Introduction
Mitochondrial dysfunction has been suggested to be
involved in the development of insulin resistance (1). However, whether mitochondrial defects
are the cause or consequence of insulin resistance remains
controversial. One of the first studies connecting mitochondrial
activity to diabetes mellitus was the identification of a mutation
in the mitochondrial DNA that caused a maternal-inherited form of
diabetes and associated deafness (2,3).
Studies in human subjects concerning this putative association
indicated that the mitochondrial defect observed in diabetic muscle
may be secondary to the insulin-resistant state instead of being a
causal factor (4). In addition,
functional defects of mitochondria, particularly reduced oxidative
and phosphorylation capacities, which are secondary to insulin
resistance, may aggravate insulin resistance.
Mitofusin 2 (Mfn2) is a mitochondrial membrane
protein that plays a role in mitochondrial fusion and regulates
mitochondrial metabolism in mammalian cells (5–7). It
is highly expressed in a number of tissues, including skeletal
muscle, heart and brain, with high energy demands (5). Mfn2 is relevant to numerous human
diseases and is important in glucose metabolism and the development
of insulin resistance. Mfn2 deficiency induces mitochondrial
dysfunction, increases H2O2 concentration and
activates c-Jun N-terminal kinase (JNK), leading to insulin
resistance in skeletal muscle and liver (8). In addition, in myoblasts with a
limited oxidative capacity, Mfn2 gain of function causes an
increase in the glucose oxidation rate and a parallel increase in
mitochondrial membrane potential, indicative of elevated pyruvate
oxidation in mitochondria and enhanced Krebs cycle and oxidative
phosphorylation (9).
Skeletal muscle glucose uptake and metabolism play a
key role in the regulation of whole-body glucose homeostasis in
normal and diabetic subjects (10,11).
Glucose uptake is the rate-limiting step of glucose utilization and
is depressed in cases of insulin resistance, a process
characterized by the reduced availability of sarcolemmal glucose
transporter 4 (GLUT4) translocation and consequential lower glucose
uptake (12,13). GLUT4 is a glucose transport
protein, highly expressed in adipose tissue and skeletal muscle, in
which translocation is induced by two separate signal transduction
pathways, an insulin-dependent and -independent pathway. The
insulin-dependent pathway results in GLUT4 translocation via the
activation of phosphatidylinositol-3 kinase (PI3K) and protein
kinase B/Akt, whereas the AMP-activated protein kinase (AMPK)
signaling pathway provides an alternative to the glucose uptake
pathway in muscle. A defect in GLUT4 expression and translocation
has been reported to be the primary metabolic abnormality in
diabetic skeletal muscle (14).
The aim of the present study was to examine the effects of Mfn2 on
glucose homeostasis and correlative GLUT4 expression and
translocation in high-fat diet (HFD) rats.
Materials and methods
Animals and experimental design
Forty male 4-week-old Sprague-Dawley rats (80–100 g)
were obtained from the Animal Center of Hebei Medical University
(Shijiazhuang, China) and maintained in an optimal environment for
1 week prior to experimentation. Animals were randomly divided into
four groups: negative control (NC; n=10), high-fat diet (HF; n=10),
high-fat diet plus adenoviral vectors (HF + Ado; n=10) and high-fat
diet plus adenoviral vectors encoding Mfn2 (HF + AdMfn2, n=10). The
rats were housed in a 12-h light-dark cycle and provided free
access to a rodent standard diet (65.5% carbohydrate, 10.3% fat and
24.2% protein) or a HFD (20.1% carbohydrate, 59.8% fat and 20.1%
protein), starting at 5 weeks of age, for 8 weeks. Following 8
weeks of HFD treatment, rats were subjected to a
euglycemic-hyperinsulinemic clamp, as described previously, to
assess insulin sensitivity (15).
Briefly, following collection of two basal samples,
insulin was infused intravenously at a constant rate (0.25 U/kg/h).
Blood samples (50 μl) were collected every 10 min to determine
blood glucose and to adjust glucose infusion until the infusion
rate was stabilized. Following 8 week HFD treatment, rats in the
intervention groups (HF + Ado and HF + AdMfn2) received 0.1 ml Ado
or AdMfn2 adenoviruses at a dose of 1×1010
plaque-forming U/ml via tail vein injection for 3 weeks and the
non-intervention rats (NC and HF) were injected with saline buffer
only. At the end of the 11-week study, the rats were fasted
overnight and the euglycemic-hyperinsulinemic clamp technique was
applied to evaluate improvement in insulin resistance. Rats were
anesthetized by intraperitoneal injections of pentobarbital sodium
(50 mg/kg body weight) and blood samples were drawn from the
abdominal aorta to measure the concentrations of glucose, insulin
and free fatty acids (FFA) in plasma. Muscle tissues were
collected, quickly placed in a liquid nitrogen container and stored
at −80°C for analysis. Experimental protocols were approved by the
Animal Welfare Committee of the University and conducted in
accordance with the institutional guidelines for animal
research.
