Introduction
The thymus is one of the central organs of the
immune system where T cells develop, differentiate and mature. In
recent years, it has been demonstrated that stromal cells of the
thymus are important in the differentiation and selection of thymic
lymphocytes. Macrophages are a type of thymic stromal cell that are
involved in phagocytosis and antigen presentation (1,2).
Additionally, macrophages secrete cytokines, which may affect
thymocyte proliferation, maturation, differentiation and the
negative selection of potential self-reactive T-cell clones
(3,4). Furthermore, macrophages are important
in immune reactions, particularly tumor immunity (5).
Previous studies have shown that macrophages are
generally heterogeneous in their phenotype and function (6). Immunohistochemical examination in
rats employing ED1, ED2 and ED3 monoclonal antibodies (mAbs) has
demonstrated that there are distinct subpopulations of thymic
macrophages (7). Compared with rat
thymic macrophages, fewer studies have focused on the macrophages
of the mouse thymus. To understand the characteristics of these
cells, we examined mouse thymic macrophages by immunofluorescence
staining, immunochemistry and acid phosphatase (ACP) activity
double staining using various mAbs.
Materials and methods
Animals
Female BALB/c mice (4–6 weeks old) were obtained
from the Experimental Animal Center of Harbin Medical University
(Harbin, Heilongjiang, China). All of the animal experiments
performed were reviewed by the Ethics Committee for Animal
Experiments at Harbin University School of Medicine and every
procedure followed the Guidelines for the Care and Use of
Laboratory Animals.
Antibodies
Affinity purified anti-mouse F4/80 antibody, rabbit
anti-CD68 (ED1), FITC-labeled goat anti-rat IgG and goat
anti-rabbit IgG/RBITC were purchased from BD Biosciences (Franklin
Lakes, NJ, USA). The antibody against LGALS3Bp (Mac-2) and
biotin-SP-conjugated affinity purified donkey anti-rabbit IgG were
purchased from PTGLabs (Chicago, IL, USA). ExtrAvidin-Cy3 conjugate
was purchased from Sigma (St. Louis, MO, USA).
Mouse model of thymic apoptosis
The BALB/c mice were randomly allocated into two
groups: the experimental and the normal control groups. The mice in
the experimental group were treated with an intraperitoneal
injection of dexamethasone (30 mg/kg), while those in the normal
control group were treated with physiological saline at the same
dose. The thymus was removed 15 h post-injection when typical
apoptotic morphology was observed (8). The mice were then divided into three
groups for analysis using transmission electron microscopy,
immunofluorescence and immunohistochemistry.
Preparation of frozen sections
Immediately following dissection, the thymic tissues
were fixed with periodate-lysine-paraformaldehyde fixative for 4 h
at 4°C. After washing with 0.01 M phosphate-buffered saline (PBS;
pH 7.4), the tissues were immersed in 5% sucrose phosphate buffer
for 1 h, 15% sucrose phosphate buffer for 2 h and finally in 30%
sucrose phosphate buffer overnight at 4°C. The tissues were
embedded in optimal cutting temperature compound for 30 min and
immersed in a mixture of acetone and dry ice.
Tissue preparation for transmission
electron microscopy
The mouse thymic tissues were fixed in 2.5%
glutaraldehyde through arterial perfusion. Post-fixation was
performed with 1% osmium trioxide. The thymic tissues were
dehydrated with acetone and then embedded in Epon 812. Thin
sections (0.5 μm) were observed under a light microscope to
identify the location of positive cells in the tissues. Ultra-thin
sections were observed under an H-600 transmission electron
microscope.
