Introduction
Bortezomib is a novel and highly selective
proteasome inhibitor. It is a dipeptide boronic acid inhibitor,
selectively blocking the chymotrypsin-related activity of the
proteasome (1). Data from clinical
studies showed that the 26S proteasome inhibitor bortezomib has
therapeutic potential against various types of cancer including
breast, colorectal, lymphoma, multiple myeloma, ovarian,
pancreatic, prostate and squamous cell carcinomas (2). Findings of previous studies have
shown that the proteasome inhibitor bortezomib exhibits antitumor
activities by inducing the accumulation of pro-apoptotic proteins
or cell cycle inhibitors (such as
Phorbol-12-myristate-13-acetate-induced protein 1, BH3
interacting-domain death agonist, Bcl-2-associated X protein, p53,
Bcl-2-associated death promoter and cyclin- dependent kinase
inhibitors p21 and p27) depending on the cell line used (2–5). In
addition, inhibition of the proteasome by bortezomib results in
inhibition of the activation of nuclear factor
κ-light-chain-enhancer of activated B cells (NFκB) by preventing
the degradation of IκB (an inhibitor of NFκB). NFκB is an important
transcription factor for cell survival (6,7).
Therefore, in addition to the stabilization of the above-mentioned
pro-apoptotic proteins, bortezomib promotes the apoptosis of cancer
cells through inhibition of the activation of NFκB.
In a number of preclinical murine tumor models,
bortezomib was identified to exhibit promising antitumor activities
as a single agent. LeBlanc et al(8) examined the efficacy, toxicity and
in vivo mechanism of action of bortezomib using a human
plasmacytoma xenograft mouse model. They observed that the median
overall survival was significantly prolonged compared with
controls. Their results showed that bortezomib has significant
in vivo antimyeloma activity at well-tolerated doses in a
murine model. The antitumor activity of bortezomib in combination
with other therapies has also been evaluated. For example,
Denlinger et al(9) observed
that a combined treatment with histone deacetylase inhibitor
suberoylanilide hydroxamic acid and bortezomib induced greater
reactive oxygen species generation and more apoptosis than either
drug alone. Teicher et al(10) also evaluated the efficacy of
bortezomib in combination with 5-fluorouracil, cisplatin, taxol and
adriamycin. Results of that study showed that bortezomib produced
primarily additive tumor growth delays against the EMT-6/parent
mouse mammary carcinoma grown as a solid tumor subcutaneously in
the flanks of female Balb/c mice. The combinations were also highly
effective against metastasis to the lungs.
The purpose of this study was to determine the
cytotoxic effects of bortezomib, cisplatin and 5-fluorouracil as
monotherapies or in combination in a highly metastatic and p53-null
4T1 breast cancer cell line. The results obtained demonstrated that
additional studies should be performed on the combination of
bortezomib and cisplatin in highly aggressive and metastatic
cancers bearing mutated p53 gene.
Materials and methods
Materials
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium
bromide (MTT), RPMI-1640 cell culture media, fetal bovine serum
(FBS), trypsin and penicillin/streptomycin were purchased from
Sigma-Aldrich (St. Louis, MO, USA). Agarose was from Life Sciences
Advanced Technologies, Inc. (St. Petersburg, FL, USA). The Stericup
vacuum filtration system was obtained from Millipore Inc., (St.
Quentin, France).
Cell culture maintenance
4T1 breast cancer cells were cultured in RPMI-1640
with 10% FBS, 10 mM Hepes, 4.5 g/l glucose, 1 mM sodium pyruvate,
0.15% sodium bicarbonate, 100 μg/ml streptomycin and 100 U/ml
penicillin. Cells were incubated at 37°C with 5% CO2.
Stock cultures were grown in 25 cm2 corning flasks and
experimental cultures were plated in 60×15 or 35×10 mm corning
plates.
IC50 determination
Fifty thousand cells were seeded in 35×10 mm plates.
