Introduction
Renal cancer is a urinary tumor that affects
individuals worldwide, accounting for ~3% of all systemic
malignancies (1). Furthermore, it
is one of the most common forms of cancer in China. Exosomes are
classically defined as membranous vesicles with a diameter of
30–100 nm and cup-shaped morphology that are secreted by a broad
array of cells during physiological and pathological conditions
(2). These organelles exert
versatile functions due to significant variations in their contents
from the originating cell, including a large array of proteins,
RNA, mRNA and lipids (2,3). Previous studies reported that
extracellular organelles are important mediators of intercellular
community (4–9). Tumor cell-derived exosomes are
associated with numerous events in cancer pathogenesis and
development, including tumor angiogenesis (10).
Beginning at the early phases of the neoplastic
process, tumor cells begin to manipulate the host environment to
favor their survival and growth (11). Vessels are markedly associated with
the pathogenesis and development of tumors and it has been
demonstrated that cells at the preneoplastic stage must acquire
angiogenic capacity to become malignant cells. Without blood
vessels, tumors cannot grow and form metastases (12–14).
Vascular endothelial growth factor (VEGF) is the most important
factor for the induction and regulation of proliferation of
vascular endothelial cells as well as angiogenesis in physiological
and pathological conditions. High expression of VEGF has been
detected in kidney cancer tissue and serum (3,15).
Thus, VEGF is an important target for studies in renal cancer
immunity therapy. Hepatocyte cell adhesion molecule (hepaCAM) was
previously identified as a novel member of the immunoglobulin super
family and was undetectable or expressed at low levels in a number
of cancer cells and tissues, including renal cancer (16–18).
Therefore, hepaCAM has been hypothesized to be a candidate tumor
suppressor gene. In our previous study, hepaCAM was undetectable in
transitional cell carcinoma of bladder cell lines T24 and BIU-87,
and low hepaCAM levels were found to correlate with increased VEGF
levels (19), indicating that
hepaCAM is important in suppression of tumor angiogenesis.
Thus, the aim of the current study was to determine
whether renal cancer-derived exosomes upregulate VEGF expression
via the downregulation of hepaCAM expression, leading to the
promotion of angiogenesis.
Materials and methods
Cell lines and culture
Human renal cancer cell line, 786-0 and human
umbilical vein endothelial cell (HUVEC) line, hy-926, were gifts
from the College of Laboratory Medicine (Chongqing Medical
University). The cell lines were maintained in RPMI-1640 medium
(Gibco-BRL, Shanghai, China) supplemented with 10% fetal bovine
serum (Hyclone Laboratories, Inc., Logan, UT, USA) in a 5%
CO2 humid incubator at 37°C. Experiments were performed
at the cell logarithmic growth phase.
Adenovirus transfection
When cell confluence reached 90%, serum-free medium
was exchanged and the adenovirus solution (recombinant adenovirus
AdI-EGFP and AdI-hepaCAM) was added to the flask. Complete medum
(RPMI-1640 medium supplemented with 10% fetal bovine serum) was
added following 1.5 h and protein from each group was extracted
following 72-h incubation.
Extraction and identification of
exosomes
Supernatants of cultured 786-0 cells were collected
and subsequently centrifuged at 4°C at 300 × g for 10 min, 800 × g
for 30 min and 10,000 × g for 30 min to deposit cells and debris.
Supernatants were concentrated by ultrafiltration using a 100 kDa
MWCO Centriplus centrifugal ultrafiltration tube (Millipore,
Billerica, MA, USA) at 1,000 × g for 30 min. Remaining supernatants
were concentrated and subjected to ultracentrifugation in a
centrifugal ultrafiltration tube containing 30% sucrose in heavy
water (Tenglong Weibo Technology, Qingdao, China) at 100,000 × g
for 1 h at 4°C. The sucrose solution was collected and diluted with
phosphate-buffered saline, followed by concentration using an
additional 100 kDa MWCO Centriplus centrifugal ultrafiltration tube
at 1,000 × g for 30 min. Finally, the remaining exosome-containing
solution was collected, filtered through a 0.22 μm filter,
aliquoted and stored at −80°C. Exosomes were characterized by
transmission electron microscopy.
Exosome suspension (20 μl) was dropped on the
copper-net and the sample was dried using filter paper 1 min later.
Subsequently, the sample was negatively stained by 2% Salkowski’s
solution for 1 min and dried using incandescent lights for 10 min.
The sample was then observed and images were captured using
transmission electron microscopy.
Matrigel tubular assay
Matrigel (BD Biosciences, Franklin Lakes, NJ, USA)
was thawed at 4°C, applied to a 24-well plate and incubated at 37°C
for 12 h to allow solidification. Then, hy926 cells were seeded
onto the matrigel at 1×105 cells/well with or without
renal cell-derived exosomes. Following incubaton for 72 h, tubular
formation of the cells was observed and images were captured. The
assay was performed in 5 wells/group.