Immunofluorescence assay
Frozen sections (8 μm thick) were placed on
poly-L-lysine-coated glass slides and dried in air. Following
fixation with acetone, the sections were incubated in 0.25% Triton
X-100 for 15 min. Regarding the F4/80 group, the sections were
incubated with the anti-F4/80 mAb overnight at 4°C. After washing
with PBS, FITC-labeled goat anti-rat IgG was added and incubated
for 1 h at room temperature. For Mac-2 staining, the sections were
incubated with the anti-Mac-2 antibody overnight at 4°C. After
washing with PBS, biotin-SP-conjugated affinity purified donkey
anti-rabbit IgG was added and incubated for 1 h at room
temperature, washed again with PBS, and then ExtrAvidin-Cy3 was
added and incubated for 1 h at room temperature. For the ED1 group,
the sections were incubated with the anti-ED1 mAb overnight at 4°C.
After washing with PBS, the goat anti-rabbit IgG/RBITC secondary
antibody was added and incubated for 1 h at room temperature.
Double staining was performed for the F4/80 + Mac-2
group, in which sections were incubated with the anti-F4/80 mAb
overnight at 4°C. The sections were washed with PBS and the
FITC-labeled goat anti-rat IgG was added and incubated for 1 h.
They were then washed with PBS, blocked with 5% normal goat serum
for 30 min prior to the addition of the anti-Mac-2 antibody and
incubated for 1 h. The sections were washed with PBS and the
biotin-SP-conjugated donkey anti-rabbit IgG was added and incubated
for 1 h, washed with PBS, and finally ExtrAvidin-Cy3 was added and
incubated for 1 h. In the F4/80 + ED1 group, the sections were
incubated with the anti-F4/80 mAb overnight at 4°C. Following
washing with PBS, the FITC-labeled goat anti-rat IgG was added and
incubated for 1 h, washed again with PBS and blocked with 5% normal
goat serum for 30 min; the anti-ED1 mAb was added and incubated for
1 h. The samples were then washed with PBS and the secondary goat
anti-rabbit IgG/RBITC antibody was added and incubated for 1 h.
Sections from the normal control group were only incubated with the
