Introduction
Atherosclerotic arterial disease is the leading
cause of morbidity and mortality in Western countries and is
rapidly increasing in developing nations (1). An important step in atherogenesis is
the infiltration of monocytes within the subendothelial space of
large arteries and phagocytosis of local lipids. This is followed
by differentiation into monocyte-derived foam cells, which
simultaneously release large amounts of inflammatory cytokines that
further promote monocyte aggregation. These steps contribute to a
vicious cycle and are known to directly result in the development
of myocardial infarction and stroke (2). In this process, lipid metabolism
disorders and the inflammatory response were considered key factors
in atherosclerosis formation.
A number of previous studies have described a mouse
model of regression (2–4). First, plaques were developed in ApoE
null (ApoE−/−) mice and a segment of thoracic aortic or
aortic arch was transplanted into the abdominal aorta of a wild
type (WT) recipient, rapidly altering the plasma lipid environment
from hyperlipidemia to a normal lipid level. As a control, an
aortic segment was transplanted into an ApoE−/− mouse.
In the regression environment (WT recipient), the majority of foam
cells disappeared from plaques after 3 days, with no longer visible
after 1 month (5). By contrast, in
the progression environment (ApoE−/− recipient), plaque
size and foam cell content increased in a time-dependent
manner.
A more recent study observed that C-C chemokine
receptor type 7 (CCR7) is induced in foam cells and is functionally
required for regression (6). In
addition, in the regression environment, foam cells were found to
overexpress liver X receptor (LXR) α and ATP-binding cassette (ABC)
A1 and scavenger receptor BI(SR-BI). Inflammation-related factors,
including nuclear factor κB (NF-κB) targets, vascular cell adhesion
protein 1, monocyte chemotactic protein 1 and other inflammatory
factors, were downregulated at the mRNA level in vivo.
NF-κB, is a core nuclear transcription factor in the
inflammatory response, enhancing the expression of various
cytokines and chemical factors in the formation of atherosclerosis
and promoting initiation and progression of atherosclerotic lesions
(7). In a previous study, gene
knockout of the NF-κB subunit, p50, was found to lead to a
significant reduction in atherosclerotic lesions and almost no foam
cells were observed in the lesions of a mouse atherosclerosis model
(LDLR−/−) (8).
Caloric restriction (CR) is known to slow the aging
process and may have the potential to prevent a wide range of
diseases associated with aging, including to reduce the risk of
atherosclerotic disease (9,10).
One of the key factors involved in the mechanism of CR is Sirtuin 1
(SIRT1) (11). SIRT1 transgenic
mice exhibit a number of phenotypes similar to mice on a CR diet.
Mice are leaner than littermate controls, more metabolically
active, exhibit reductions in blood cholesterol, adipokines,
insulin and fasting glucose levels and have increased glucose
tolerance (12). SIRT1 activation
represents a promising therapeutic approach for the prevention of
atherosclerosis via multiple pathways (13,14).
Li et al previously reported that SIRT1
positively regulates LXRs by deacetylation at lysine K432 and is
important for cholesterol homeostasis. SIRT1−/− cells
were found to exhibit defective cholesterol efflux and reduced
ABCA1 gene expression (15). In
addition, a more recent study found that SIRT1 overexpression in
transgenic mice inhibited NF-κB activity induced by high-fat foods
and reduced specific proinflammatory cytokines, including tumor
necrosis factor α (TNFα) and interleukin (IL)-6, promoting fatty
liver (16). By contrast,
SIRT1-knockout mice administered with a high-fat diet were found to
be prone to inflammation of the liver and fatty liver formation
(17).
Therefore, in the present study, we hypothesized
that SIRT1 expression is inhibited during monocyte-derived foam
cell formation, resulting in downregulation of LXR-ABCA1/ABCG1/CCR7
and upregulation of NF-κB proinflammatory signaling pathways,
leading to foam cell formation and blockage of foam cell
elimination from atherosclerotic plaques. To verify this, mRNA and
protein levels of genes involved in monocyte-derived foam cell
formation in U937 cells treated with palmitate and Ox-LDL were
analyzed and observations confirmed the association of SIRT1 with
LXR and NF-κB.
Materials and methods
Materials
RPMI-1640 medium was obtained from Gibco-BRL
(Carlsbad, CA, USA). Oil Red O was purchased from Bio Basic Inc.
(Markham, ON, Canada). Sodium palmitate, nicotinamide and phorbol
12-myristate 13-acetate (PMA) were purchased from Sigma-Aldrich
(St. Louis, MO, USA). SRT1720 was purchased from Selleck Chemicals
LLC (Houston, TX, USA). For cell treatment, SRT1720 stock solution
was prepared in ultra-pure water; stock solution of sodium
palmitate was dissolved in a bovine serum albumin/Hanks Balanced
Salt Solution (BSA/HBSS) mixture, as described previously (18,19).