Reverse transcription-polymerase chain
reaction (RT-PCR) analysis
Total RNA was isolated from cells using TRIzol
(Takara Bio, Inc., Shiga, Japan) and semi-quantitative RT-PCR was
performed using the Two-step RT-PCR kit (Takara Bio, Inc.)
following the manufacturer’s instructions. Primers were designed
using Primer Premier 5.0 (Premier Biosoft, Palo Alto, CA, USA) and
gene primer specificity was confirmed by BLAST search using the
GeneBank database. The primers used were: hepaCAM, forward: 5′-TAC
TGT AGA TGT GCC CAT TTC G-3′ and reverse: 5′-CTT CTG GTT TCA GGC
GGT C-3′; VEGF, forward: 5′-GTC CAA CTT CTG GGC TGT TCT-3′ and
reverse: 5′-ACC ACT TCG TGA TGA TTC TGC-3′; and β-actin (loading
control), forward: 5′-TGA CGT GGA CAT CCG CAA AG-3′ and reverse:
5′-CTG GAA GGT GGA CAG CGA GG-3′. Amplified hepaCAM, VEGF and
β-actin fragments were 461, 497 and 205-bp in length, respectively.
Total RNA was reverse transcribed and RT-PCR was performed using 1
μl cDNA and primers for relevant genes under the following
optimized conditions: predenaturation, 95°C for 5 min; 35 cycles of
denaturation at 95°C for 30 sec, annealing at 56°C, 59°C or 56°C
for 30 sec and extension at 72°C for 1 min; and final extension,
72°C for 5 min. Products were analyzed by 1.5% gel electrophoresis
using a Bio-Rad imaging plate (Bio-Rad, Hercules, CA, USA).
Western blot analysis
Cells were solubilized in lysis buffer (Beyotime
Institute of Biotechnology, Jiangsu, China) containing 1 μl
phenylmethanesulfonyl fluoride and then centrifuged at 4°C at 13200
× g for 5 min to obtain the supernatant. The concentration was
determined by BCA method. Proteins were separated using 10% sodium
dodecyl sulfate-polyacrylamide gel electrophoresis and the protein
bands were transferred to polyvinylidene fluoride membranes
(Amersham Pharmacia Biotech, Amersham, UK). Membranes were blocked
in 5% skimmed milk for 2 h and incubated with anti-hepaCAM (Wuhan
Sanying Biotechnology Inc., Wuhan, China), anti-VEGF and
anti-β-actin (Wuhan Boster Biological Technology, Ltd., Wuhan,
China) antibodies overnight at 4°C. Following three 10 min washes
with TBST, membranes were incubated with HRP-conjugated secondary
antibody for 1.5 h. Membranes were washed again (three 10 min
washes with TBST) and the immunoreactive bands were detected using
an enhanced chemoluminescence kit (Beyotime Institute of
Biotechnology, China) in the dark. β-actin was used as an internal
control. The intensity of the protein bands was quantified using
Quantity-One software (Bio-Rad).
Statistical analysis
Statistical differences between the groups were
analyzed using the Kruskal-Wallis test. Data are presented as mean
± SD. P<0.05 was considered to indicate a statistically
significant difference.
Results
Morphological identification of
exosomes
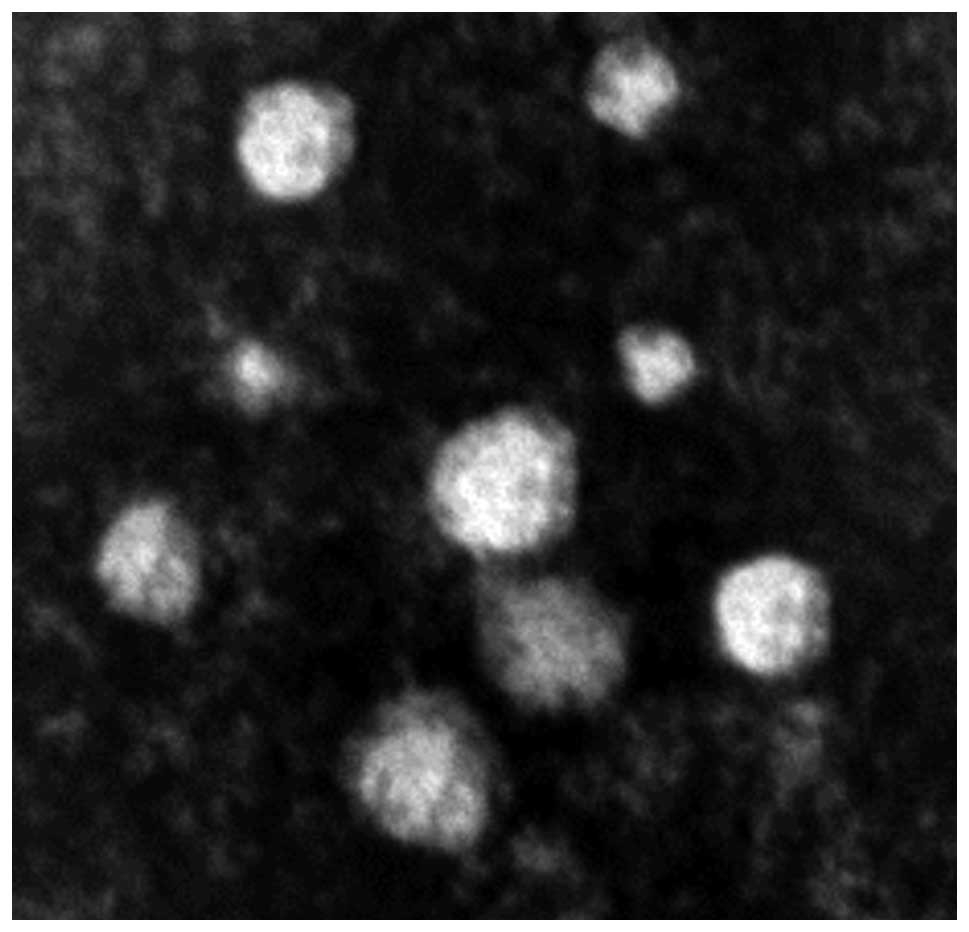
TEM analysis of exosomes indicated that typical
characteristics of a cup-shaped or saucer-like structure with a
size ranging from 30–100 nm in diameter (Fig. 1).