fluorescent-conjugated antibody. All the sections were observed
under a fluorescence microscope.
Immunohistochemistry and ACP double
staining
Frozen sections, treated with 5% normal goat serum
for 15 min, were incubated with the anti-F4/80, anti-Mac-2 and
anti-ED1 mAbs. Following washing with PBS, the sections were
incubated with the rabbit anti-rat IgG and the goat anti-rabbit IgG
antibodies. The sections were washed with PBS and incubated with
DAB solution. The reaction was terminated with deionized water and
then incubated with ACP for 45 min at 37°C. Counterstaining with
methyl green was performed after the sections were washed with PBS.
The sections were immediately immersed in 100% alcohol and xylene,
and mounted with India rubber. The prepared sections were observed
under a light microscope.
Results
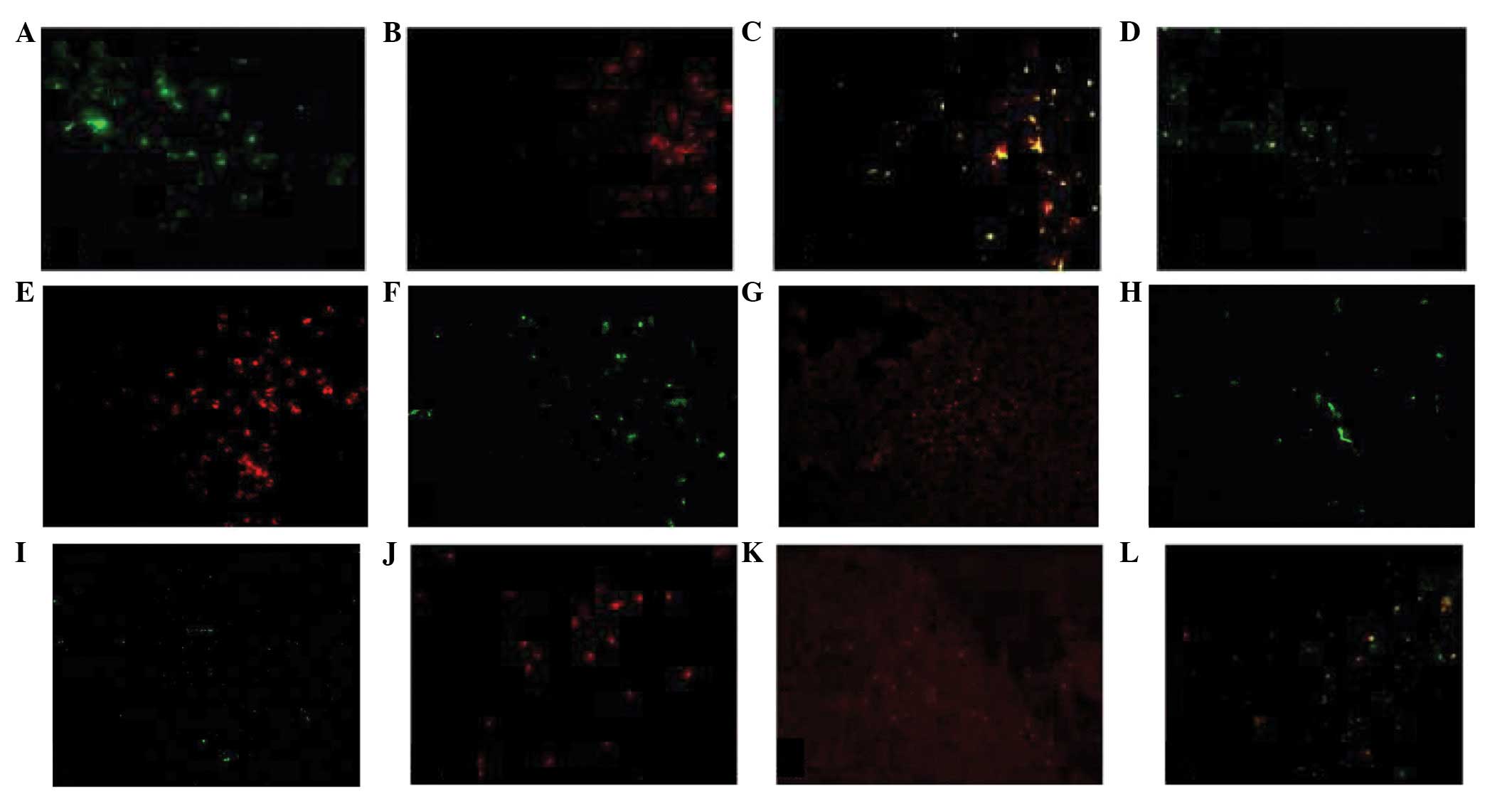
Morphology and distribution of distinct
subpopulations of thymic macrophages
The majority of mouse thymic macrophages exhibit the
shape of dendritic cells with well-developed cell processes
extending into the narrow spaces around thymocytes. These types of
macrophages were identified with anti-F4/80 and anti-Mac-2
antibodies (Fig. 1A-C), and they
were distributed in the entire thymus (Fig. 1D). The second type of macrophage
appeared as small oval cells, which notably lacked cell processes.
These cells were strongly stained by anti-Mac-2; however, weakly
stained by anti-F4/80 antibodies (Fig.
1E and F). Small oval macrophages were distributed in the
thymic medulla and corticomedullary region (CMR; Fig. 1G). The third type of macrophage
exhibited a flat shape and was distributed in the subcapsular
region of the thymic cortex. These cells were F4/80+ but
Mac-2− (Fig. 1H and I).
The fourth type of macrophage exhibited an irregular shape and was
distributed in the CMR. The number of these types of macrophage
which exhibited ED1− and F4/80+ staining was
low (Fig. 1J-L). Fluorescent cells
were not observed in the negative control group (data not
shown).