Cell culture and treatment
Human mononuclear U937 cells were grown and
maintained in culture in RPMI-1640 medium (Life Technologies, Grand
Island, NY, USA) supplemented with 10% FBS, penicillin (100 U/ml)
and streptomycin (100 mg/ml) in a 5% CO2 atmosphere at
37°C. For each experiment, U937 cells were plated in 6-well plates
(3×105 cells/ml) in RPMI-1640 medium containing 0.2 μM/l
PMA. After 24 h, cells were harvested.
Cells were divided into five groups: the control,
CR, high fat (HF) + OxLDL, HF + Ox-LDL + SRT1720 and HF + Ox-LDL +
SRT1720 (SRT) + nicotinamide (Nam) groups. Control cells were
cultured in RPMI-1640 medium containing 0.45% glucose only. In HF
groups, cells were exposed to a medium containing 0.2 mM palmitate
and 0.45% glucose. Similarly, for groups treated with Ox-LDL, cells
were exposed to medium containing 80 μg/ml Ox-LDL. CR groups were
treated with medium containing 0.1% glucose. For groups without
palmitate, the same concentration of BSA/HBSS mixture was added to
the medium. In all the experiments, cells were incubated for 24
h.
Cell viability assay
The viability of U937 cells was measured by an MTT
assay. The cells were grown in 96-well plates (2.5×104
cells/well) with 100 μl medium. The medium was refreshed with
various concentrations of sodium palmitate (0.05–0.8 mM). For the
control group, the same concentration of vehicle was added to the
medium. Following culture for 24 h, cells were incubated with MTT
for 4 h at 37°C. Next, triple liquid (10% SDS, 5% isobutanol, 0.012
mol/l hydrochloric acid, dissolved in distilled water) was added to
each well. The absorbance of samples was measured at 570 nm using a
microtiter plate reader (Multiskan MK3, Thermo Scientific,
Helsinki, Finland). All the experiments were performed
independently in triplicate.
Oil Red O staining
Cells were fixed in 4% paraformaldehyde in PBS for
20 min and stained with freshly diluted Oil Red O solution (6 parts
0.1% Oil Red O in isopropyl alcohol and 4 parts water) for 2 h at
room temperature. Cell images were captured under a microscope. For
quantitative analysis of cellular triglycerides, 2.5×104
cells were stained with freshly diluted Oil Red O solution for 2 h
at room temperature in every Eppendorf tube. Oil Red O was removed
by centrifugation and cells were extensively rinsed with water.
Excess water was evaporated by placing the stained culture in an
incubator at 37°C. Next, 200 μl isopropyl alcohol was added to
every EP tube. The extracted dye was immediately removed by gentle
pipetting and absorbance was monitored using a spectrophotometer at
510 nm.
Reverse transcription-polymerase chain
reaction (RT-PCR) analysis
Total cellular RNA was isolated from cells using an
RNA extraction kit (Aidlab Biotechnologies Co., Ltd., Beijing,
China) according to the manufacturer’s instructions. cDNA was
synthesized from 1 μg total RNA using a cDNA synthesis kit
(TransGen Biotech Co., Ltd., Beijing, China). Primer sequences are
presented in Table I and were
synthesized by GeneCore Biotechnologies Co., Ltd. (Shanghai,
China). Each PCR was performed with 1 μl cDNA product and 20 pM of
each primer in a final volume of 25 μl using a PCR kit (Aidlab
Biotechnologies Co., Ltd.) as follows: initial denaturation at 94°C
for 3 min; 32 or 35 cycles of denaturation for 30 sec at 94°C,
annealing for 30 sec at 58°C and elongation for 1 min at 72°C;
followed by a final extension step for 5 min at 72°C. PCR products
were electrophoresed on 1% agarose gel and visualized by ethidium
bromide staining. The relative intensities of the bands were
quantified by densitometric analysis and normalized against
corresponding 18S band densities.
 | Table ISpecific primers used in PCR. |
Table I
Specific primers used in PCR.
| Genes | Forward (5′-3′) | Reverse (5′-3′) |
|---|
| SIRT1 |
AGTGGCATTCCAGACTTCAGA |
GGGCTTGTAGTTTCCAGGGTA |
| NF-κB |
ACATGGTGGTCGGCTTCGCA |
TGCAGAGCTGCTTGGCGGAT |
| IL-1β |
GGACAGGATATGGAGCAACAAG |
TTCAACACGCAGGACAGGTA |
| TNFα |
TCAGCAAGGACAGCAGAGG |
CCACGATCAGGAAGGAGAAGA |
| LXRα |
TCTGCGGTGGAGCTGTGGAA |
TGACGCTGGGCGGAAGAAT |
| ABCA1 |
GGAGCAGGCAATCATCAG |
ACACGGACAGGAAGACAA |
| ABCG1 |
GGTCATCCTCTCCATCTATG |
CAATCTGCCTACATCTTCCT |
| CCR7 |
ACACCAGACAGACAACAC |
CTCACCAAGCCAAGAAGT |
| 18S rRNA |
TTGGTGGAGCGATTTGTCTG |
AATGGGGTTCAACGGGTTAC |
Statistical analysis
All experiments were performed at least three times.