In vitro tube formation of
exosome-treated HUVECs is markedly increased by treatment with
exosomes
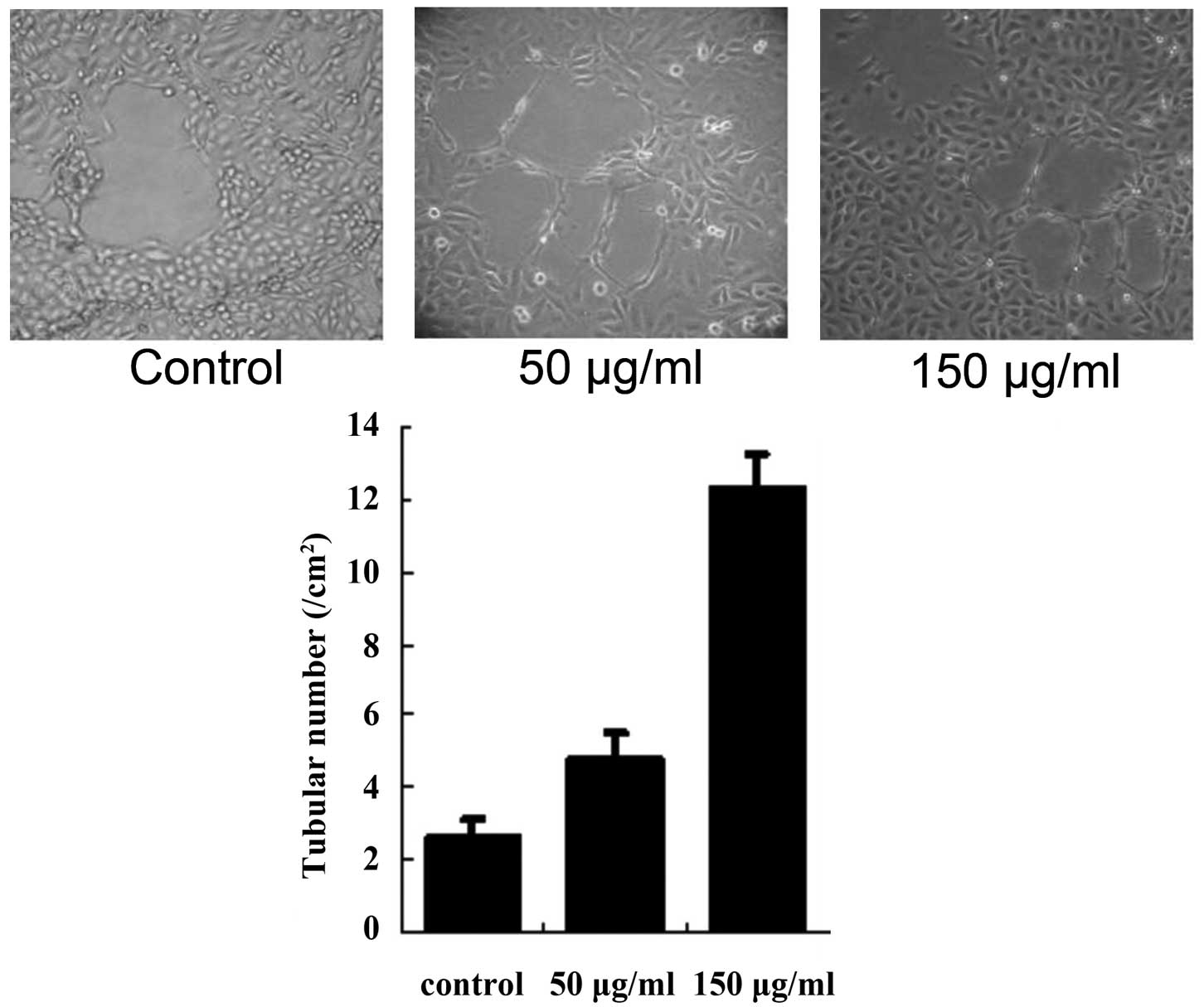
HUVECs formed tubular structures in the matrigel and
the effect was examined 72 h following treatment. Compared with the
control group, cells treated with 50 or 150 μg/ml exosomes
exhibited a marked increase in the formation of tubular structures
(both P<0.01; Fig. 2).