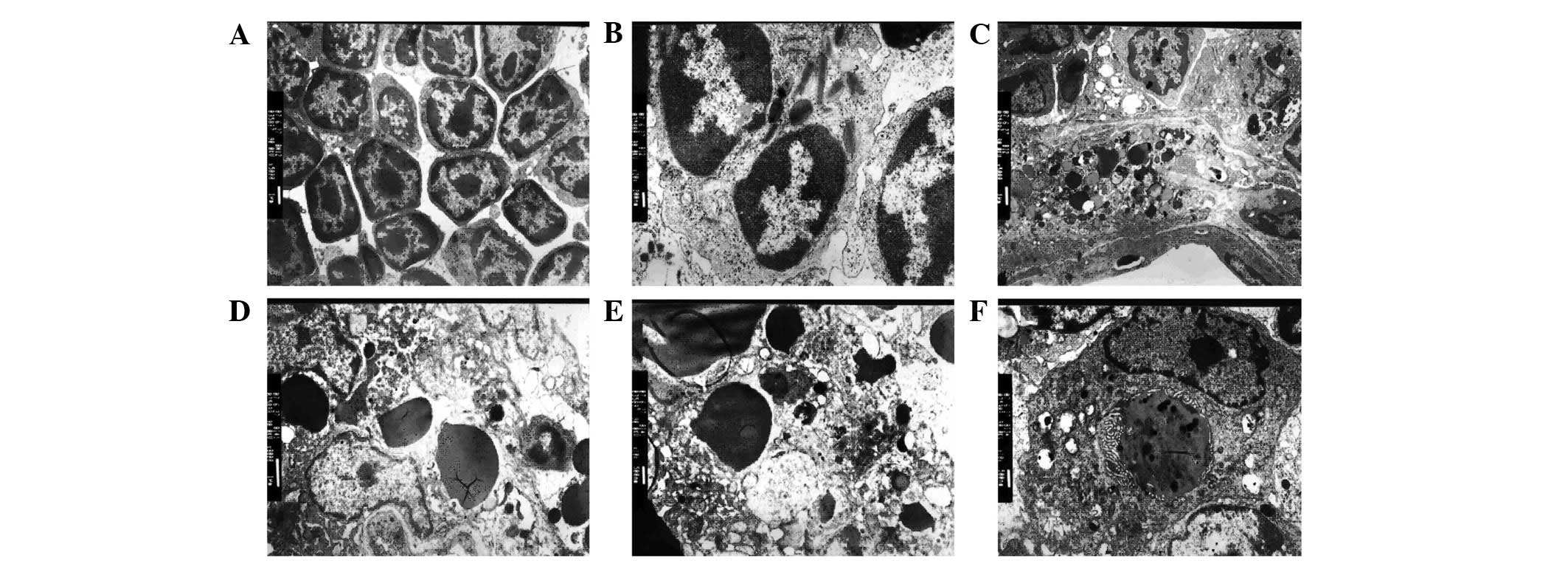
Phagocytes of thymic macrophages
In the control group, the shape of thymocytes was
normal, and the cell membrane and nucleus of thymocytes were
integrated (Fig. 2A). By contrast,
in the dexamethasone-injected mice, the membranes of phagocytes
fused with lymphocytes (Fig. 2B).
Additionally, numerous apoptotic bodies were observed in the
phagocytes along with hyperdense grains and nuclear chromatin.