Quantitative real-time polymerase chain
reaction (qPCR)
Total RNA from rat muscle tissue was obtained using
TRIzol reagent (Invitrogen Technologies, Carlsbad, CA, USA),
according to the manufacturer’s instructions. RNA concentration was
determined by measuring the absorbance of a diluted sample at 260
nm in a UV spectrometer (ND 2000, Thermo, USA). Reverse
transcription was performed on total RNA (2 μg) using random
primers to obtain the first-strand cDNA template. Real-time PCR was
performed with 0.8 μl cDNA (diluted 1:10), 2 μl specific primers
and 2X GoTaq® Green Master mix (Promega Corporation,
Madison, WI, USA) in a final volume of 20 μl. PCR was performed as
follows: an initial cycle at 95°C for 10 min, followed by 40 cycles
at 95°C for 15 sec, 58°C for 20 sec and 72°C for 27 sec. PCR
products were analyzed by melting curve to confirm the specificity
of amplification. Expression of Mfn2 and GLUT4 genes was analyzed
and GAPDH was used as an internal control. The following sets of
primers were used: Mfn2, 5′-AGCGTCCTCTCCCTCT GACA-3′ and
5′-TTCCACACCACTCCTCCGAC-3′; GLUT4, 5′-CCCACAAGGCACCCTCACTA-3′ and
5′-TGCCACCC ACAGAGAAGATG-3′; GAPDH, 5′-TGAACGGGAAGC TCACTG-3′ and
5′-GCTTCACCACCTTCTTGATG-3′
Western blot analysis
Frozen-dried muscle tissues (50 mg) were homogenized
in 500 μl ice-cold homogenization buffer [1% NP-40, 150 mmol/l
NaCl, 50 mmol/l Tris (pH 8.0), 0.1% aprotinin, 0.1% leupeptin,
0.035% pepstain A and 100 μg/ml PMSF] for 30 min at 4°C. The
samples were subjected to centrifugation at 11,600 × g for 10 min
at 4°C. Protein concentrations of supernatants were determined
using a BCA protein assay (Zomanbio, Beijing, China). Muscle
protein fractions (30 μg) were separated by 10% SDS-polyacrylamide
gel electrophoresis and transferred to PVDF membranes (Millipore,
USA). Following protein transfer, the membranes were blocked with
5% non-fat dry milk in TBST containing 0.05% Tween-20 overnight.
Following blocking, membranes were incubated overnight with
anti-IRβ (1:500), anti-AKT (1:800), anti-p-AKT (Ser-473; 1:200),
anti-AMPKα (1:500), anti-p-AMPKα (Thr-172; 1:200), anti-Mfn2
(1:500) and anti-GLUT4 (1:500; All Bioworld Technology, Inc.
Minneapolis, Mn, USA), followed by incubation with appropriate
HRP-conjugated secondary antibodies (Santa Cruz Biotechnology,
Santa Cruz, CA, USA) at room temperature for 1 h. The membranes
were then washed three times for 10 min in 0.05% Tween-20 in TBST.
The immunoreactive proteins were visualized by enhanced
chemiluminescence (Pierce Biotechnology, Rockford, IL, USA). X-ray
film was exposed to the PVDF membranes for 5 min. The reaction
product of each blot was analyzed by densitometry using the
Bandscan 4.3 Software.
Membrane preparations of skeletal
muscle
Following euglycemic hyperinsulinemic clamp
experiments, muscle tissues were excised and immediately frozen in
liquid nitrogen. Plasma membrane (PM) fractions of skeletal muscles
were isolated from the muscles according to a method described
previously (16).
Statistical analysis
Data were presented as the mean ± SD and were
analyzed using one-way analysis of variance followed by post hoc
comparisons. P<0.05 was considered to indicate a statistically
significant difference.
Results
Plasma metabolic parameters
Following a total of 11-week-HFD treatment (shown in
Table I), the HF group had higher
plasma glucose, insulin and FFA levels than the NC group. In
addition, plasma parameters following Ado or AdMfn2 administration
were analyzed in subsets of HFD rats. The parameters did not alter
in the HF+Ado group compared with HF (P>0.05), but decreased in
HF+AdMfn2 compared with HF (P<0.05).
 | Table IPlasma parameters at the end of the
11-week study. |
Table I
Plasma parameters at the end of the
11-week study.
| Plasma
parameters | NC | HF | HF + Ado | HF + AdMfn2 |
|---|
| Glucose (mmol/l) | 6.00±0.64 | 7.18±0.63a | 7.20±0.73a | 5.78±0.83b |
| Insulin (mU/l) | 28.88±4.81 | 40.62±6.89a | 40.80±5.45a | 30.70±4.20b |
| Fatty acids
(mmol/l) | 0.64±0.09 | 0.85±0.08a | 0.82±0.04a | 0.65±0.08b |
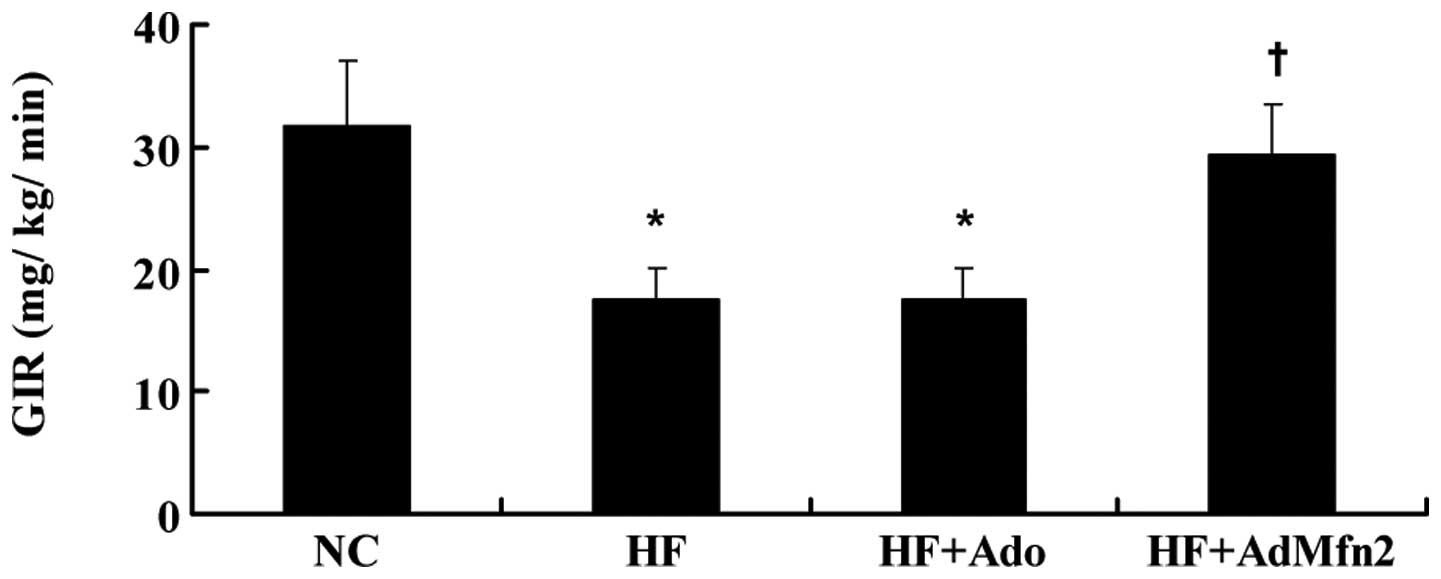
AdMfn2 increases the clamp glucose
infusion rate (GIR)
HFD caused a decrease in the clamp GIR required to
maintain euglycemia (Fig. 1).