Re-expression of hepaCAM in 786-0 cells
downregulates VEGF protein expression
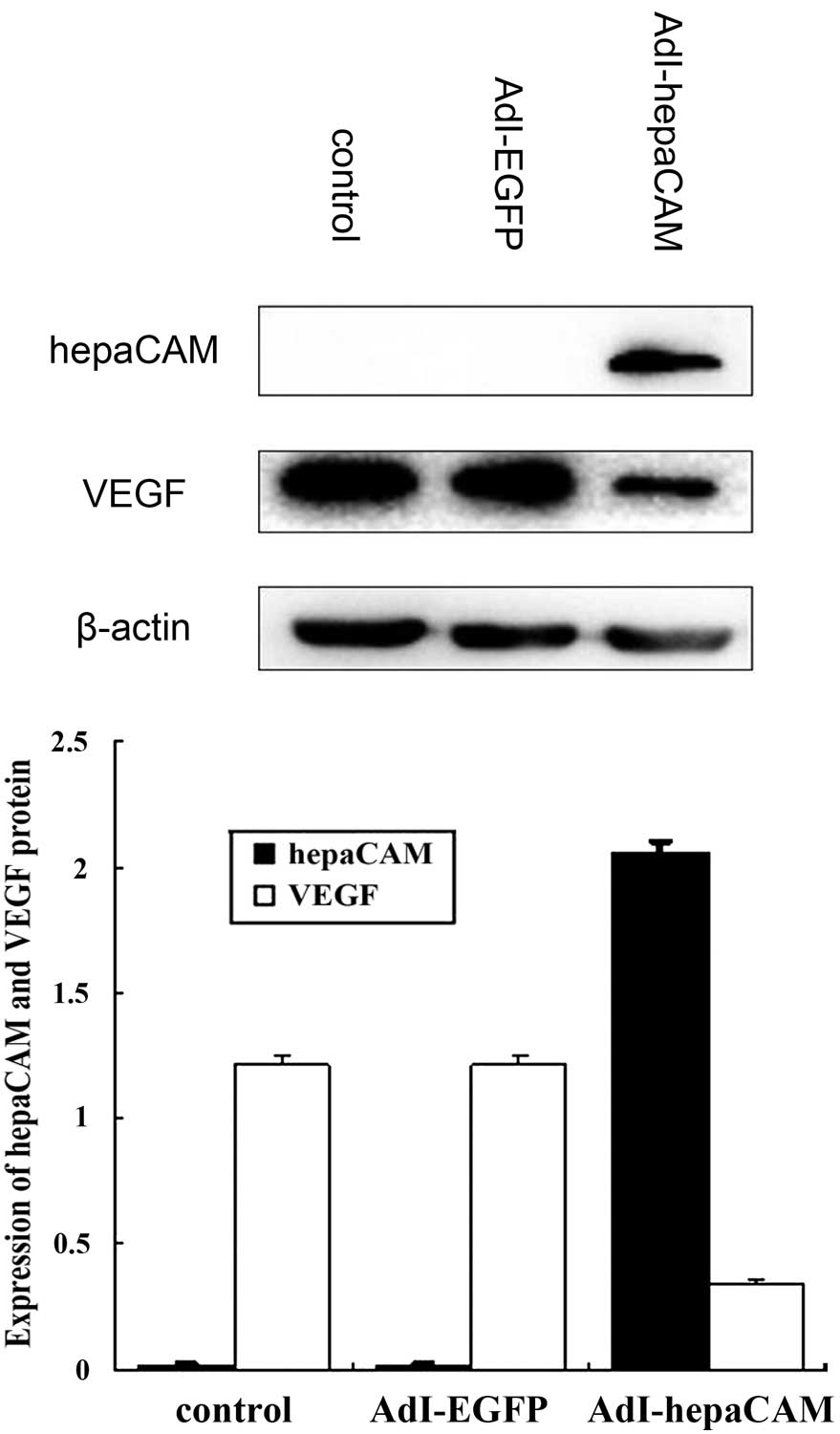
hepaCAM expression was not detected in 786-0 cells
and high VEGF expression was observed. Following transfection with
AdI-hepaCAM, cells revealed increasing hepaCAM expression and
decreasing VEGF expression compared with the AdI-EGFP group (both
P<0.01; Fig. 3).