After 48 h of plating, cells were treated with various doses of
cisplatin or 5-fluorouracil (0.01, 0.1, 0.5, 1, 10, 50, 100 or 200
μM) for 24 h. The cells were incubated for 4 h with RPMI-1640 media
containing 0.5% FBS + 0.5 mg/ml MTT at 37°C with 5% CO2
to determine the number of surviving cells. After removing the
medium containing MTT, the cells were lysed with 3% SDS and 40 mM
HCl/isopropanol for 15 min. The lysate was pipetted well to
dissolve the MTT-formazan crystals completely and centrifuged at
11,000 × g for 5 min. Absorbance at 570 nm was recorded with a
Bio-Rad (Hercules, CA, USA) Smartspec Plus spectrophotometer
(5,11). The IC50 values of each
agent were determined with a Prism 3.03 program.
Soft agar assay
Similarly, 100,000 cells were seeded in 60×15 mm
petri dishes. At logarithmic phase, the cells were treated with 10
nM bortezomib, 1 μM cisplatin, 1 μM 5-fluorouracil or in
combination (1 μM cisplatin + 10 nM bortezomib or 1 μM fluorouracil
+ 10 nM bortezomib) for 24 h. After the drug treatment, the cells
were counted and 0.75 ml of 2X RPMI-1640 + 20% FBS (containing
5,000 cells) was mixed with 0.75 ml of 0.7% agarose in a tube for
each plate. The mixture was added to the base agar containing 0.5%
agar + 1X RPMI-1640 + 10% FBS. Plates were incubated for 3 weeks at
37°C in a humidified incubator and fed twice a week (12). Plates were then stained with 0.1%
crystal violet in 10% ethanol for 1 h and washed extensively with
PBS until colonies become apparent for counting.
Statistical analysis
Data were analyzed and presented using GraphPad
Prism 3.03 program. One-way ANOVA with the Bonferroni post-test or
two-tailed Student’s t-test was used to evaluate the statistical
significance. P<0.05 was considered to indicate a statistically
significant difference.
Results
Determination of IC50
values
In previous studies, we determined the p53 status of
4T1 breast cancer cells and verified that they are p53-deficient by
examining the induction of both p53 and its downstream target p21
in response to various concentrations of the proteasome inhibitor
bortezomib (5,13). Additionally, we identified the
IC50 value of bortezomib in 4T1 cells as 71 nM,
indicating that these p53-deficient cells are sensitive to the
inhibition of the proteasome (5).
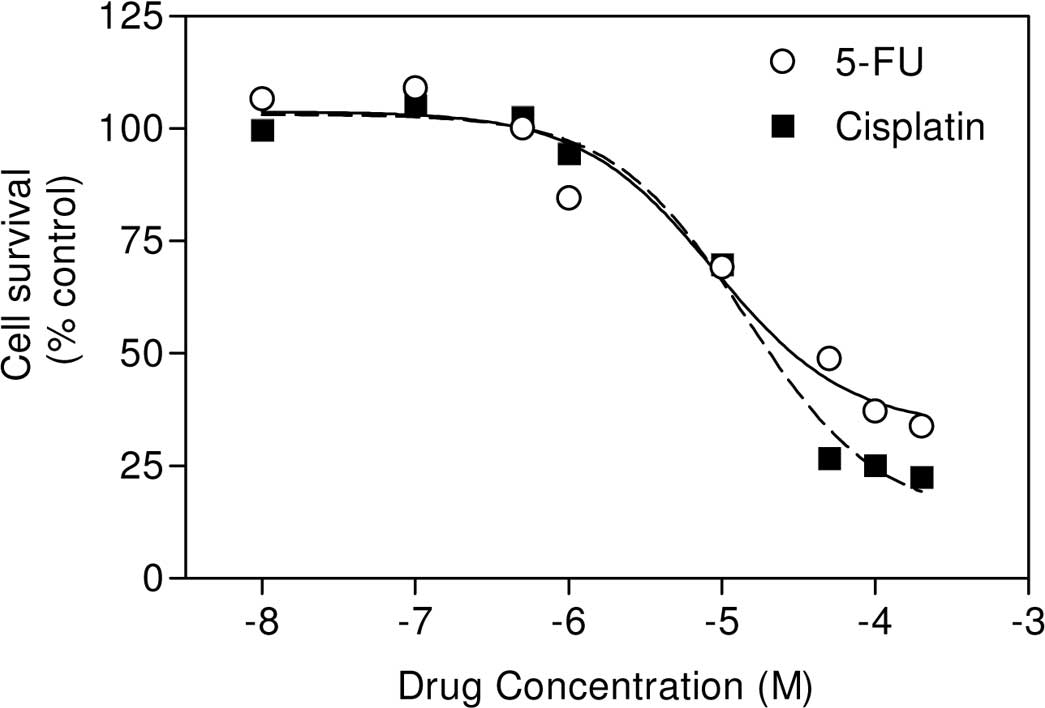
In the present study, we first determined whether the
chemotherapeutic agents (cisplatin and 5-fluorouracil) were also
cytotoxic to the highly metastatic and p53-null 4T1 breast cancer
cells. Results showed that these drugs are highly cytotoxic to 4T1
cells (Fig. 1). Using these data,
we calculated the IC50 values of cisplatin and
5-fluorouracil to be 14.2 and 8.9 μM.