AdMfn2 administration significantly increased GIR (61%), whereas
treatment of rats with Ado did not cause any alteration compared
with the HF group.
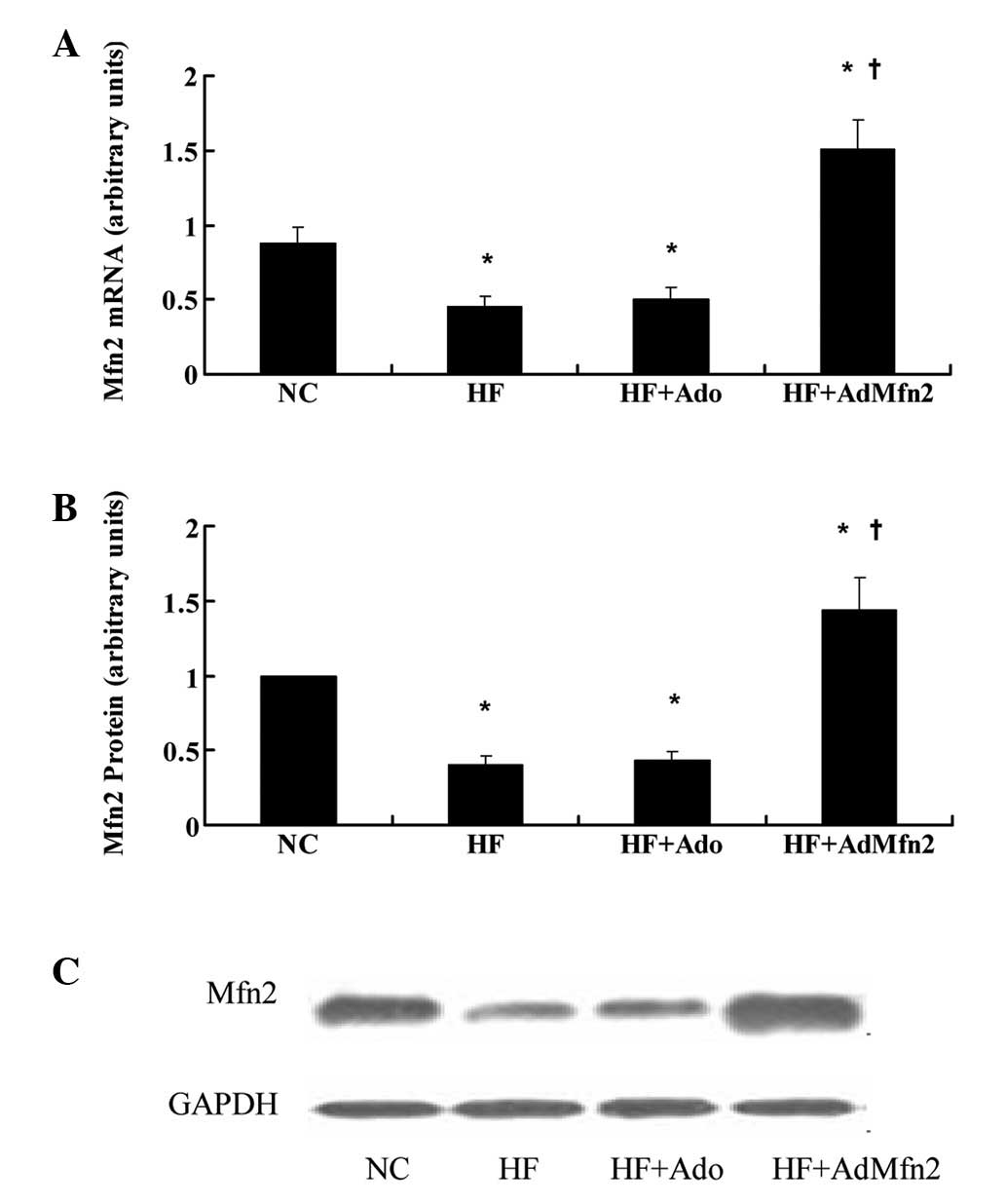
Mfn2 is repressed in skeletal muscle in
response to treatment with HFD and induced by administration of
AdMfn2
To study the regulatory profile of Mfn2, its
expression was analyzed under specific conditions, including
exposure to HFD or treatment with AdMfn2, to stimulate whole-body
Mfn2 expression. Exposure to HFD for 11 weeks caused downregulation
of Mfn2 mRNA and protein levels in skeletal muscles (Fig. 2; 51 and 44% of NC, respectively;
P<0.05), consistent with a previous study (1). Administration of AdMfn2 for three
weeks led to a 3.4- and 3.3-fold increase in Mfn2 mRNA and protein
expression in muscle tissues, respectively. In the HF + Ado group,
mRNA and protein levels were not altered in muscle tissue compared
with the HF group.