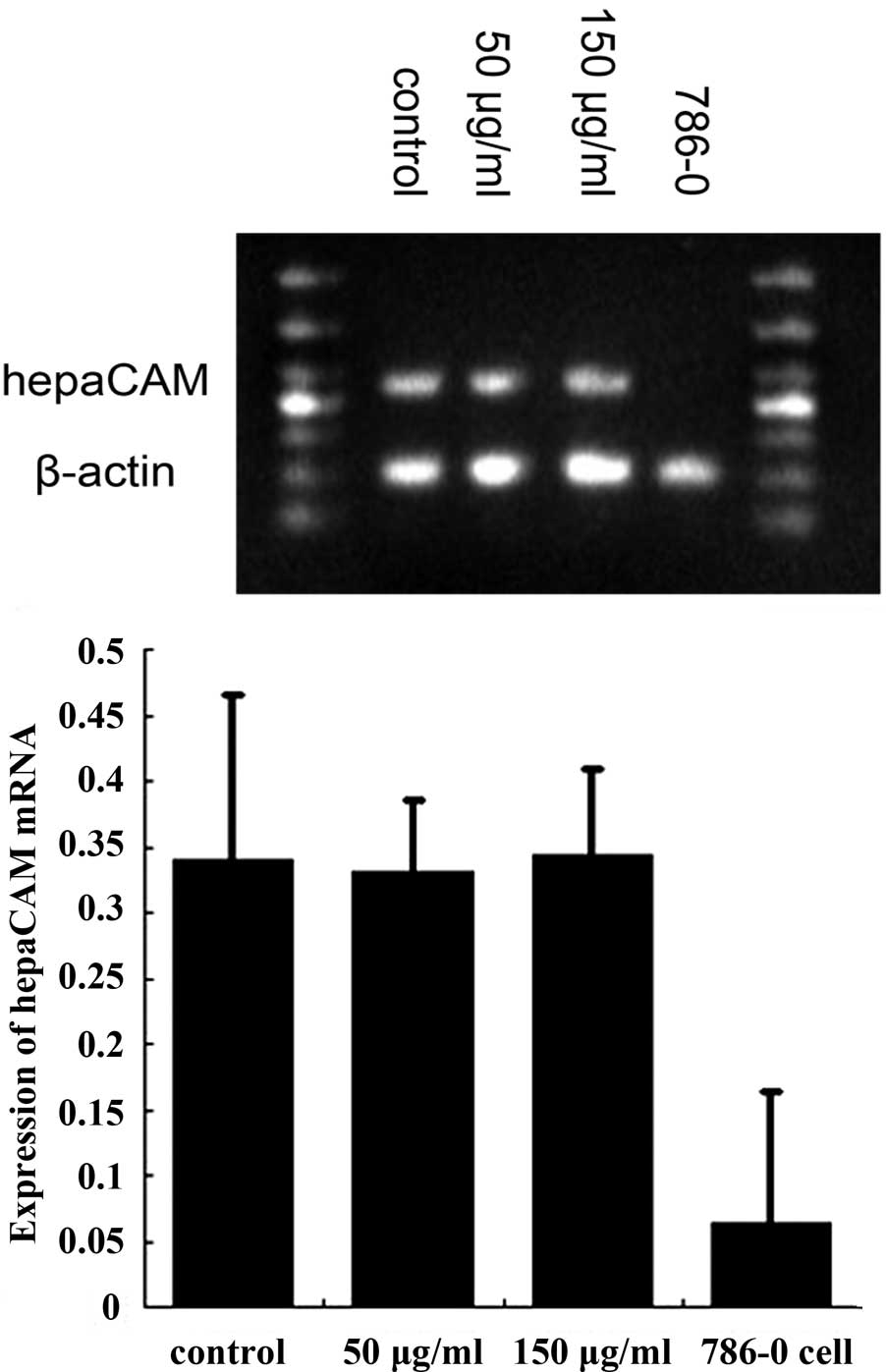
hepaCAM and VEGF expression in HUVECs
following treatment with exosomes
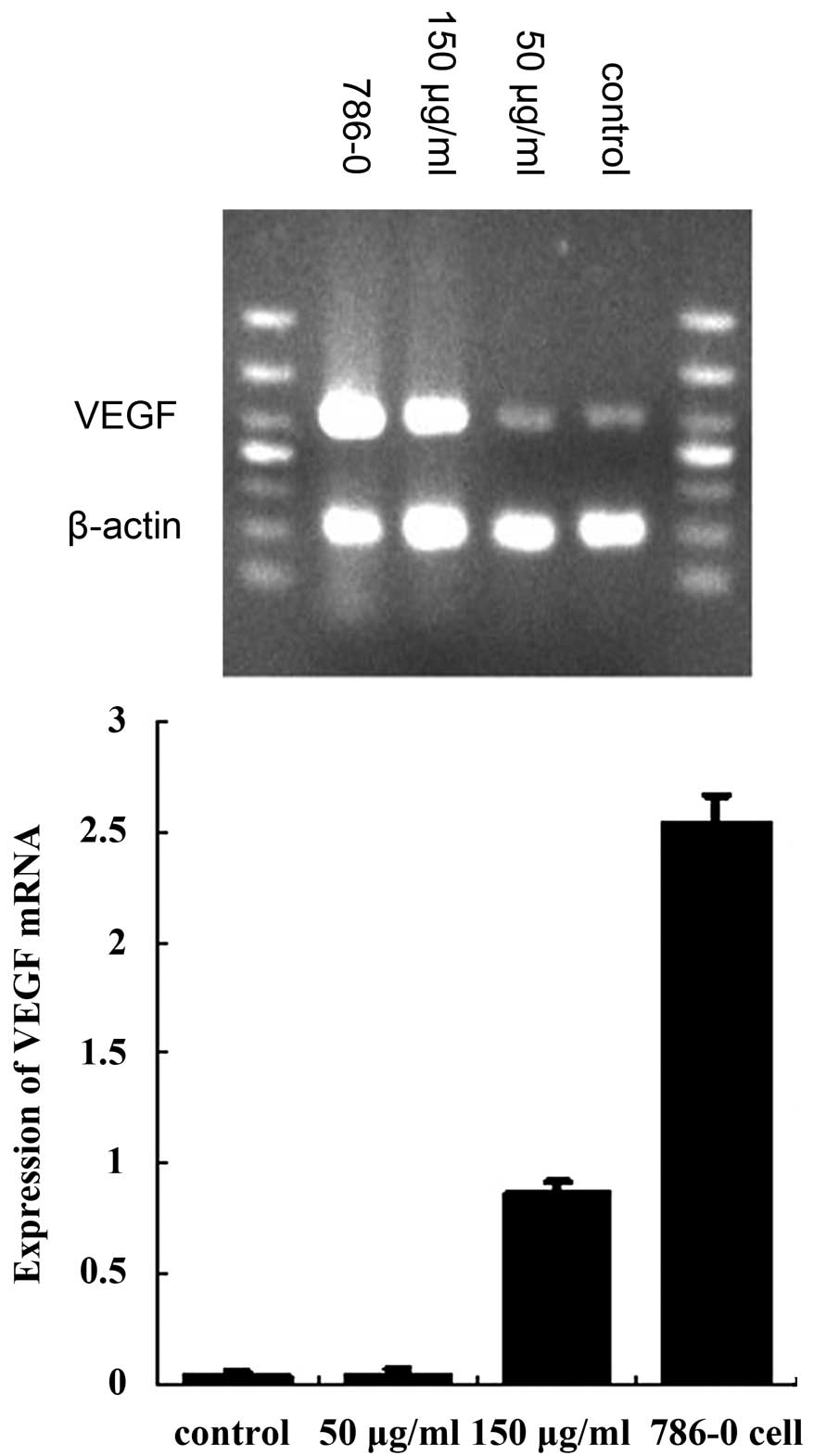
HUVECs expressed high levels of hepaCAM mRNA and a
low expression of VEGF mRNA. Following treatment with renal cancer
cell-derived exosomes, VEGF mRNA expression was found to be
markedly decreased compared with the control group (50 and 150
μg/ml, both P<0.01; Fig. 4).
Expression of hepaCAM mRNA was not found to be statistically