Inclusion bodies surrounded by the membrane structure were also
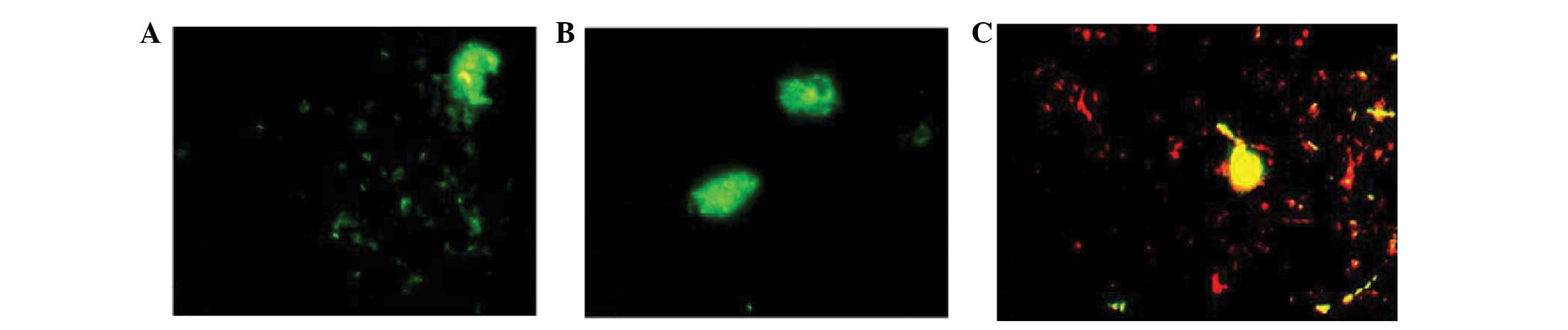
observed in the cytoplasm of phagocytes (Fig. 2C-F). Phagocytosis was observed in
dendritic macrophages, which presented as swollen cells exhibiting
strong fluorescent staining (Fig.
3).
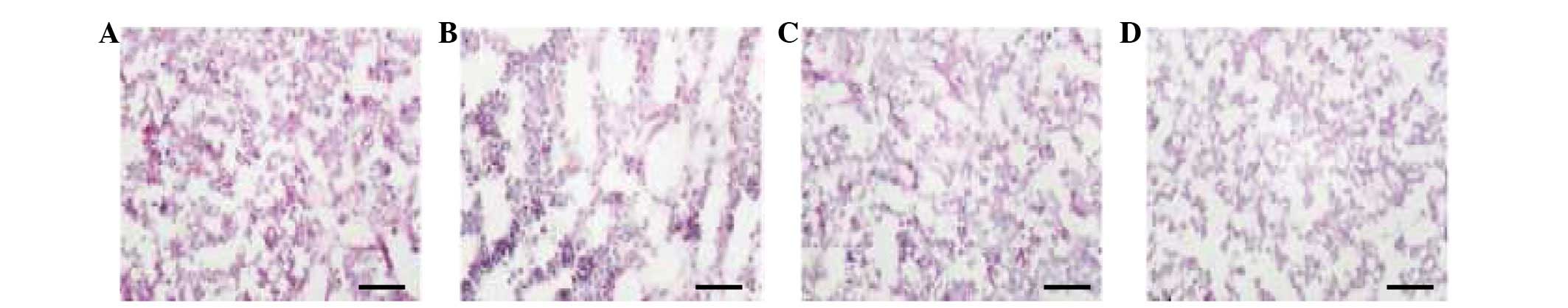
Mouse thymic tissue assessment using
double antibody and ACP staining
All of the four subpopulations of mouse thymic
macrophages described above exhibited ACP activity. There were a
large number of F4/80+ cells (Fig. 4A). Compared with the
F4/80+ cells, there were fewer Mac-2+ cells
stained positive for ACP (Fig.
4B). The ED1+ cells were mainly distributed in the
CMR (Fig. 4C). ACP+
cells were not observed in the negative control group (Fig. 4D). The subpopulations of mouse
thymic macrophages are listed in Table
I.
 | Table ICharacteristics of the subpopulations
of mouse thymic macrophages. |
Table I
Characteristics of the subpopulations
of mouse thymic macrophages.
| Cell subpopulation
(shape) |
|---|
|
|
|---|
| Characteristic | Dendritic | Round | Flat | Irregular |
|---|
| Phenotype |
| F4/80 | + | − | + | − |
| Mac-2 | + | + | − | − |
| ED1 | − | − | − | + |
| Distribution |
| Cortex | ++ | +− | +− | + |
| Corticomedullary
region | ++ | + | − | ++ |
| Medulla | + | + | − | +− |
| Phagocytosis | ++ | − | ND | + |
Discussion
In the present study, morphological and
immunohistochemical analysis of macrophages in the mouse thymus was
performed using anti-F4/80, anti-Mac-2 and anti-ED1 mAbs. The cell
types of this organ were not adequately identified by staining the
thymus with a single fluorescent antibody. Therefore, the
application of double-antibody staining using fluorescent
antibodies was required. Generally, the anti-ED1 mAb has been
considered valuable in the identification of rat macrophages.