AdMfn2 specifically induces GLUT4 mRNA
and protein expression in HFD rat muscle tissue
GLUT4 mediates glucose uptake in muscle tissue. To
determine the role of the transporter in AdMfn2-induced insulin
sensitivity improvement, GLUT4 expression levels were examined
(Fig. 3). GLUT4 mRNA levels were
measured by real-time PCR and normalized against the internal
reference gene, GAPDH. Results presented in Fig. 3A indicate a significant
downregulation of GLUT4 mRNA by HFD (56% of NC, P<0.05) and that
Ado treatment did not have a significant effect on GLUT4 mRNA
levels compared with the HF group. By contrast, following
intervention of AdMfn2, GLUT4 mRNA was significantly higher in HF +
AdMfn2 compared with HF (149% of HF; P<0.05; Fig. 3A). Consistent with previous
observations (17), muscle
expression of GLUT4 protein was markedly reduced in the HF group
(66% of NC, P<0.05; Fig. 3B).
Following AdMfn2 treatment, total GLUT4 protein levels were
significantly increased to 131% of the HF group (P<0.05;
Fig. 3B). By contrast, there was
no difference in total GLUT4 protein levels between the HF + Ado
and HF groups (P>0.05; Fig.
3B). Therefore, administration of AdMfn2 for 3 weeks enhanced
GLUT4 gene expression and stabilized GLUT4 protein expression.
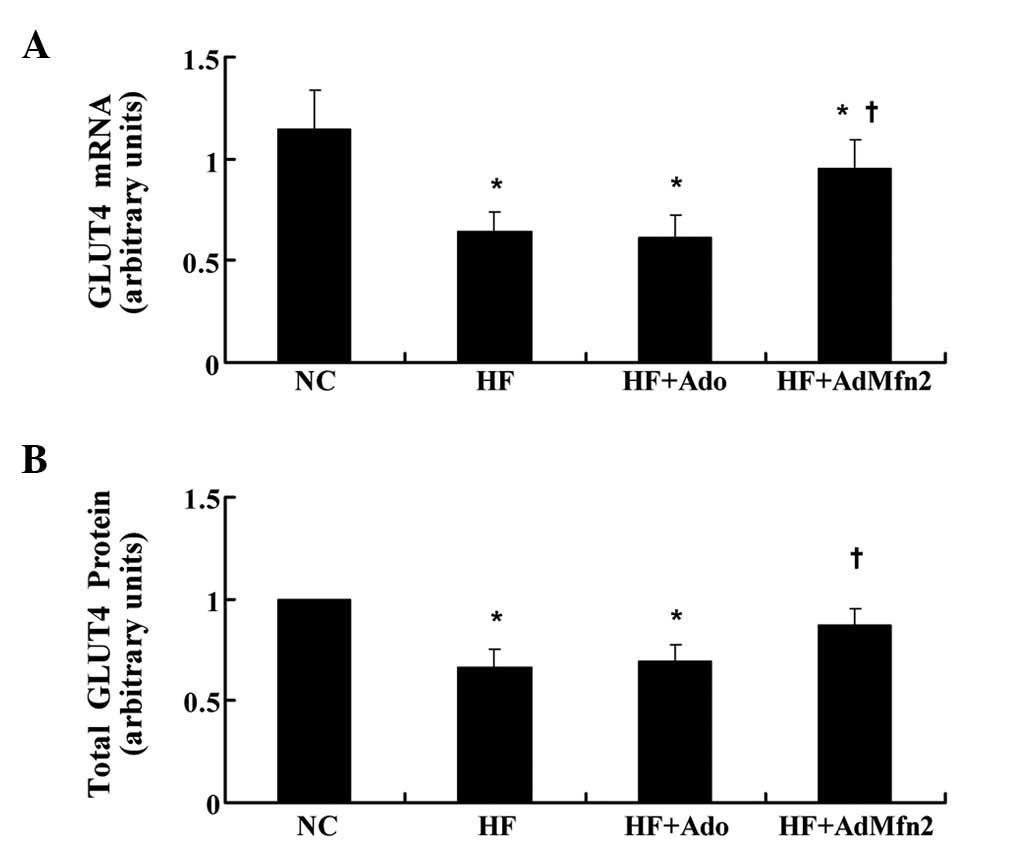 | Figure 3Effects of AdMfn2 on GLUT4 mRNA and
total protein expression in rats muscle tissues from the NC, HF, HF
+ Ado, HF + AdMfn2 groups. (A) Expression of GLUT4 mRNA was
measured by real-time PCR. (B) Total GLUT4 protein was measured by
western blot analysis. Data are shown as the average of three
separate experiments performed in duplicate (mean ± SD).