Effect of bortezomib and cisplatin
combination on cell viability
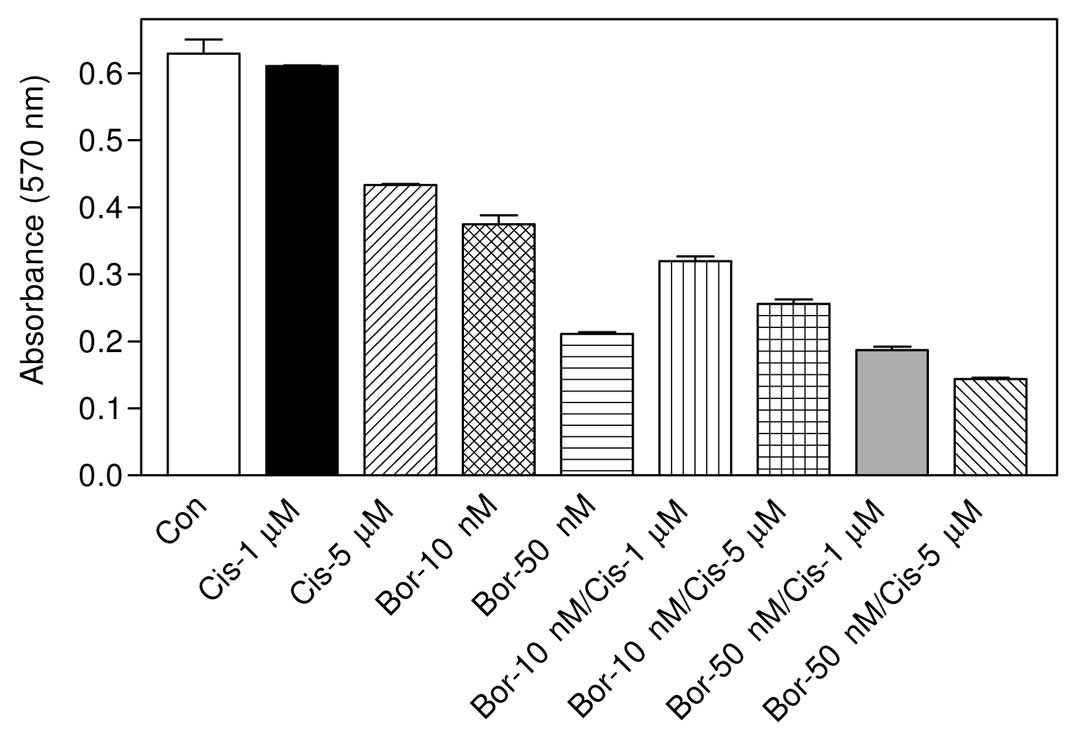
The effect of the combination of each drug with
bortezomib was investigated. Based on the IC50 values,
the cells were first treated with two different doses of cisplatin
and bortezomib as well as the combinations of each dose tested. As
shown in Fig. 2, 1 μM cisplatin
treatment did not significantly reduce cell survival (P>0.05 vs.
control) after 24 h of treatment and 5 μM treatment of cisplatin
caused a 31% reduction in the cell number (P<0.001 vs. control).