Values are expressed as mean ± SD. Results were analyzed with
unpaired Student’s t-test or one-way ANOVA using the statistical
software GraphPad Prism 5.01. P<0.05 was considered to indicate
a statistically significant difference.
Results
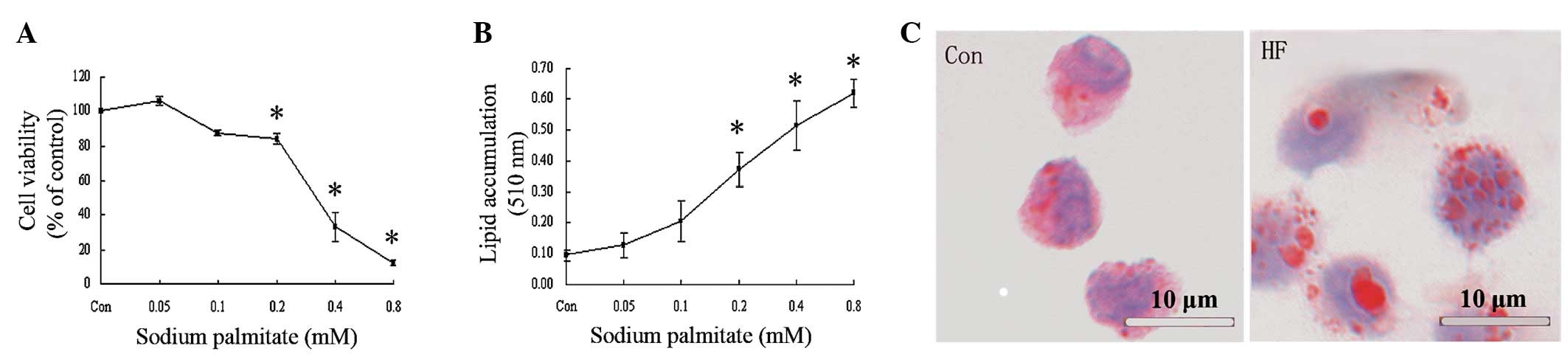
Effect of palmitate on cell viability and
cellular triglyceride accumulation
The effect of palmitate on cell viability was
determined by an MTT assay (Fig.
1A) and 0.05 mM palmitate was revealed to slightly promote cell
growth. Cell survival rate decreased with increasing concentrations
of palmitate, for example, 0.2 and 0.4 mM palmitate decreased cell
viability in a dose-dependent manner by 15.75±3.07 and 66.97±8.53%,
respectively. Quantitative analysis of cellular triglycerides was
performed to investigate the dose-dependent effect of palmitate on
lipid accumulation in U937 cells. As demonstrated in Fig. 1B, ≥0.2 mM palmitate was found to
significantly increase cellular lipid accumulation compared with
the control. Oil Red O staining revealed that an increased number
and larger lipid droplets accumulated in cells cultured with 0.2 mM
palmitate (Fig. 1C). Based on
these observations, 0.2 mM palmitate was used to induce the cell
model of monocyte-derived foam cell formation to achieve maximal
fat accumulation with minimal cytotoxicity.
mRNA expression of SIRT1 and its target
genes during foam cell formation
The foam cell model was induced by palmitate and
Ox-LDL treatment (20,21). U937 cells were cultured in control,
CR or HF + Ox-LDL for 24 h and mRNA expression of SIRT1, LXRα,
NF-κB and their targets was assessed by RT-PCR. As demonstrated in
Fig. 2, the expression of SIRT1,
LXRα and its targets, ABCA1, ABCG1 and CCR7, was downregulated;
however, NF-κB and its targets, IL-1β and TNFα, were upregulated in
the HF + OxLDL culture. By contrast, in the CR group, SIRT1
expression was upregulated, whereas NF-κB and its targets, IL-1β
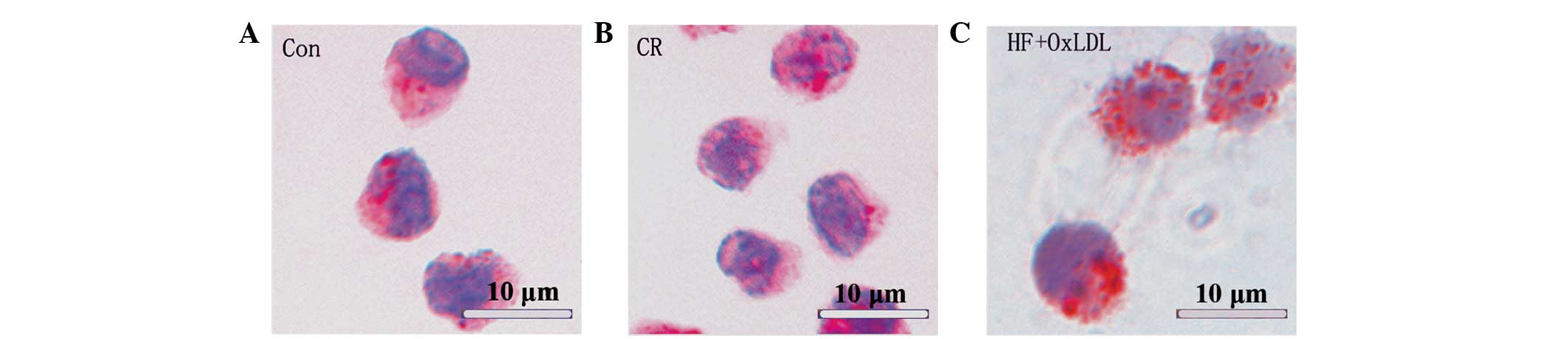
and TNFα, were downregulated. Oil red O staining revealed that
triglyceride accumulation in cells of the HF + OxLDL group was
significantly higher than that of the control and CR groups
(Fig. 3).