*P<0.05, vs. NC; †P<0.05, vs. HF;
one-way ANOVA. GLUT4, glucose transporter 4; Mfn2, mitofusin 2; NC,
negative control; HF, high-fat diet; Ado, adenoviral vector; PCR,
polymerase chain reaction. |
AdMfn2 promotes GLUT4 translocation in
HFD rat muscle tissues
To investigate the effect of AdMfn2 on GLUT4
translocation, the ratio of PM to total GLUT4 protein was analyzed
(Fig. 4). The PM and total GLUT4
protein of muscle tissues was fractionated and subjected to western
blot analysis (Fig. 4A). Following
administration of AdMfn2 to HFD rats, the ratio was significantly
increased to 187% of the HF group (P<0.05; Fig. 4B). However, the ratio was not
significantly altered in the HF + Ado group compared with the HF
group (P>0.05). These results demonstrate that AdMfn2 induces
GLUT4 redistribution into the PM fraction in skeletal muscle
tissue.
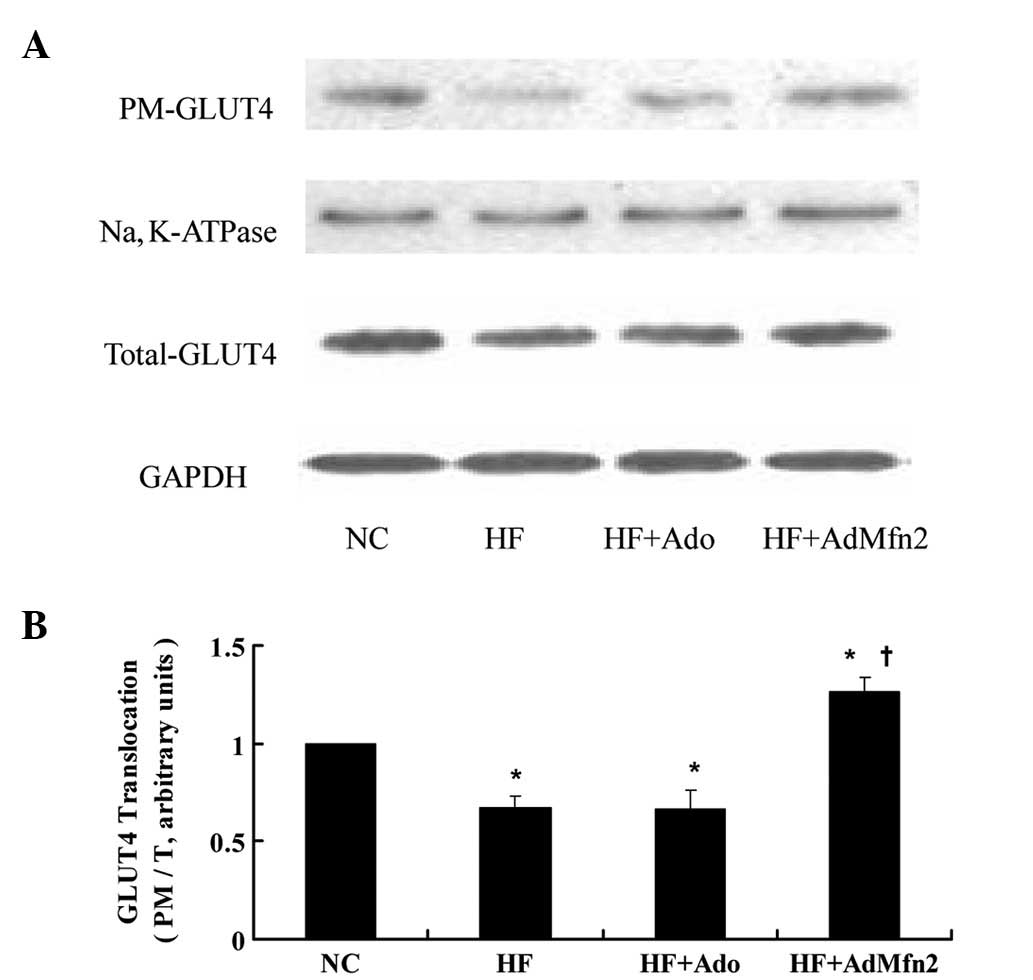 | Figure 4Effects of AdMfn2 on GLUT4
translocation in rat muscle tissues from the NC, HF, HF + Ado, HF +
AdMfn2 groups. PM and total GLUT4 protein of muscle tissues was
fractionated and subjected to western blot analysis. (A)
Representative immunoblots are presented. (B) Ratio of PM to total
GLUT4 protein is shown as mean ± SD for three experiments.
*P<0.05, vs. NC; †P<0.05, vs. HF;
one-way ANOVA. GLUT4, glucose transporter 4; Mfn2, mitofusin 2; NC,
negative control; HF, high-fat diet; Ado, adenoviral vector. |
Role of Akt and AMPK pathways in
AdMfn2-induced glucose uptake and GLUT4 translocation
To elucidate the effect of AdMfn2 on the signaling
pathways involved in GLUT4 translocation, the IRβ-PI3K-Akt and AMPK
pathways were analyzed by western blot analysis (Fig. 5A). Results presented in Fig. 5B and C demonstrate that AdMfn2 had
no effect on the phosphorylation of Akt (89% of HF; P>0.05) but
significantly increased the phosphorylation of AMPKα (276% of HF;
P<0.05). No differences were detected with regard to the
expression of AMPKα (P>0.05; Fig.
5C). Expression of IRβ, PI-3K, p-AKT and AKT protein was
significantly decreased in the HF group compared with NC
(P<0.05; Fig. 5B and D). No
significant differences were found in the expression levels of IRβ,
PI-3K, p-AKT and AKT protein among the three groups (HF, HF + Ado
and HF + AdMfn2; P>0.05; Fig. 5B
and D). These results demonstrate that the effect of AdMfn2 on
GLUT4 translocation contributes, in part, to increased AMPK
activation.
