Introduction
Peritoneal dialysis (PD) is one of the most
effective ways to treat end-stage renal disease (ESRD) patients.
Peritoneal structure and function are essential factors in the
maintenance of PD; however, the daily instillation of PD fluid
(PDF) leads to morphological and functional alterations of the
peritoneal membrane. These alterations ultimately lead to
ultrafiltration (UF) failure (UFF), which results in PD patients
opting out of treatment, or even in mortality. For long-term PD
patients, increasing angiogenesis is one of the significant changes
to the peritoneum (1). According
to a previous report, peritoneal angiogenesis is important in the
mechanism of UFF (2).
Angiopoietin (Ang) is important in the initiation of
pathological neoangiogenesis (3,4).
Within the Ang family, Ang-2 has been shown to be associated with
neoangiogenesis (5). Under hypoxic
conditions or in the presence of vascular endothelial growth factor
(VEGF), Ang-2 leads to angiogenesis through promotion of the
proliferation and migration of vascular endothelial cells, and by
increasing the sensitivity of endothelial cells to VEGF (6,7). In
the absence of VEGF, Ang-2 may lead to vessel regression (8). Ang-2 binds to the tyrosine kinase
receptor Tie2, forming the Ang-2-Tie2 complex, which is involved in
angiogenesis. However, there are limited reports with regard to the
impact of the Ang/Tie system in peritoneal angiogenesis (9,10).
The extracellular Tie2 domain is released by proteases, forming
soluble Tie2 (sTie2) that competitively inhibits the binding of
Ang-2 to Tie2 (11).
To identify new therapeutic modalities against
PD-induced angiogenesis, a uremic PD rat model treated with sTie2
fusion protein (sTie2/Fc) was used in this study.
Materials and methods
Reagents
Silicon-based (GB 18671-2002; Shandong Weigao
Medical Polymer Co., Shandong, China) ethylene oxide sterilized PD
catheters were manufactured in our laboratory (12,13).
Reagents used in this study were as follows: Ang-2 or CD34
polyclonal antibodies, DAB, the ultra-sensitive SP kit (Maixin
Inc., Fuzhou, China), total RNA extraction reagent TRIzol (Bio
Basic Inc., Amherst, NY, USA), reverse transcription kit, RT-PCR
amplification kit (Takara Co., Shiga, Japan), recombinant rat
sTie2/Fc (R&D systems, Minneapolis, MN, USA), and 4.25%
peritoneal dialysate (Baxter, Deerfield, IL, USA).
Model establishment and group
distribution
This study was approved by Experimental Animal
Center of Medical School, Zhengzhou University (Zhengzhou, Henan).
We purchased 48 male Sprague-Dawley rats from the Experimental
Animal Center of the Medical School, Zhengzhou University
(Zhengzhou, China), with initial weights of 180–200 g. The rats had
free access to food and water throughout the study. The rats were
randomly divided into 6 groups: normal rats as a control group
(control, n=8); rats with sham surgery (sham, n=8); uremic rats
without PD (uremic, n=8); uremic rats dialyzed with 4.25% PD
solution (dialyze, n=8); uremic rats dialyzed with 4.25% PD
solution and treated with subcutaneous injections of 0.25 μg/100 g
sTie2/Fc (dialyze + 0.25Tie2, n=8); and uremic rats dialyzed with
4.25% PD solution and treated by subcutaneous injections of 0.50
μg/100 g sTie2/Fc (dialyze + 0.50Tie2, n=8). The rats in the sham
surgery group underwent removal of the renal capsule from both
kidneys.
Rats in the uremic groups were subjected to a 5/6
nephrectomy, performed by removing the upper and lower thirds of
the left kidney, and then the removed renal tissue was weighed. One
week later, the entirety of the right kidney was resected (14).
Five weeks after the subtotal nephrectomy, a
peritoneal catheter, connected to a subcutaneous mini-vascular
access port, was implanted into rats of the PD groups (10,15).
One week after implantation, the PD rats were infused with 4.25% PD
solution (3 ml/100 g body weight) each day and the PD fluid was
retained for 2 h before draining.