By contrast, 10 nM bortezomib reduced the cell number by ~40%
(P<0.001 vs. control), and 50 nM bortezomib resulted in a 66%
reduction in the cell viability (P<0.001 vs. control),
altogether indicating that bortezomib is a more cytotoxic agent
than cisplatin in this highly mestastatic breast carcinoma cell
line. When cells were treated with 10 nM bortezomib + 1 μM
cisplatin, a statistically significant cell death was observed as
compared with single drug treatments (for example, P<0.05 vs. 10
nM treated bortezomib). When a higher concentration (5 μM) of
cisplatin was combined with 10 nM bortezomib, again more
cytotoxicity was detected (P<0.001 vs. 10 nM bortezomib). In
cells treated with 50 nM bortezomib + 1 μM cisplatin, no
significant results were observed when compared with 50 nM
bortezomib as a monotherapy (P>0.05). However, when cells were
treated with 50 nM + 5 μM cisplatin, a statistically significant
result was obtained as compared with 50 nM bortezomib-treated cells
(P<0.01) (Fig. 2).
Effect of combination of bortezomib and
5-fluorouracil on cell viability
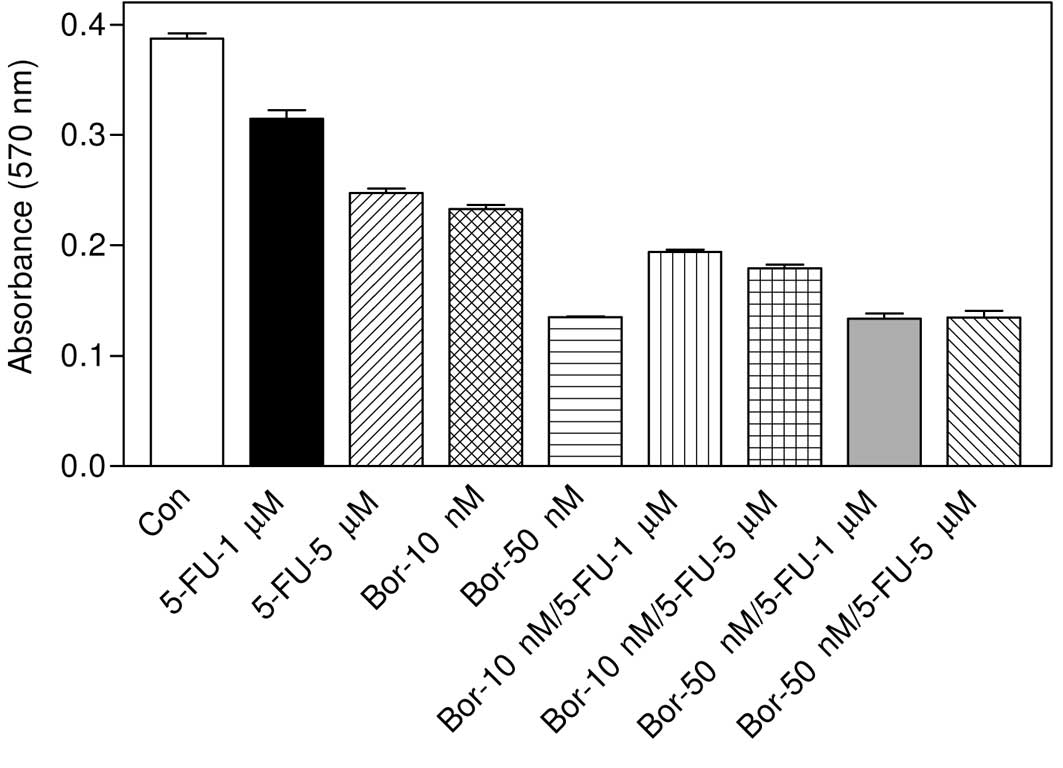
As can be seen in Fig.
3, 1 or 5 μM 5-fluorouracil caused significant cell death as
compared to the control group (P<0.001 in both cases). However,
a 10 or 50 nM concentration of bortezomib was more effective
compared with 5-fluorouracil. Results showed that 1 μM
5-fluorouracil potentiated the cytotoxicity of 10 nM bortezomib
when used in combination (P<0.001 vs. 10 nM bortezomib).