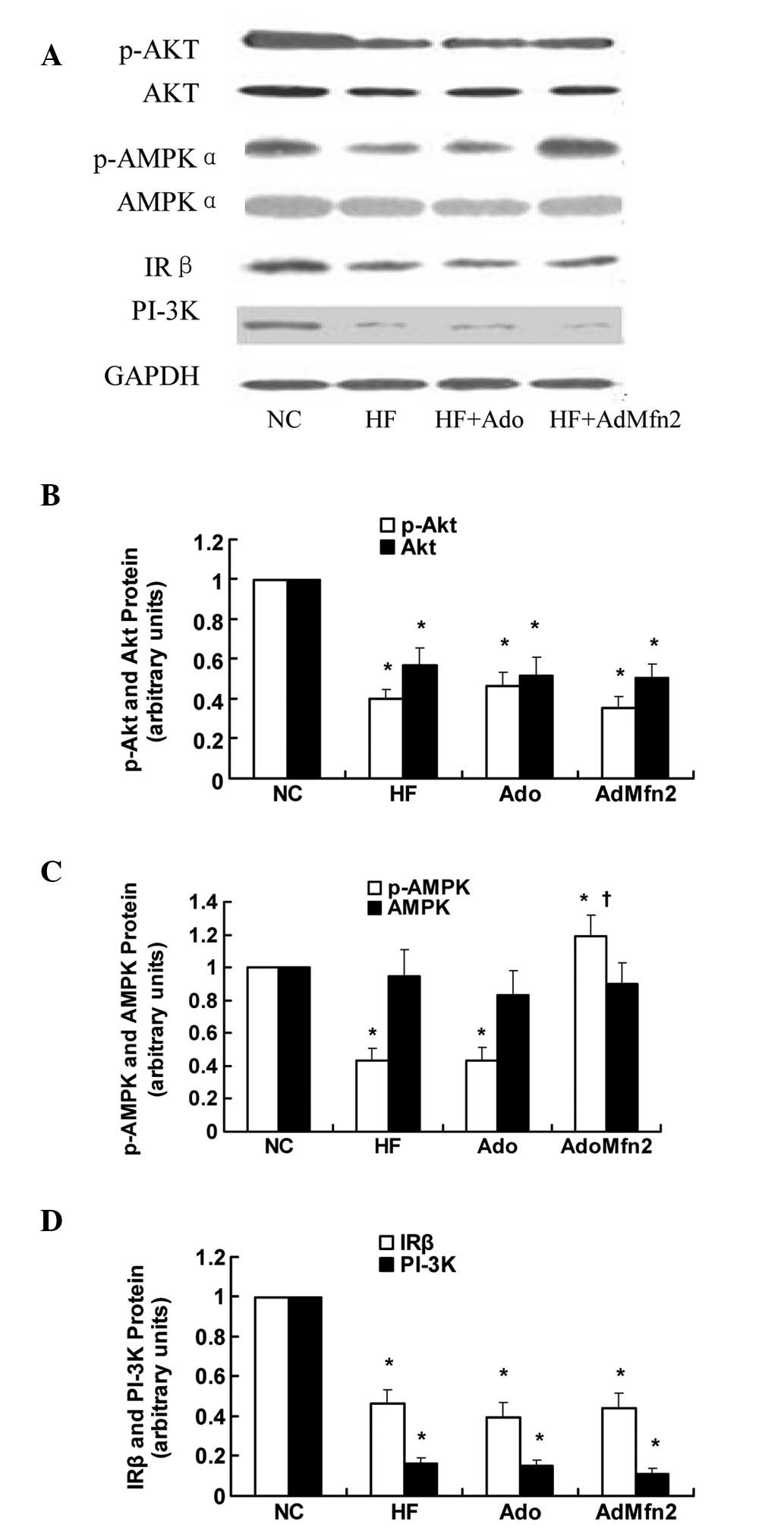 | Figure 5Effects of AdMfn2 on the signaling
pathways involved in GLUT4 translocation in rat muscle tissues from
the NC, HF, HF + Ado and HF + AdMfn2 groups. (A) Expression of
phosphorylated Akt (Ser473), Akt, phosphorylated AMPK (Thr172),
AMPK, IRβ and PI3K were measured by western blot analysis. (B-D)
Phosphorylated Akt, Akt, phosphorylated AMPK, AMPK, IRβ and PI-3K
protein levels were calculated as ratios. Data are shown as the
average of three separate experiments performed in duplicate (mean
± SD). *P<0.05, vs. NC; †P<0.05, vs.
HF; one-way ANOVA. GLUT4, glucose transporter 4; AMPK,
AMP-activated protein kinase; IRβ, insulin receptor β; PI3K,
phosphatidylionositol-3 kinase; NC, negative control; HF, high-fat
diet; Ado, adenoviral vector. |
Discussion
Results of the present study indicate that Mfn2
improves insulin sensitivity and may regulate GLUT4 translocation
in an AMPK-dependent manner in skeletal muscles of HFD rats. In the
present study, HFD treatment in rats was found to induce insulin
resistance and reduce Mfn2 expression in insulin-resistant rats.
These results are in agreement with the previous observations that
muscle Mfn2 is repressed in Zucker rats and diabetes patients
(1,18,19).
Previous studies have indicated that Mfn2 plays a
positive role in maintaining glucose homeostasis. In addition,
aberrant expression of Mfn2 may be involved in the pathophysiology
of insulin resistance. Therefore, consistent with Mfn2/shRNA BALB/c
mice, hepatic glucose production and insulin resistance was
significantly increased (20). An
additional study has also reported that Mfn2 deficiency causes
mitochondrial dysfunction, leading to enhanced reactive oxygen
species production and JNK activity and inactivation of insulin
receptor substrate 1, a key protein in insulin signaling (8). A positive correlation between Mfn2
expression and insulin sensitivity was also previously detected in
non-diabetic and type 2 diabetic subjects (1). Consistent with these observations a
67% increase in whole-body insulin sensitivity was noted in the
current study, following administration of AdMfn2 for three weeks
in HFD rats (as indicated by clamp GIR). However, the mechanism by
which Mfn2 overexpression ameliorates GIR must be further
investigated.