significant compared with the control (50 and 150 μg/ml, both
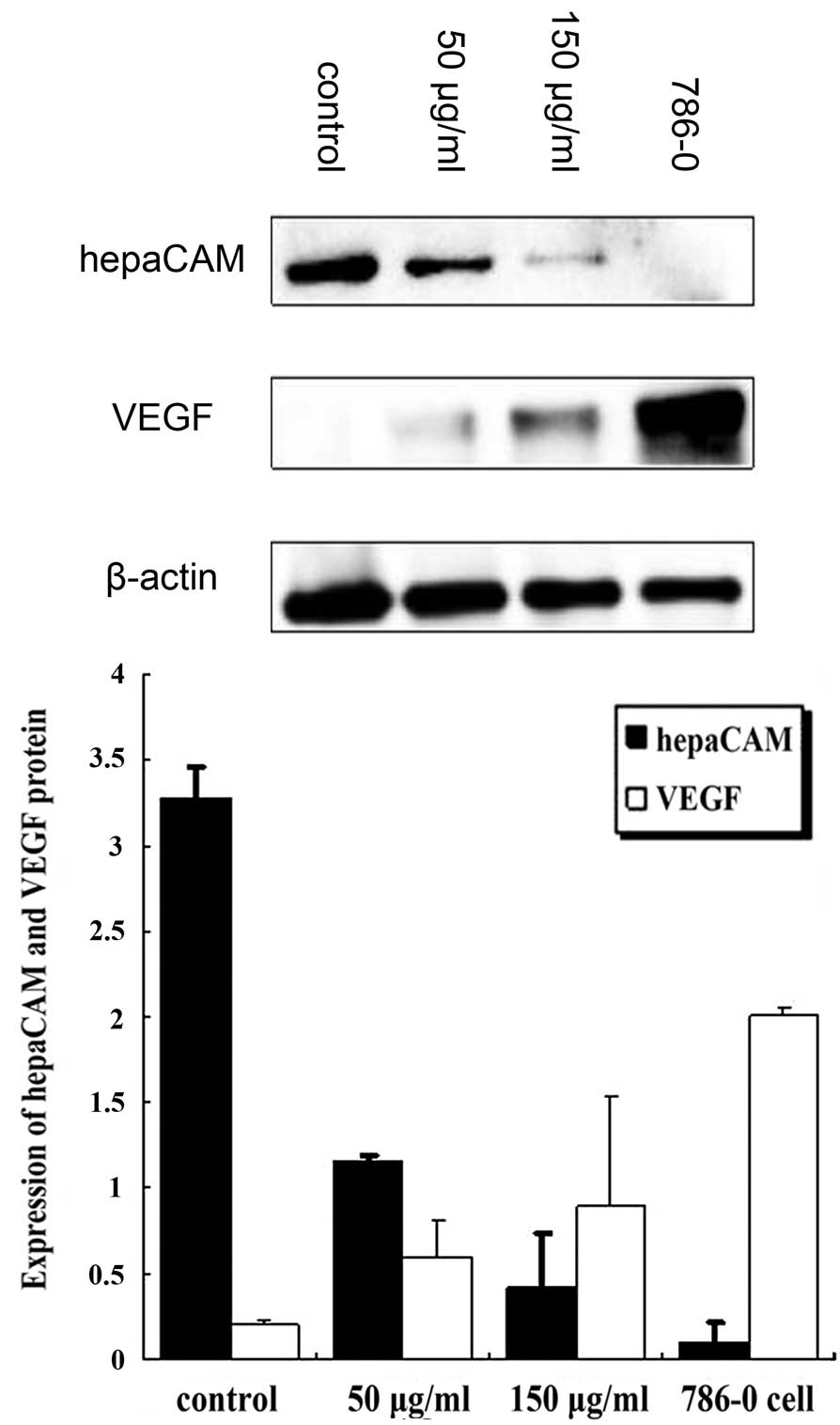
P<0.01; Fig. 5). Western blot
analysis revealed that, compared with the control group, VEGF
protein expression was markedly increased (50 and 150 μg/ml, both
P<0.01) and hepaCAM protein expression was significantly
decreased (50 and 150 μg/ml, both P<0.01). Levels of VEGF were
found to inversely correlate with that of hepaCAM (Fig. 6).