However, whether mouse thymic macrophages are able to be identified
using the anti-ED1 antibody still remains to be elucidated. In the
present study, we used anti-ED1 mAb for the identification of mouse
thymic macrophages to investigate the subpopulations of these
cells.
In the present study, all of the F4/80+,
Mac-2+ and ED1+ cells exhibited an ACP
activity. These cells were mainly distributed in the cortex and the
CMR. Compared with F4/80+ and ED1+ cells,
Mac-2+ cells were fewer in number and smaller in size.
The results indicate that the macrophages with positive ACP
activity contained a number of phagosomes. The majority of thymic
macrophages were large in size and possessed rich cell processes.
These cells were designated as dendritic macrophages and were
observed to be distributed throughout the thymic parenchyma,
expressing Mac-2 and F4/80. These macrophages have been considered
as phagocytes, as they may play a role in the phagocytosis of dying
thymocytes (9,10). Dexamethasone-induced apoptosis of
thymic lymphocytes in mice was observed by electron microscopy. It
was shown that a number of phagosomes containing ingested
thymocytes at various stages of degradation were present in the
thymic macrophages with well-developed cell processes (11,12).
Furthermore, a subtype of dendritic macrophages was observed in the
CMR. However, it still remains unknown whether dendritic and
interdigitating dendritic cells are part of the same group of
thymic macrophages (13,14).
Small round macrophages lacking cell processes were
mainly distributed in the medulla and the CMR. These cells were
stained positive for Mac-2 and negative for F4/80. However, in the
cortex, there were some weakly stained F4/80+ cells.
These cells may be involved in the final stage of thymocyte
differentiation (8,15). Electron microscopy showed that
these small oval macrophages exhibited limited phagocytic activity,
indicating that they may constitute immature macrophages that
recently entered the thymus via blood vessels running through the
septa to the medulla and/or the CMR (13).
The third type of mouse thymic macrophages were
slender- and flat-shaped cells, extending their processes along and
underneath the capsule. These macrophages were low in quantity and
were found to be distributed in the subcapsular region. They were
stained positive with anti-F4/80 antibody; however, negative with
anti-Mac-2 and anti-ED1 mAbs. This type of macrophage was
considered as an independent subset of thymic macrophages, which
were different from the dendritic and oval macrophages described
above. Thymocytes are extensively divided at the subcapsular region
of the thymus; therefore, these flat macrophages may be important
in thymocyte proliferation and selective differentiation (8).
The fourth type of thymic macrophage with an
irregular form was observed to be mainly distributed in the CRM.
They were diffused in the cortex and a small number of these cells
were located in the medulla. Double-antibody staining showed that
these cells were stained positive for F4/80 or ED1, while few cells
were F4/80+ and ED1+. According to the
characteristic morphology and distribution of ED1+
cells, this type of macrophage was considered as an independent
subset of thymic macrophages. The CMR macrophages that strongly
express ED1 and F4/80 antigens have been suggested to be involved
in the ingestion of dying thymocytes (8,16).
In conclusion, the present study identified four
subpopulations of mouse thymic macrophages and provided the
platform to further investigate the function of thymic macrophages
in the proliferation and differentiation of lymphocytes.
Acknowledgements
This study was supported by funding from the
Education Bureau of Heilongjiang Province (no. 2011-318) and the
Health Bureau of Heilongjiang Province (no. 12511585).