HFD rats demonstrated glucose intolerance, which is,
at least in part, attributable to decreased glucose uptake. Glucose
transport mediated by GLUT4 is a major rate-limiting step in
glucose metabolism in skeletal muscle, which may be activated by
two separate signaling pathways. Impaired glucose transport in
skeletal muscle leads to impaired whole body glucose uptake and
contributes to the pathogenesis of type 2 diabetes mellitus.
Previous studies have reported whole-body glucose uptake as a
linear function of GLUT4 expression in skeletal muscle (21). A previous study of six morbidly
obese females who underwent malabsorptive bariatric surgery,
revealed an increase in Mfn2 mRNA levels, associated with the
improvement of whole-body glucose uptake, as well as with an
increase in GLUT4 expression (19). In the present study, Mfn2
overexpression increased GLUT4 expression and translocation in
skeletal muscles of HFD rats, which coincided with an increase in
AMPK phosphorylation rather than Akt phosphorylation. A number of
previous studies have described an association between Mfn2
expression and Akt phosphorylation (8,22–25).
Current results demonstrate that overexpression of Mfn2 has no
effect on Akt activation in skeletal muscles of HFD rats,
consistent with previous observations indicating that upregulated
Mfn2 does not affect Akt phosphorylation in cultured cardiomyocytes
(22). However, other studies have
reported Mfn2 overexpression depresses the activation of Akt or,
Mfn2 knockdown attenuated the activation of Akt (8,23–25).
Thus, the correlation between Mfn2 expression and Akt
phosphorylation remains poorly understood due to signaling
discrepancies and cell selection.
Physiological and pathophysiological conditions
characterized by altered glucose utilization (diabetes, obesity,
insulin resistance, exercise and weight loss) leads to changes in
Mfn2 expression. However, direct evidence of the association of
Mfn2 in glucose uptake and insulin sensitivity has not been
reported. In the present study, a positive correlation between Mfn2
expression and insulin sensitivity assessed by clamp GIR was
reported, consistent with previous studies (1,19).
In addition, GLUT4 mRNA levels have been found to positively
correlate with Mfn2 mRNA concentrations and linearly correlate with
whole-body glucose uptake (19).
However, the direct correlation between Mfn2 and GLUT4 remains
unclear. In the present study, it was demonstrated that Mfn2
overexpression induced GLUT4 expression and translocation via AMPK
activation in skeletal muscles of HFD rats, which was important for
improving insulin sensitivity.
Based on the role of Mfn2 in improvement of glucose
uptake, one of the limitations of this study was that the
hypothesis of a potential signaling pathway by which Mfn2
overexpression caused increased GLUT4 translocation was made,
however, we did not reach a final conclusion as to whether the
increase of GLUT4 translocation correlates with AMPK
phosphorylation via Mfn2 upregulation in muscle tissues. In
addition, a specific mechanism by which Mfn2 regulates GLUT4
expression by AMPK phosphorylation in skeletal muscles has yet to
be established. Thus, future studies are required to establish the
specific cause-and-effect correlation between Mfn2 expression and
AMPK phosphorylation and to identify the detailed signaling
pathways by which Mfn2 regulates GLUT4 expression.
In summary, results of the current study indicate
that insulin resistance is associated, at baseline, with a reduced
expression of Mfn2 in skeletal muscle, which is associated with a
concomitant reduction in expression and translocation activity of
GLUT4. In addition, Mfn2 overexpression increased glucose uptake by
enhanced GLUT4 translocation, which was induced via increased AMPK
phosphorylation. Increase in Mfn2 expression and the concomitant
stimulation of AMPK activity may be relevant to enhanced glucose
uptake capacity in insulin-resistant rats.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundations of China (nos. 30971391 and 81170742)
and Hebei Natural Science Foundation of China (nos. C2010001638 and
C2011307008).
References
|
1
|
Bach D, Naon D, Pich S, et al: Expression
of Mfn2, the Charcot-Marie-Tooth neuropathy type 2A gene, in human
skeletal muscle: effects of type 2 diabetes, obesity, weight loss
and the regulatory role of tumor necrosis factor alpha and
interleukin-6. Diabetes. 54:2685–2693. 2005. View Article : Google Scholar
|
|
2
|
van den Ouweland JM, Lemkes HH, Ruitenbeek
W, et al: Mutation in mitochondrial tRNA(Leu)(UUR) gene in a large
pedigree with maternally transmitted type II diabetes mellitus and
deafness. Nat Genet. 1:368–371. 1992.PubMed/NCBI
|
|
3
|
Zorzano A, Liesa M and Palacin M:
Mitochondrial dynamics as a bridge between mitochondrial
dysfunction and insulin resistance. Arch Physiol Biochem. 115:1–12.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hoeks J and Schrauwen P: Muscle
mitochondria and insulin resistance: a human perspective. Trends
Endocrinol Metab. 23:444–450. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bach D, Pich S, Soriano FX, et al:
Mitofusin-2 determines mitochondrial network architecture and
mitochondrial metabolism. A novel regulatory mechanism altered in
obesity. J Biol Chem. 278:17190–17197. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen H, Detmer SA, Ewald AJ, Griffin EE,
Fraser SE and Chan DC: Mitofusins Mfn1 and Mfn2 coordinately
regulate mitochondrial fusion and are essential for embryonic
development. J Cell Biol. 160:189–200. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Soriano FX, Liesa M, Bach D, Chan DC,
Palacin M and Zorzano A: Evidence for a mitochondrial regulatory
pathway defined by peroxisome proliferator-activated receptor-gamma
coactivator-1 alpha, estrogen-related receptor-alpha and mitofusin
2. Diabetes. 55:1783–1791. 2006. View Article : Google Scholar
|
|
8
|
Sebastián D, Hernández-Alvarez MI, Segalés
J, et al: Mitofusin 2 (Mfn2) links mitochondrial and endoplasmic
reticulum function with insulin signaling and is essential for
normal glucose homeostasis. Proc Natl Acad Sci USA. 109:5523–5528.