STie2/Fc was administered every day during PD
treatment, for a total of 14 doses. After 28 days of regular PD
treatment, all rats were sacrificed and the peritoneal tissues were
collected.
Real-time polymerase chain reaction (RT-PCR) and
tissue immunohistochemical staining were used to detect Ang-2 mRNA
and protein expression in peritoneal tissues in each rat. The
microvessel density (MVD) of the peritoneum was detected and
quantified with immunohistochemical staining using anti-CD34
antibody.
Detection of Ang-2 protein and MVD in rat
peritoneal tissue
Tissue specimens were embedded in paraffin and 5-μm
sections were obtained. From each rat, one section of the
peritoneum was randomly selected for immunohistochemical
pathological examination to detect Ang-2 protein expression and
MVD.
Specimens were dewaxed and rehydrated, treated with
3% H2O2 to block endogenous peroxidase and
incubated in 5% goat antiserum for 30 min at 37°C. The sections
were marked with Ang-2 antibody (1:80 dilution) and incubated
overnight at 4°C. The sections were washed once with PBS (0.01 M,
pH 7.4), then a biotin-labeled secondary antibody was added and
incubated at 37°C for 35 min. Avidin biotin-peroxidase complex and
DAB were used for the color reaction. PBS (0.01 M, pH 7.4) without
primary antibody was used as a negative control (16).
Detection of Ang-2 mRNA in peritoneal
tissues
Total RNA was isolated with a single-step method
using TRIzol reagent, according to the manufacturer’s instructions
(17). An ultraviolet
spectrophotometer XO-1101 (Kai-Di high-speed analytical instruments
Co., Ltd., Nanjing, China) was used to measure the total RNA
concentration. Complementary DNA (cDNA) was synthesized according
to standard procedures (18). The
primers were as follows: Ang-2 (100 bp product) sense,
5′-CGGCCACAGTCAACAACTCA-3′ and antisense,
5′-GCTCTTATAGTCGGGCGATGA-3′; and β-actin (222 bp product) sense,
5′-AGCCATGTACGTAGCCATCC-3′ and antisense,
5′-GCTGTGGGCGTGAAGCTGTA-3′.
Electrophoresis was performed and imaging system
analysis software was used to capture images and determine the
absorbance (A) value (Bio-Rad, Berkeley, CA, USA). Ang-2 mRNA was
expressed as the ratio of its amplicon to the β-actin amplicon and
analyzed by densitometry using Image J software (National
Institutes of Health, Bethesda, MD, USA).
Immunohistochemical staining for CD34 and
MVD value
The omentum was removed from the sacrificed rats and
fixed in a sufficient amount of 4% phosphate-buffered formaldehyde
for 24 h. Tissue samples were embedded in paraffin and 4-μm
sections were cut. Cut sections were stained for
immunohistochemical examination with CD34-related antigen antibody
(1:100 dilution).
The MVD was assessed according to Weidner (19), and the microscopic image was fixed
at the position with the greatest amount of vessels at ×100
magnification. Vessels were counted in five random fields at ×200
magnification and the average number of microvessels was assessed.
A single CD34-positive cell, or a cluster of endothelial cells
clearly separated from adjacent microvessels and other connective
tissue elements were considered to be vessels.
Statistical analysis
Data were expressed as the mean ± SD. Multiple
comparisons were initially subjected to one-way analysis of
variance (ANOVA). Correlations were analyzed using the Pearson’s
correlation test. Statistical analysis was performed using SPSS13.0
software (SPSS Inc., Chicago, IL, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Renal function of the uremic rat
model
There were no statistically significant differences
in the serum creatinine (SCr) levels between the control and sham
surgery groups. However, serum creatinine levels were increased
2–3-fold in uremic rats (P<0.05) compared with the control group
(Table I).
 | Table IRenal function and body weight of rats
(mean ± SD). |
Table I
Renal function and body weight of rats
(mean ± SD).