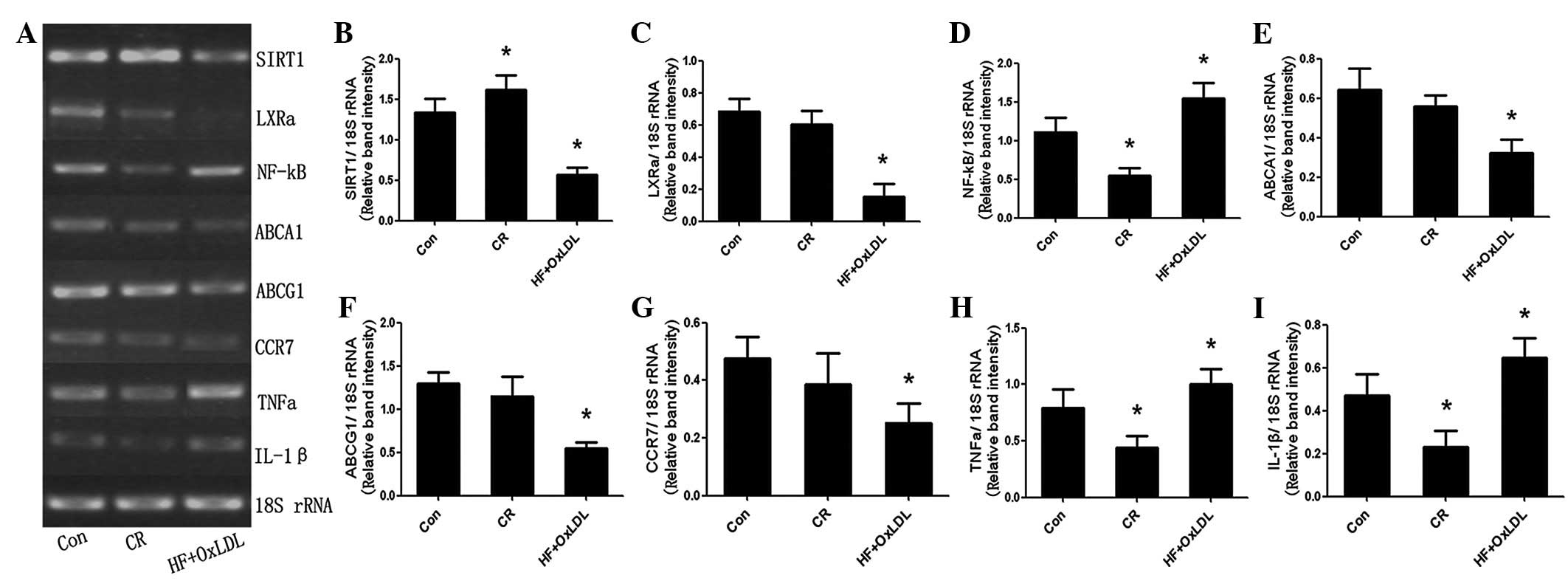 | Figure 2mRNA expression levels in control, CR
and HF + OxLDL cultures, as determined by RT-PCR. (A)
Electrophoresis and quantification of (B) SIRT1, (C) LXRα, (D)
NF-κB, (E) ABCA1, (F) ABCG1, (G) CCR7, (H) TNFα and (I) IL-1β
expression. *P<0.05 vs. control. Con, control; CR,
caloric restriction; SIRT1, Sirtuin 1; NF-κB, nuclear factor κB;
IL-1β, interleukin-1β; TNFα, tumor necrosis factor α; LXRα, liver X
receptor α; ABC, ATP-binding cassette; CCR7, C-C chemokine receptor
type 7; HF, high fat. |
mRNA expression of LXRα, NF-κB and their
targets following activation of SIRT1 under HF + Ox-LDL culture
conditions
Results of RT-PCR demonstrated that the expression
of SIRT1, LXRα and its targets, ABCA1, ABCG1 and CCR7, was
downregulated; however, NF-κB and its targets, IL-1β and TNFα, were
upregulated 24 h after incubation in the HF culture with 80 μg/ml
Ox-LDL. SRT1720 has been identified as a specific activator of
SIRT1 and the activity of SRT1720 is increased 1,000-fold compared
with that of resveratrol (22).