Discussion
Extensive studies on tumor cell-derived exosomes and
their roles in intercellular communication in the tumor
microenvironment have been performed (6,8,9,20–24).
The tumor cell-derived exosome is known to manipulate the
surrounding microenvironment to promote angiogenesis, invasion and
metastasis, as well as escape immune surveillance (10). In the present study, exosomes
secreted from the human renal cancer cell line, 786-0, were found
to facilitate tubular formation via regulation of hepaCAM and VEGF
expression of HUVECs.
Consistent with a number of previous studies
(10,25–27),
786-0 cell-derived exosomes were observed to increase the formation
of tubular stuctures in HUVECs compared with the control group.
However, the underlying molecular mechanism of this effect remains
unclear. Al-Nedawi et al revealed that exosomes transfer the
oncogenic form of EGFR, EGFRvIII, from glioblastoma multiforme
cells to endothelial cells (24),
resulting in EGFRvIII-driven endothelial expression of autocrine
VEGF. In the present study, expression of VEGF, an important factor
in angiogenesis in physiological and pathological conditions, was
upregulated in HUVECs at the mRNA and protein levels following
treatment with cancer cell-derived exosomes, while hepaCAM protein
levels decreased. In addition, re-expression of hepaCAM markedly
reduced the expression of VEGF in 786-0 cells, which was consistent
with our previous study (19).
Induction of angiogenesis by VEGF has been found to be facilitated
by the downregulation of expression (28) or decreasing stability of p53
(29) and is inhibited when p53
protein is upregulated (30).
Re-expression of hepaCAM elevates p53 protein levels, while the
knockdown of endogenous p53 expression via small-interfering RNA
alleviates the proliferation inhibition of hepaCAM (17). In the current study, decreased
hepaCAM partly induced increased levels of VEGF in HUVECs and the
p53 signaling pathway was hypothesized to be be involved in this
process.
In addition, no significant change in hepaCAM mRNA
expression was identified and the lower protein level may be
associated with post-transcriptional regulation. The specific
mechanism by which hepaCAM protein is reduced and the associated
signaling pathway requires further analysis. In a previous study,
Zhang et al analyzed human breast carcinoma MCF7 cells,
identifying a cleaved form of hepaCAM associated with the
proteasome, calpain-1 and cathepsin B (18). Tumor cell-generated exosomes may
directly modify adhesion molecules following transfer of these
enzymes from parent to recipient cells. By contrast, specific
immunoglobulin superfamily adhesion molecules, including ICAM-1,
from activated endothelial cells are shed in soluble form, which
may promote angiogenesis (31).
Exosomes may also affect hepaCAM expression by activating the
endothelial cells and shedding them from the membrane. In addition,
the signaling pathways associated with exosome regulation of
hepaCAM and VEGF remain unknown and require additional
analysis.
In the present study, renal cancer 786-0
cell-derived exosomes significantly promoted angiogenesis via
upregulation of VEGF expression in HUVECs, which may be induced by
the downregulation of hepaCAM.
Acknowledgements
The authors would like to thank Dr Shali Shen for
the kind support throughout the study and Professor Weixue Tang for
providing technical guidance.
References
|
1
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar
|
|
2
|
Keller S, Sanderson MP, Stoeck A and
Altevogt P: Exosomes: from biogenesis and secretion to biological
function. Immunol Lett. 107:102–108. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Iero M, Valenti R, Huber V, et al:
Tumour-released exosomes and their implications in cancer immunity.
Cell Death Differ. 15:80–88. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ratajczak J, Wysoczynski M, Hayek F,
Janowska-Wieczorek A and Ratajczak MZ: Membrane-derived
microvesicles: important and underappreciated mediators of
cell-to-cell communication. Leukemia. 20:1487–1495. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Quesenberry PJ and Aliotta JM: The
paradoxical dynamism of marrow stem cells: considerations of stem
cells, niches and microvesicles. Stem Cell Rev. 4:137–147. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cocucci E, Racchetti G and Meldolesi J:
Shedding microvesicles: artefacts no more. Trends Cell Biol.