Similarly, 10 nM bortezomib + 5 μM 5-fluorouracil was also more
cytotoxic (P<0.001 vs. 10 nM bortezomib). However, the effect of
50 nM bortezomib + 1 μM 5-fluorouracil was not statistically
different compared with the effect of 50 nM bortezomib as a
monotherapy (P>0.05). Similarly, 50 nM bortezomib + 5 μM
5-fluorouracil did not produce significant cell death as compared
with 50 nM bortezomib-treated group (P>0.05).
Soft agar assay for the determination of
cytotoxicity
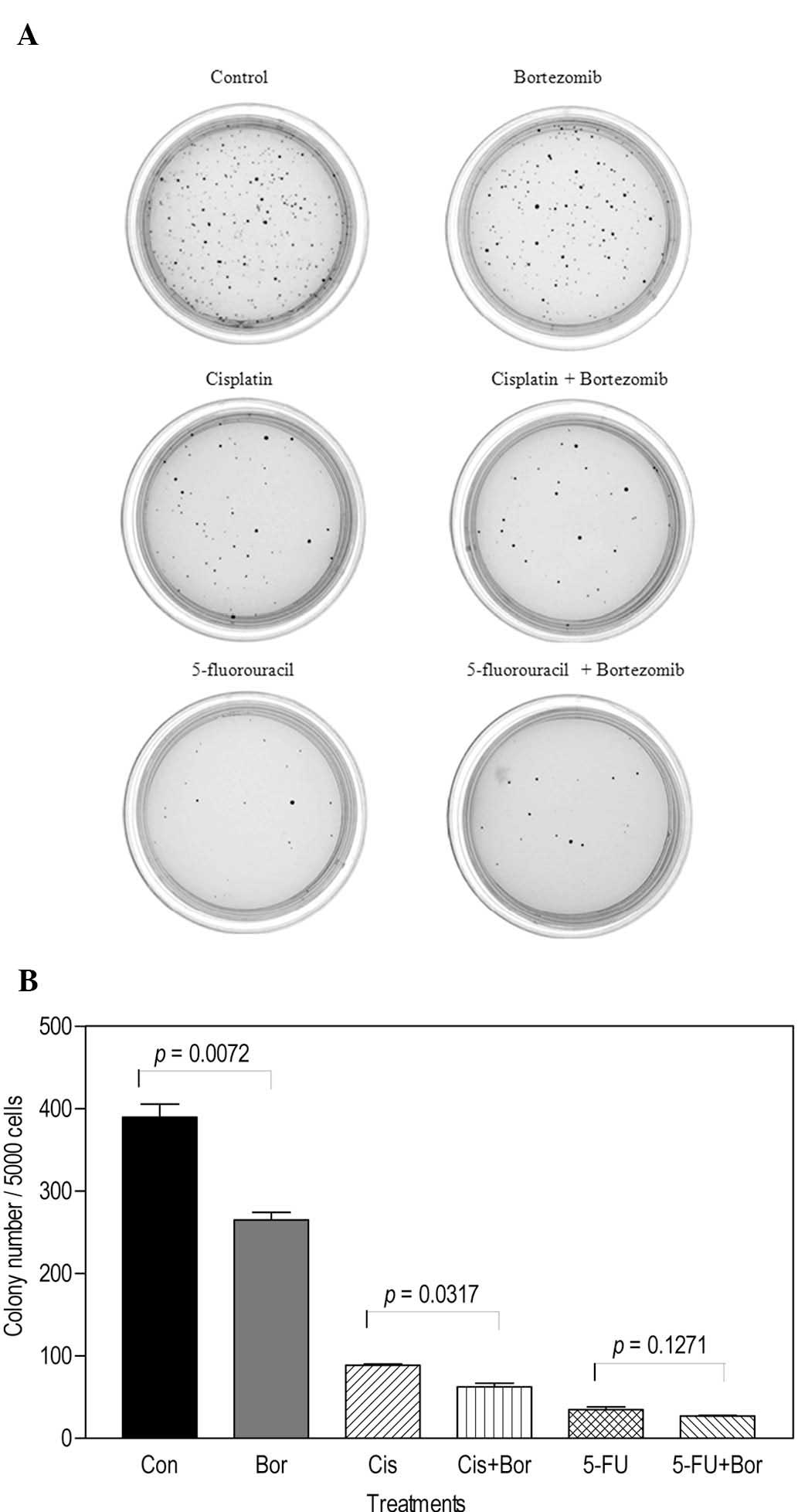
The effect of the combination of cisplatin or
5-fluorouracil with bortezomib was examined using soft agar assay.