Following stimulation with 6.0 μM/ml SRT1720 for 24 h, the
expression of SIRT1 was unchanged. However, the expression of LXRα
and its target genes (ABCA1, ABCG1 and CCR7)increased, whereas
expression of NF-κB and its target genes (IL-1β and TNFα)
decreased. Treatment for 24 h with 20 mM nicotinamide, the most
potent inhibitor of Sir2 enzymes (23), was found to eliminate the effects
of SRT1720 (Fig. 4).
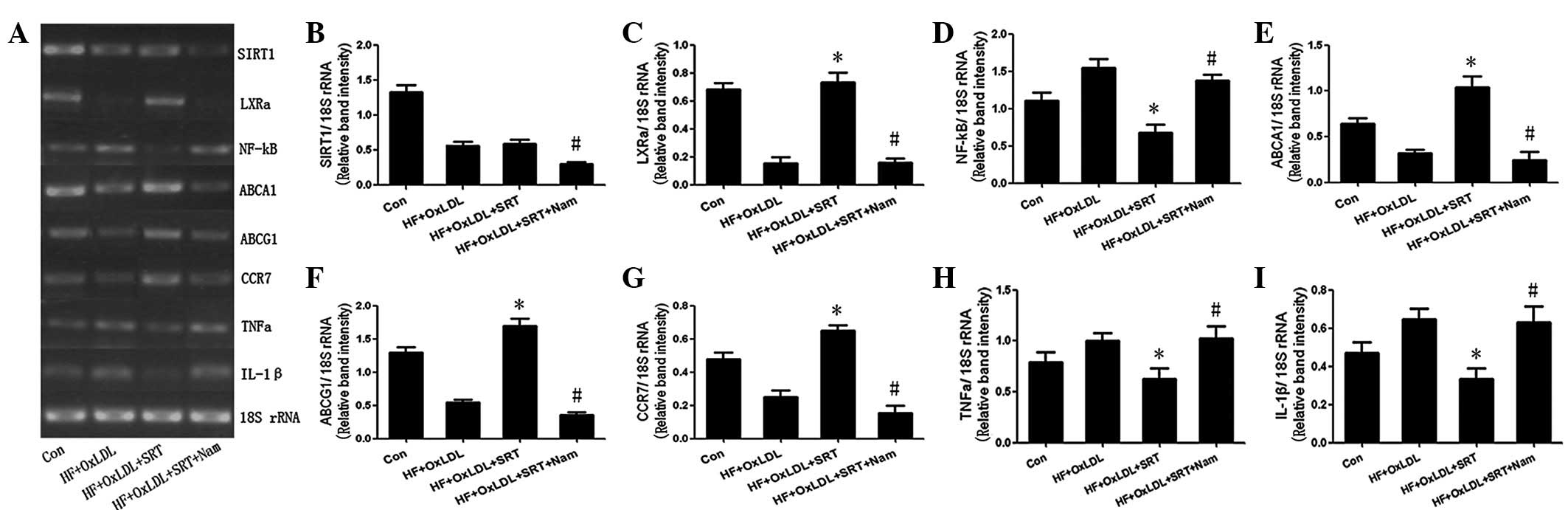 | Figure 4mRNA expression levels in control, HF
+ OxLDL, HF + OxLDL + SRT and HF + OxLDL + SRT + Nam cultures, as
determined by RT-PCR. (A) Electrophoresis and quantification of (B)
SIRT1, (C) LXRα, (D) NF-κB, (E) ABCA1, (F) ABCG1, (G) CCR7, (H)
TNFα and (I) IL-1β expression. *P<0.05, vs. HF +
OxLDL; #P<0.05, vs. HF + OxLDL + SRT. SIRT1, Sirtuin
1; NF-κB, nuclear factor κB; IL-1β, interleukin-1β; TNFα, tumor
necrosis factor α; LXRα, liver X receptor α; ABC, ATP-binding
cassette; CCR7, C-C chemokine receptor type 7; HF, high fat; CR,
caloric restriction; Con, control; SRT, SRT1720; Nam,
nicotinamide. |
Discussion
An increasing number of studies have demonstrated
that SIRT1 is a key regulator in protection from atherosclerosis
formation and progression (13,14).
SIRT1 not only regulates inflammatory processes and cholesterol
metabolism in macrophages, but also suppresses the expression of
the scavenger receptor Lox-1 in macrophages, reduces the uptake of
Ox-LDL and prevents monocyte-derived foam cell formation (13,24).