19:43–51. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Théry C: Exosomes: secreted vesicles and
intercellular communications. F1000 Biol Rep. 3:152011.PubMed/NCBI
|
|
8
|
Mathivanan S, Ji H and Simpson RJ:
Exosomes: extracellular organelles important in intercellular
communication. J Proteomics. 73:1907–1920. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fevrier B and Raposo G: Exosomes:
endosomal-derived vesicles shipping extracellular messages. Curr
Opin Cell Biol. 16:415–421. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Marleau AM, Chen CS, Joyce JA and Tullis
RH: Exosome removal as a therapeutic adjuvant in cancer. J Transl
Med. 10:1342012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lin WW and Karin M: A cytokine-mediated
link between innate immunity, inflammation and cancer. J Clin
Invest. 117:1175–1183. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Goth MI, Hubina E, Raptis S, Nagy GM and
Toth BE: Physiological and pathological angiogenesis in the
endocrine system. Microsc Res Tech. 60:98–106. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pircher A, Medinger M and Drevs J: Liver
cancer: Targeted future options. World J Hepatol. 3:38–44. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Carmeliet P and Jain RK: Angiogenesis in
cancer and other diseases. Nature. 407:249–257. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang R, Zhang H and Zhu L: Inhibitory
effect of resveratrol on the expression of the VEGF gene and
proliferation in renal cancer cells. Mol Med Rep. 4:981–983.
2011.PubMed/NCBI
|
|
16
|
He Y, Wu X, Luo C, Wang L and Lin J:
Functional significance of the hepaCAM gene in bladder cancer. BMC
Cancer. 10:832010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Moh MC, Zhang T, Lee LH and Shen S:
Expression of hepaCAM is downregulated in cancers and induces
senescence-like growth arrest via a p53/p21-dependent pathway in
human breast cancer cells. Carcinogenesis. 29:2298–2305. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang T, Moh MC, Lee LH and Shen S: The
immunoglobulin-like cell adhesion molecule hepaCAM is cleaved in
the human breast carcinoma MCF7 cells. Int J Oncol. 37:155–165.
2010.PubMed/NCBI
|
|
19
|
Yang S, Wu X, Luo C, Pan C and Pu J:
Expression and clinical significance of hepaCAM and VEGF in
urothelial carcinoma. World J Urol. 28:473–478. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Belting M and Wittrup A: Nanotubes,
exosomes and nucleic acid-binding peptides provide novel mechanisms
of intercellular communication in eukaryotic cells: implications in
health and disease. J Cell Biol. 183:1187–1191. 2008. View Article : Google Scholar
|
|
21
|
Al-Nedawi K, Meehan B and Rak J:
Microvesicles: messengers and mediators of tumor progression. Cell
Cycle. 8:2014–2018. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Skog J, Würdinger T, van Rijn S, et al:
Glioblastoma microvesicles transport RNA and proteins that promote
tumour growth and provide diagnostic biomarkers. Nat Cell Biol.
10:1470–1476. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Al-Nedawi K, Meehan B, Micallef J, et al:
Intercellular transfer of the oncogenic receptor EGFRvIII by
microvesicles derived from tumour cells. Nat Cell Biol. 10:619–624.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Al-Nedawi K, Meehan B, Kerbel RS, Allison
AC and Rak J: Endothelial expression of autocrine VEGF upon the
uptake of tumor-derived microvesicles containing oncogenic EGFR.
Proc Natl Acad Sci USA. 106:3794–3799. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Corrado C, Flugy AM, Taverna S, et al:
Carboxyamidotriazole-orotate inhibits the growth of
imatinib-resistant chronic myeloid leukaemia cells and modulates
exosomes-stimulated angiogenesis. PLoS One. 7:e423102012.
View Article : Google Scholar
|
|
26
|
Grange C, Tapparo M, Collino F, et al:
Microvesicles released from human renal cancer stem cells stimulate
angiogenesis and formation of lung premetastatic niche. Cancer Res.
71:5346–5356. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Martinez MC and Andriantsitohaina R:
Microparticles in angiogenesis: therapeutic potential. Circ Res.
109:110–119. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Song H, Yin D and Liu Z: GDF-15 promotes
angiogenesis through modulating p53/HIF-1alpha signaling pathway in
hypoxic human umbilical vein endothelial cells. Mol Biol Rep.
39:4017–4022. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ma J, Xue Y, Cui W, et al: Ras homolog
gene family, member A promotes p53 degradation and vascular
endothelial growth factor-dependent angiogenesis through an
interaction with murine double minute 2 under hypoxic conditions.
Cancer. 118:4105–4116. 2012. View Article : Google Scholar
|
|
30
|
Ling Y, Chen Y, Chen P, et al: Baicalein
potently suppresses angiogenesis induced by vascular endothelial
growth factor through the p53/Rb signaling pathway leading to G1/S
cell cycle arrest. Exp Biol Med (Maywood). 236:851–858. 2011.
View Article : Google Scholar
|
|
31
|
Reinmuth N, Thomas M, Meister M, Schnabel
PA and Kreuter M: Current data on predictive markers for
anti-angiogenic therapy in thoracic tumours. Eur Respir J.
36:915–924. 2010. View Article : Google Scholar : PubMed/NCBI
|