy was performed successfully in
6–7-week-old rats weighing <200 g.
Bowel preparation prior to colonoscopy is essential
for a successful procedure. Bowel preparation varies between
studies, from no bowel preparation at all (2,3) to
lavage with water through a gastric feeding tube (4), fasting for 24 h and flushing with
water prior to colonoscopy (1) and
fasting for 56 h and administration of oral phosphosoda once or
twice in addition to a saline solution enema prior to colonoscopy
(5,8). The latter regime, which is the most
rigorous, results in a clean bowel, however, often distresses the
rats (5,8). In total, 1.6% of rats subjected to
this regime died during bowel preparation (8). Rats have a tendency to ingest their
feces during starvation. To prevent this in the present study, the
rats were housed on a grid floor in order that feces would fall
through to the bottom of the cage and become inaccessible, however,
following fasting for 36 h, a number of rats were able to ingest
their feces. Fasting for 24 h with 2 doses of Picoprep provided the
most effective preparation.
The endoscope used to perform colonoscopy in rats is
an important factor with regard to successful procedure, with three
of its properties particularly relevant, i.e., its diameter,
flexibility and tip movements in the up and down, and right to left
direction. A small endoscope is simpler to insert and minimizes the
risk of perforation, as would a flexible endoscope which is capable
of more readily following the course of the colon. Movement of the
endoscope tip facilitates its navigation through the sharp bend of
the hepatic flexure. Rat colons have been examined in a number of
studies using a rigid endoscope with a diameter of 2.2 or 2.7 mm
(2,4), and with a flexible endoscope
exhibiting tip mobility in four directions (with a diameter of
5.2–5.9 mm) (1,5,8). A
total colonoscopy was achieved when using a flexible endoscope with
the ability to maneuvre the tip in four directions and with a
diameter of 5.9 mm (5,8). In the present study, colonoscopy was
performed using an endoscope with a diameter of 4.9 mm, which is
the smallest available with a working channel for collecting biopsy
samples. The tip of the endoscope was only able to be maneuvered up
and down. The diameter of the endoscope did not cause any
complications, however, the endoscope had to be manually rotated
and shifted right and left to enable it to pass the hepatic
flexure.
While previous studies have reported the collection
of biopsies during colonoscopy in rats (2,3),
several factors including the rate of perforation and other
complications were not reported. Preliminary experiments with the
same biopsy forceps used in the present study demonstrated that
colonoscopy was feasible without a risk of perforation (1). The present findings demonstrate that
the risk of perforation is high when collecting biopsy samples,
however, the procedure can be successful if caution is
exercised.
Acknowledgements
This study was supported by a grant from
Helse-Fonna.
References
|
1
|
Vermeulen W, De Man JG, Nullens S,
Pelckmans PA, De Winter BY and Moreels TG: The use of colonoscopy
to follow the inflammatory time course of TNBS colitis in rats.
Acta Gastroenterol Belg. 74:304–311. 2011.PubMed/NCBI
|
|
2
|
Mann NS, Mann SK and Cheung EC: Fiberoptic
colonoscopic study of experimental chemical colitis. Gastrointest
Endosc. 26:28–40. 1980.PubMed/NCBI
|
|
3
|
Mann NS and Demers LM: Experimental
colitis studied by colonoscopy in the rat: effect of indomethacin.
Gastrointest Endosc. 29:77–82. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ahn BO, Ko KH, Oh TY, Cho H, Kim WB, Lee
KJ, Cho SW and Hahm KB: Efficacy of use of colonoscopy in dextran
sulfate sodium induced ulcerative colitis in rats: the evaluation
of the effects of antioxidant by colonoscopy. Int J Colorectal Dis.
16:174–181. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hamilton SR, Zhang SZ, O’Ceallaigh D and
McAvinchey D: Growth characteristics of autochthonous experimental
colonic tumors as assessed by serial colonoscopic measurement in
rats. Gastroenterology. 91:1511–1520. 1986.
|
|
6
|
Hull CC, Stellato TA, Ament AA, Gordon N
and Galloway P: Endoscopic and radiographic evaluation of the
murine colon. Cancer. 66:2528–2532. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Karas JR, Essani R, Haughn C, Uchal M,
Bishawi MM and Bergamaschi R: Colonoscopic injection for murine
solid cecal cancer model. Surg Endosc. 25:2956–2959. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Haughn C, Uchal M, Raftopoulos Y, Rossi S,
Santucci T, Torpey M, Pollice A, Yavuz Y, Marvik R and Bergamaschi
R: Development of a total colonoscopy rat model with endoscopic
submucosal injection of the cecal wall. Surg Endosc. 20:270–273.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Narisawa T, Wong CQ and Weisburger JH:
Evaluation of endoscopic examination of colon tumors in rats. Am J
Dig Dis. 20:928–934. 1975. View Article : Google Scholar : PubMed/NCBI
|