However, the mechanisms by which these processes are mediated
remain unknown. In the current study, the mechanisms of SIRT1 in
the prevention of atherosclerosis were analyzed.
The function of peripheral blood monocyte
infiltration into the subendothelial space is to clear local
lipids. During atherosclerosis, these cells not only lose their
clearance function, but are also unable to be removed from
atherosclerotic plaques to return to the lymphatic system or
bloodstream. Regardless of this, monocytes within the arterial wall
may be able to maintain their original clearance potential,
although it may be blocked or inhibited. de Kreutzenberg et
al(20) found that mRNA and
protein expression of SIRT1 in peripheral blood mononuclear cells
was significantly reduced in association with insulin resistance
and metabolic syndrome. Song et al(25) also revealed that the expression of
SIRT1 in blood mononuclear cells was significantly lower in
patients with high cholesterol, hyperglycemia or diabetes than in
healthy individuals. By contrast, under CR conditions, SIRT1
expression was increased (26). In
the present study, expression of SIRT1 was found to decrease in
high glucose and high lipid environments, representative of the
blood of patients with atherosclerosis, compared with cells
cultured in control conditions. In addition, SIRT1 expression
significantly increased under conditions of CR. These results are
consistent with previous studies and demonstrate that SIRT1
downregulation may be associated with development of
atherosclerosis.
LXR serves as a cholesterol sensor to protect the
organism from cholesterol overload. LXR is deacetyled by SIRT1 at a
single conserved lysine (K432 in LXRα and K433 in LXRβ) adjacent to
the ligand-regulated activation domain. SIRT1 interacts with LXR
and promotes its deacetylation and subsequent activation (17). In the present study, expression of
LXRα was found to be significantly reduced, consistent with SIRT1
expression, in U937 cells during foam cell formation. In addition,
exposure of cells to 6.0 μM/ml SRT1720 was observed to lead to
rapid activation of SIRT1, which subsequently enhanced expression
of LXRα and its target genes, ABCA1, ABCG1 and CCR7. These results
indicate that SIRT1 is located upstream of LXRα signaling, which
may be important for regulation of the development and progression
of atherosclerosis.
The SIRT1-mediated activation of LXR may result in
several positive effects in lipid metabolic and cardiovascular
diseases, including the stimulation of cholesterol efflux from
cells to high-density lipoproteins through the ABC transporters,
ABCA1 and ABCG1, activating the conversion of cholesterol to bile
acids in the liver and facilitating excretion (27). Therefore, we hypothesize that
SIRT1-LXR-ABCA1/ABCG1 is one of the signaling pathways involved in
the regulation of cholesterol efflux and is inhibited during
atherosclerosis formation and progression, leading to cholesterol
loading in macrophages and subsequent foam cell formation. In a
mouse model of regression, rapid loss of plaque foam cells was
observed to be due to migration to lymph nodes, a process
reminiscent of dendritic cells. CCR7 is considered an essential
factor for dendritic cell migration (6). Results of the present study indicate
that CCR7 expression was enhanced, in addition to an increased
expression of SIRT1 and LXR, which may promote foam cell migration
from atherosclerotic plaques. These observations may represent a
potential therapeutic target for the reversal of atherosclerotic
plaques.
SIRT1 is known to physically bind the p65/RelA
subunit of NF-κB and deacetylate p65 at lysine 310. This
interaction downregulates the transcriptional activity of NF-κB,
leading to a reduced inflammatory response (28). In the current study, NF-κB
expression was enhanced in U937 cells by palmitate and Ox-LDL
stimulation. In addition, activation of SIRT1 by SRT1720 suppressed
NF-κB signaling. These results are consistent with previous studies
which revealed that SIRT1-deficient macrophages exhibit NF-κB
hyperacetylation, resulting in enhanced expression of various
pro-inflammatory genes (28–30).
In addition, results of the current study revealed that the SIRT1
inhibitor nicotinamide eliminated the effects of SRT1720, which
restored the expression of inflammatory genes, including NF-κB,
providing further evidence that SIRT1 functions upstream of NF-κB
during foam cell formation.
Atherosclerosis is also a progressive chronic
inflammatory disease (31). Active
NF-κB is detected in aortae with clear atherosclerotic lesions;
however, it is absent in normal, nonlesional aortae (32). A previous study by Gareus et
al(33) demonstrated that
inhibition of NF-κB abrogated the induction of adhesion molecules
in endothelial cells, impaired macrophage recruitment to
atherosclerotic plaques and reduced expression of cytokines and
chemokines in the aorta of ApoE−/− mice. Thus,
endothelial NF-κB signaling may orchestrate proinflammatory gene
expression in the arterial wall and promote the pathogenesis of
atherosclerosis. Inhibition of NF-κB activity is accompanied by a
significant reduction in atherosclerotic lesion formation in
ApoE−/− mice (33). The
present study demonstrated that SIRT1 inhibited the expression and
activity of the transcription factor NF-κB in foam cells, thus
inhibiting the expression of proinflammatory factors, including
TNFα and IL-1β. Therefore, in the process of monocyte-derived foam
cell formation, the expression and activity of SIRT1 was
suppressed, which may promote atherosclerotic plaque formation and
progression by enhanced inflammation.