Of note, in contrast to the MTT assay results, cells formed a low
number of colonies when treated with 1 μM cisplatin or 1 μM
5-fluorouracil alone (Fig. 4A).
The colony numbers were lower than those in the plate treated with
bortezomib alone. Additionally, the number of colonies in the 10 nM
bortezomib + 1 μM cisplatin-treated group was significantly lower
than that of colonies in the plate treated with 1 μM cisplatin
alone (P=0.0317), indicating that cisplatin potentiated the effect
of bortezomib (Fig. 4B). By
contrast, the number of colonies in the 10 nM bortezomib + 1 μM
5-fluorouracil-treated plate was not significantly lower than that
in the 1 μM 5-fluorouracil alone-treated plate (P=0.1271), a result
consistent with those obtained with the MTT assay.
Discussion
The IC50 value of cisplatin, and
5-fluorouracil was previously determined as 43.5 and 10 μM,
respectively, in MCF cells (14–16),
which are comparable to the values obtained in this study with the
4T1 breast cancer cells. Since we have previously shown that 4T1
cells are p53-null cells (5), the
present results indicate that these anticancer chemotherapeutic
agents are able to induce cell death in 4T1 cells in a
p53-independent manner. In terms of the combination treatments, the
results have shown that cisplatin + bortezomib is more potent than
the combination of bortezomib with 5-fluorouracil since 5 μM
cisplatin potentiated the cytotoxic effects of 50 nM bortezomib. By
contrast, 5 μM 5-fluorouracil did not affect the degree of toxicity
of 50 nM bortezomib significantly. Cisplatin is a widely used
anticancer agent. To the best of our knowledge, combination of the
cisplatin and bortezomib has not been previously tested in this
metastatic 4T1 breast cancer cell line, a cell line commonly used
in animal tumor models. In addition, the effect of bortezomib and
cisplatin combination has not been widely tested in other breast
cancer cells. However, in a study with the EMT-6 murine mammary
carcinoma cell line, Teicher et al(10) showed that bortezomib (also known as
Velcade or PS-341) increased the tumor cell killing of cisplatin.
Based on the results of the MTT assay, we expected more colonies in
cisplatin- or 5-fluorouracil alone-treated plates (with ~14 or 9
times lower concentrations of cisplatin or 5-fluorouracil than the
IC50 values, respectively) in soft agar assay. Of note,
we observed only a 24% colony formation in cisplatin-treated plates
and 17% colony formation in 5-fluorouracil-treated plates, which
may be due to the irreversible binding of cisplatin or
5-fluorouracil to the cell targets. In 10 nM bortezomib-treated
plates, we observed 72% colony formation, which was consistent with
the results obtained with the MTT assay. Since bortezomib is known
to be a highly selective and reversible inhibitor of the 26S
proteasome (17), the
discrepancies between the number of colonies in soft agar assay and
MTT results obtained for cisplatin and 5-fluorouracil may therefore
be explained by the reversibility of the drugs. Similar results may
be obtained with other irreversible drugs and the assays used may
be taken into account when comparing the efficacy of the drugs.
The data presented in this study suggest that
p53-null 4T1 cells can be used to delineate the mechanism of
p53-independent induction of apoptosis by cisplatin and
5-fluorouracil and bortezomib. The studies also suggest that the
combination of cisplatin + bortezomib is more effective and that
additional investigations should be conducted in clinical
settings.
Acknowledgements
We would like to thank Prof. Dr. Engin Ulukaya
(Uludağ University, Bursa, Turkey) for providing bortezomib.