References
|
1
|
Castor A, Nilsson L, Astrand-Grundström I,
Buitenhuis M, Ramirez C, Anderson K, Strömbeck B, Garwicz S,
Békássy AN, Schmiegelow K, Lausen B, Hokland P, Lehmann S,
Juliusson G, Johansson B and Jacobsen SE: Distinct patterns of
hematopoietic stem cell involvement in acute lymphoblastic
leukemia. Nat Med. 11:630–637. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hung FM, Chuang YY, Lee CS, Chen YL, Yang
JS, Lin JJ, Lu KW, Huang HY, Yu CC, Lu HF and Chung JG: Butylated
hydroxyanisole affects immunomodulation and promotes macrophage
phagocytosis in normal BALB/c mice. Mol Med Report. 5:683–687.
2012.PubMed/NCBI
|
|
3
|
de Pooter RF, Cho SK, Carlyle JR and
Zúñiga-Pflücker JC: In vitro generation of T lymphocytes from
embryonic stem cell-derived prehematopoietic progenitors. Blood.
102:1649–1653. 2003.
|
|
4
|
Lee CK, Kim JK, Kim Y, Lee MK, Kim K, Kang
JK, Hofmeister R, Durum SK and Han SS: Generation of macrophages
from early T progenitors in vitro. J Immunol. 166:5964–5969. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Luo H, Hao Y, Tang B, Zeng D, Shi Y and Yu
P: Mouse forestomach carcinoma cells immunosuppress macrophages
through transforming growth factor-β1. Mol Med Report. 5:988–992.
2012.
|
|
6
|
Naito M, Umeda S, Yamamoto T, Moriyama H,
Umezu H, Hasegawa G, Usuda H, Shultz LD and Takahashi K:
Development, differentiation, and phenotypic heterogeneity of
murine tissue macrophages. J Leukoc Biol. 59:133–138.
1996.PubMed/NCBI
|
|
7
|
Dijkstra CD, Dopp EA, Joling P and Kraal
G: The heterogeneity of mononuclear phagocytes in lymphoid organs:
distinct macrophage subpopulations in the rat recognized by
monoclonal antibodies ED1, ED2 and ED3. Immunology. 54:589–599.
1985.
|
|
8
|
Soga H, Nakamura M, Yagi H, Kayaba S,
Ishii T, Gotoh T and Itoh T: Heterogeneity of mouse thymic
macrophages: I. Immunohistochemical analysis. Arch Histol Cytol.
60:53–63. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jamieson CH, Ailles LE, Dylla SJ,
Muijtjens M, Jones C, Zehnder JL, Gotlib J, Li K, Manz MG, Keating
A, Sawyers CL and Weissman IL: Granulocyte-macrophage progenitors
as candidate leukemic stem cells in blast-crisis CML. N Engl J Med.
351:657–667. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Perry SS, Pierce LJ, Slayton WB and
Spangrude GJ: Characterization of thymic progenitors in adult mouse
bone marrow. J Immunol. 170:1877–1886. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Akashi K, Richie LI, Miyamoto T, Carr WH
and Weissman IL: B lymphopoiesis in the thymus. J Immunol.
164:5221–5226. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Foss DL, Donskoy E and Goldschneider I:
The importation of hematogenous precursors by the thymus is a gated
phenomenon in normal adult mice. J Exp Med. 193:365–374. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hashimoto S, Suzuki T, Dong HY, Yamazaki N
and Matsushima K: Serial analysis of gene expression in human
monocytes and macrophages. Blood. 94:837–844. 1999.PubMed/NCBI
|
|
14
|
Laskin DL, Weinberger B and Laskin JD:
Functional heterogeneity in liver and lung macrophages. J Leukoc
Biol. 70:163–170. 2001.PubMed/NCBI
|
|
15
|
Hume DA, Ross IL, Himes SR, Sasmono RT,
Wells CA and Ravasi T: The mononuclear phagocyte system revisited.
J Leukoc Biol. 72:621–627. 2002.PubMed/NCBI
|
|
16
|
Huitinga I, Bauer J, Strijbos PJ, Rothwell
NJ, Dijkstra CD and Tilders FJ: Effect of annexin-1 on experimental
autoimmune encephalomyelitis (EAE) in the rat. Clin Exp Immunol.
111:198–204. 1998. View Article : Google Scholar : PubMed/NCBI
|