In summary, results of the present study indicate
that downregulation of the LXR signaling pathway during foam cell
formation is mediated by the suppression of SIRT1 in a
high-triglyceride, high-cholesterol environment. When SIRT1 is
activated, the expression of LXR and its target genes is
upregulated, and these effects may be eliminated by the SIRT1
inhibitor nicotinamide. In addition, the suppression of SIRT1 may
enhance inflammation by decreasing deacetylation of NF-κB and
enhancing signaling. In conclusion, these results demonstrate that
SIRT1 may prevent atherosclerosis by enhancing the
LXR-ABCA1/ABCG1/CCR7 and inhibiting the NF-κB signaling
pathways.
Acknowledgements
The present study was supported by a grant from the
Natural Science Foundation of China (no. 81270382/H0215) and the
211 Project of Guangdong Province (Mechanism and Prevention of New
Emergence Infection).
References
|
1
|
Yusuf S, Reddy S, Ounpuu S and Anand S:
Global burden of cardiovascular diseases: part I: general
considerations, the epidemiologic transition, risk factors, and
impact of urbanization. Circulation. 104:2746–2753. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Trogan E, Fayad ZA, Itskovich VV,
Aguinaldo JG, Mani V, Fallon JT, Chereshnev I and Fisher EA: Serial
studies of mouse atherosclerosis by in vivo magnetic resonance
imaging detect lesion regression after correction of dyslipidemia.
Arterioscler Thromb Vasc Biol. 24:1714–1719. 2004. View Article : Google Scholar
|
|
3
|
Chereshnev I, Trogan E, Omerhodzic S,
Itskovich V, Aguinaldo JG, Fayad ZA, Fisher EA and Reis ED: Mouse
model of heterotopic aortic arch transplantation. J Surg Res.
111:171–176. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Reis ED, Li J, Fayad ZA, Rong JX, Hansoty
D, Aguinaldo JG, Fallon JT and Fisher EA: Dramatic remodeling of
advanced atherosclerotic plaques of the apolipoprotein E-deficient
mouse in a novel transplantation model. J Vasc Surg. 34:541–547.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Llodrá J, Angeli V, Liu J, Trogan E,
Fisher EA and Randolph GJ: Emigration of monocyte-derived cells
from atherosclerotic lesions characterizes regressive, but not
progressive, plaques. Proc Natl Acad Sci USA. 101:11779–11784.
2004.
|
|
6
|
Trogan E, Feig JE, Dogan S, Rothblat GH,
Angeli V, Tacke F, Randolph GJ and Fisher EA: Gene expression
changes in foam cells and the role of chemokine receptor CCR7
during atherosclerosis regression in ApoE-deficient mice. Proc Natl
Acad Sci USA. 103:3781–3786. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Baker RG, Hayden MS and Ghosh S: NF-κB,
inflammation, and metabolic disease. Cell Metab. 13:11–22.
2011.
|
|
8
|
Kanters E, Gijbels MJ, van der Made I,
Vergouwe MN, Heeringa P, Kraal G, Hofker MH and de Winther MP:
Hematopoietic NF-kappaB1 deficiency results in small
atherosclerotic lesions with an inflammatory phenotype. Blood.
103:934–940. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Koubova J and Guarente L: How does calorie
restriction work? Genes Dev. 17:313–321. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Redman LM and Ravussin E: Caloric
restriction in humans: impact on physiological, psychological and
behavioral outcomes. Antioxid Redox Signal. 14:275–287. 2011.
View Article : Google Scholar
|
|
11
|
Qiu X, Brown KV, Moran Y and Chen D:
Sirtuin regulation in calorie restriction. Biochim Biophys Acta.
1804:1576–1583. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bordone L, Cohen D, Robinson A, Motta MC,
van Veen E, Czopik A, Steele AD, Crowe H, Marmor S, Luo J, Gu W and
Guarente L: SIRT1 transgenic mice show phenotypes resembling
calorie restriction. Aging Cell. 6:759–767. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Stein S and Matter CM: Protective roles of
SIRT1 in atherosclerosis. Cell Cycle. 10:640–647. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yu W, Fu YC, Chen CJ, Wang X and Wang W:
SIRT1: a novel target to prevent atherosclerosis. J Cell Biochem.
108:10–13. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li X, Zhang S, Blander G, Tse JG, Krieger
M and Guarente L: SIRT1 deacetylates and positively regulates the
nuclear receptor LXR. Mol Cell. 28:91–106. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pfluger PT, Herranz D, Velasco-Miguel S,
Serrano M and Tschöp MH: Sirt1 protects against high-fat
diet-induced metabolic damage. Proc Natl Acad Sci USA.