2012.PubMed/NCBI
|
|
9
|
Pich S, Bach D, Briones P, et al: The
Charcot-Marie-Tooth type 2A gene product, Mfn2, up-regulates fuel
oxidation through expression of OXPHOS system. Hum Mol Genet.
14:1405–1415. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
McConell GK and Wadley GD: Potential role
of nitric oxide in contraction-stimulated glucose uptake and
mitochondrial biogenesis in skeletal muscle. Clin Exp Pharmacol
Physiol. 35:1488–1492. 2008.PubMed/NCBI
|
|
11
|
Zhao HL, Liu LZ, Sui Y, et al: Fatty acids
inhibit insulin-mediated glucose transport associated with actin
remodeling in rat L6 muscle cells. Acta Diabetol. 47:331–339. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jaiswal N, Yadav PP, Maurya R, Srivastava
AK and Tamrakar AK: Karanjin from Pongamia pinnata induces
GLUT4 translocation in skeletal muscle cells in a
phosphatidylinositol-3-kinase-independent manner. Eur J Pharmacol.
670:22–28. 2011.
|
|
13
|
Kim JY, Jo KJ, Kim BJ, Baik HW and Lee SK:
17β-estradiol induces an interaction between adenosine
monophosphate-activated protein kinase and the insulin signaling
pathway in 3T3-L1 adipocytes. Int J Mol Med. 30:979–985. 2012.
|
|
14
|
Bi Y, Sun WP, Chen X, Li M, Liang H, Cai
MY, Zhu YH, He XY, Xu F and Weng JP: Effect of early insulin
therapy on nuclear factor kappaB and cytokine gene expressions in
the liver and skeletal muscle of high-fat diet,
streptozotocin-treated diabetic rats. Acta Diabetol. 45:167–178.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Iglesias MA, Ye JM, Frangioudakis G, et
al: AICAR administration causes an apparent enhancement of muscle
and liver insulin action in insulin-resistant high-fat-fed rats.
Diabetes. 51:2886–2894. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sato T, Man ZW, Toide K and Asahi Y:
Plasma membrane content of insulin-regulated glucose transporter in
skeletal muscle of the male Otsuka Long-Evans Tokushima Fatty rat,
a model of non-insulin-dependent diabetes mellitus. FEBS Lett.
407:329–332. 1997. View Article : Google Scholar
|
|
17
|
Wang ZQ, Zhang XH, Yu Y, et al: Bioactives
from bitter melon enhance insulin signaling and modulate acyl
carnitine content in skeletal muscle in high-fat diet-fed mice. J
Nutr Biochem. 22:1064–1073. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hernández-Alvarez MI, Thabit H, Burns N,
et al: Subjects with early-onset type 2 diabetes show defective
activation of the skeletal muscle PGC-1{alpha}/Mitofusin-2
regulatory pathway in response to physical activity. Diabetes Care.
33:645–651. 2010.PubMed/NCBI
|
|
19
|
Mingrone G, Manco M, Calvani M, Castagneto
M, Naon D and Zorzano A: Could the low level of expression of the
gene encoding skeletal muscle mitofusin-2 account for the metabolic
inflexibility of obesity? Diabetologia. 48:2108–2114. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen X and Xu Y: Liver-specific reduction
of Mfn2 protein by RNAi results in impaired glycometabolism and
lipid homeostasis in BALB/c mice. J Huazhong Univ Sci Technolog Med
Sci. 29:689–696. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mingrone G, Rosa G, Di Rocco P, et al:
Skeletal muscle triglycerides lowering is associated with net
improvement of insulin sensitivity, TNF-alpha reduction and GLUT4
expression enhancement. Int J Obes Relat Metab Disord.
26:1165–1172. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yu H, Guo Y, Mi L, Wang X, Li L and Gao W:
Mitofusin 2 inhibits angiotensin II-induced myocardial hypertrophy.
J Cardiovasc Pharmacol Ther. 16:205–211. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Guo YH, Chen K, Gao W, et al:
Overexpression of Mitofusin 2 inhibited oxidized low-density
lipoprotein induced vascular smooth muscle cell proliferation and
reduced atherosclerotic lesion formation in rabbit. Biochem Biophys
Res Commun. 363:411–417. 2007. View Article : Google Scholar
|
|
24
|
Lugus JJ, Ngoh GA, Bachschmid MM and Walsh
K: Mitofusins are required for angiogenic function and modulate
different signaling pathways in cultured endothelial cells. J Mol
Cell Cardiol. 51:885–893. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wan-Xin T, Tian-Lei C, Ben W, Wei-Hua W
and Ping F: Effect of mitofusin 2 overexpression on the
proliferation and apoptosis of high-glucose-induced rat glomerular
mesangial cells. J Nephrol. 25:1023–1030. 2012. View Article : Google Scholar : PubMed/NCBI
|