References
|
1
|
Burger AM and Seth AK: The
ubiquitin-mediated protein degradation pathway in cancer:
therapeutic implications. Eur J Cancer. 40:2217–2229. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Adams J: The proteasome: a suitable
antineoplastic target. Nat Rev Cancer. 4:349–360. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Combaret V, Boyault S, Iacono I, Brejon S,
Rousseau R and Puisieux A: Effect of bortezomib on human
neuroblastoma: analysis of molecular mechanisms involved in
cytotoxicity. Mol Cancer. 7:502008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pei XY, Dai Y and Grant S: The proteasome
inhibitor bortezomib promotes mitochondrial injury and apoptosis
induced by the small molecule Bcl-2 inhibitor HA14-1 in multiple
myeloma cells. Leukemia. 17:2036–2045. 2003. View Article : Google Scholar
|
|
5
|
Yerlikaya A and Erin N: Differential
sensitivity of breast cancer and melanoma cells to proteasome
inhibitor Velcade. Int J Mol Med. 22:817–823. 2008.PubMed/NCBI
|
|
6
|
Boccadoro M, Morgan G and Cavenagh J:
Preclinical evaluation of the proteasome inhibitor bortezomib in
cancer therapy. Cancer Cell Int. 5:182005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sunwoo JB, Chen Z, Dong G, Yeh N, Crowl
Bancroft C, Sausville E, Adams J, Elliott P and Van Waes C: Novel
proteasome inhibitor PS-341 inhibits activation of nuclear
factor-kappa B, cell survival, tumor growth, and angiogenesis in
squamous cell carcinoma. Clin Cancer Res. 7:1419–1428.
2001.PubMed/NCBI
|
|
8
|
LeBlanc R, Catley LP, Hideshima T,
Lentzsch S, Mitsiades CS, Mitsiades N, Neuberg D, Goloubeva O, Pien
CS, Adams J, et al: Proteasome inhibitor PS-341 inhibits human
myeloma cell growth in vivo and prolongs survival in a murine
model. Cancer Res. 62:4996–5000. 2002.PubMed/NCBI
|
|
9
|
Denlinger CE, Rundall BK and Jones DR:
Proteasome inhibition sensitizes non-small cell lung cancer to
histone deacetylase inhibitor-induced apoptosis through the
generation of reactive oxygen species. J Thorac Cardiovasc Surg.
128:740–748. 2004. View Article : Google Scholar
|
|
10
|
Teicher BA, Ara G, Herbst R, Palombella VJ
and Adams J: The proteasome inhibitor PS-341 in cancer therapy.
Clin Cancer Res. 5:2638–2645. 1999.PubMed/NCBI
|
|
11
|
Freshney RI: Cytotoxicity. Culture of
animal cells: a manual of basic techniques. 5th edition.
Wiley-Liss; New Jersey, NJ: pp. 359–374. 2005
|
|
12
|
Chua BT, Lim SJ, Tham SC, Poh WJ and
Ullrich A: Somatic mutation in the ACK1 ubiquitin association
domain enhances oncogenic signaling through EGFR regulation in
renal cancer derived cells. Mol Oncol. 4:323–334. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yerlikaya A, Okur E and Ulukaya E: The
p53-independent induction of apoptosis in breast cancer cells in
response to proteasome inhibitor bortezomib. Tumour Biol.
33:1385–1392. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Basma H, El-Refaey H, Sgagias MK, Cowan
KH, Luo X and Cheng PW: BCL-2 antisense and cisplatin combination
treatment of MCF-7 breast cancer cells with or without functional
p53. J Biomed Sci. 12:999–1011. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hernandez-Vargas H, Ballestar E,
Carmona-Saez P, von Kobbe C, Banon-Rodriguez I, Esteller M,
Moreno-Bueno G and Palacios J: Transcriptional profiling of MCF7
breast cancer cells in response to 5-fluorouracil: relationship
with cell cycle changes and apoptosis, and identification of novel
targets of p53. Int J Cancer. 119:1164–1175. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Plano D, Baquedano Y, Ibanez E, Jimenez I,
Palop JA, Spallholz JE and Sanmartin C: Antioxidant-prooxidant
properties of a new organoselenium compound library. Molecules.
15:7292–7312. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mimnaugh EG, Xu W, Vos M, Yuan X, Isaacs
JS, Bisht KS, Gius D and Neckers L: Simultaneous inhibition of hsp
90 and the proteasome promotes protein ubiquitination, causes
endoplasmic reticulum-derived cytosolic vacuolization, and enhances
antitumor activity. Mol Cancer Ther. 3:551–566. 2004.
|