105:9793–9798. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Purushotham A, Schug TT, Xu Q, Surapureddi
S, Guo X and Li X: Hepatocyte-specific deletion of SIRT1 alters
fatty acid metabolism and results in hepatic steatosis and
inflammation. Cell Metab. 9:327–338. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ramírez-Zacarías JL, Castro-Muñozledo F
and Kuri-Harcuch W: Quantitation of adipose conversion and
triglycerides by staining intracytoplasmic lipids with Oil red O.
Histochemistry. 97:493–497. 1992.PubMed/NCBI
|
|
19
|
Vock C, Gleissner M, Klapper M and Döring
F: Identification of palmitate-regulated genes in HepG2 cells by
applying microarray analysis. Biochim Biophys Acta. 1770:1283–1288.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
de Kreutzenberg SV, Ceolotto G, Papparella
I, Bortoluzzi A, Semplicini A, Dalla Man C, Cobelli C, Fadini GP
and Avogaro A: Downregulation of the longevity-associated protein
sirtuin 1 in insulin resistance and metabolic syndrome: potential
biochemical mechanisms. Diabetes. 59:1006–1015. 2010.PubMed/NCBI
|
|
21
|
Steinberg D: Atherogenesis in perspective:
hypercholesterolemia and inflammation as partners in crime. Nat
Med. 8:1211–1217. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Milne JC, Lambert PD, Schenk S, Carney DP,
Smith JJ, Gagne DJ, Jin L, Boss O, Perni RB, Vu CB, Bemis JE, Xie
R, Disch JS, Ng PY, Nunes JJ, Lynch AV, Yang H, Galonek H,
Israelian K, Choy W, Iffland A, Lavu S, Medvedik O, Sinclair DA,
Olefsky JM, Jirousek MR, Elliott PJ and Westphal CH: Small molecule
activators of SIRT1 as therapeutics for the treatment of type 2
diabetes. Nature. 450:712–716. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bitterman KJ, Anderson RM, Cohen HY,
Latorre-Esteves M and Sinclair DA: Inhibition of silencing and
accelerated aging by nicotinamide, a putative negative regulator of
yeast sir2 and human SIRT1. J Biol Chem. 277:45099–45107. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Stein S, Lohmann C, Schäfer N, Hofmann J,
Rohrer L, Besler C, Rothgiesser KM, Becher B, Hottiger MO, Borén J,
McBurney MW, Landmesser U, Lüscher TF and Matter CM: SIRT1
decreases Lox-1-mediated foam cell formation in atherogenesis. Eur
Heart J. 31:2301–2309. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Song R, Xu W, Chen Y, Li Z, Zeng Y and Fu
Y: The expression of Sirtuins 1 and 4 in peripheral blood
leukocytes from patients with type 2 diabetes. Eur J Histochem.
55:e102011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lavu S, Boss O, Elliott PJ and Lambert PD:
Sirtuins - novel therapeutic targets to treat age-associated
diseases. Nat Rev Drug Discov. 7:841–853. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nomiyama T and Bruemmer D: Liver X
receptors as therapeutic targets in metabolism and atherosclerosis.
Curr Atheroscler Rep. 10:88–95. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yeung F, Hoberg JE, Ramsey CS, Keller MD,
Jones DR, Frye RA and Mayo MW: Modulation of NF-kappaB-dependent
transcription and cell survival by the SIRT1 deacetylase. EMBO J.
23:2369–2380. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Schug TT, Xu Q, Gao H, Peres-da-Silva A,
Draper DW, Fessler MB, Purushotham A and Li X: Myeloid deletion of
SIRT1 induces inflammatory signaling in response to environmental
stress. Mol Cell Biol. 30:4712–4721. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yoshizaki T, Schenk S, Imamura T,
Babendure JL, Sonoda N, Bae EJ, Oh DY, Lu M, Milne JC, Westphal C,
Bandyopadhyay G and Olefsky JM: SIRT1 inhibits inflammatory
pathways in macrophages and modulates insulin sensitivity. Am J
Physiol Endocrinol Metab. 298:E419–E428. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ross R: Atherosclerosis is an inflammatory
disease. Am Heart J. 138:S419–S420. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Brand K, Page S, Rogler G, Bartsch A,
Brandl R, Knuechel R, Page M, Kaltschmidt C, Baeuerle PA and
Neumeier D: Activated transcription factor nuclear factor-kappa B
is present in the atherosclerotic lesion. J Clin Invest.
97:1715–1722. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gareus R, Kotsaki E, Xanthoulea S, van der
Made I, Gijbels MJ, Kardakaris R, Polykratis A, Kollias G, de
Winther MP and Pasparakis M: Endothelial cell-specific NF-kappaB
inhibition protects mice from atherosclerosis. Cell Metab.
8:372–383. 2008. View Article : Google Scholar : PubMed/NCBI
|