| | Serum creatinine
(μmol/l) | Weight (g) |
|---|
| |
|
|
|---|
| Groups | n | Day 1 | Day 28 | Day 1 | Day 28 |
|---|
| Control | 8 | 24.61±4.70 | 24.90±4.81 | 357.80±8.49 | 445.99±9.01 |
| Sham surgery | 8 | 24.67±4.56a | 24.36±4.49a | 357.16±9.05 | 446.22±8.88a |
| Uremic | 8 | 66.19±6.47b | 68.94±7.70b | 358.62±8.85 | 358.41±8.32b |
| Dialyze | 24 | 66.19±6.47b | 62.30±5.88b | 409.90±10.21 | 436.93±8.11c |
Effects of recombinant rat sTie2/Fc on
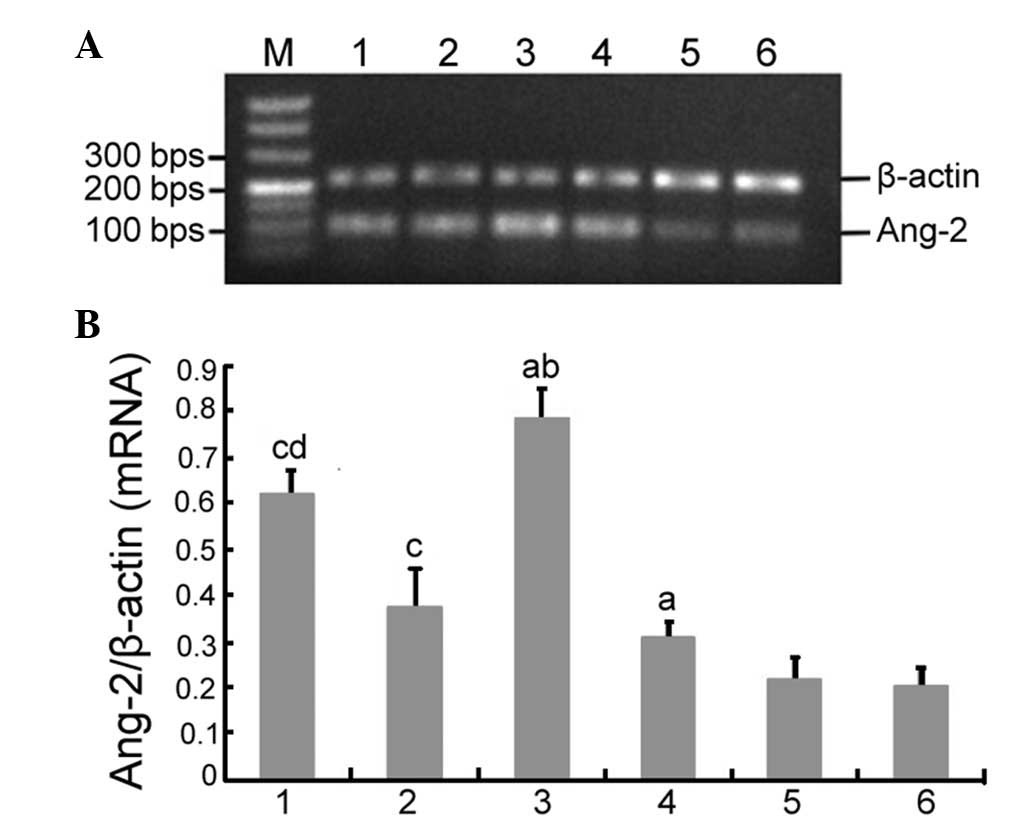
the expression of Ang-2 in peritoneal tissue
Ang-2 mRNA and protein expression was observed in
each group. There were no significant differences in Ang-2 mRNA and
protein expression between the control and sham surgery groups.
RT-PCR demonstrated that there was a significant difference in
Ang-2 mRNA expression between the control, uremic and dialyze
groups (0.196±0.036 vs. 0.334±0.041 vs. 0.796±0.019, respectively;
P<0.05). Furthermore, Ang-2 mRNA expression was significantly
different between the dialyze, dialyze + 0.25Tie2 and dialyze +
0.50Tie2 groups (0.796±0.019 vs. 0.607±0.033 vs. 0.349±0.021,
respectively; P<0.05; Fig. 1).
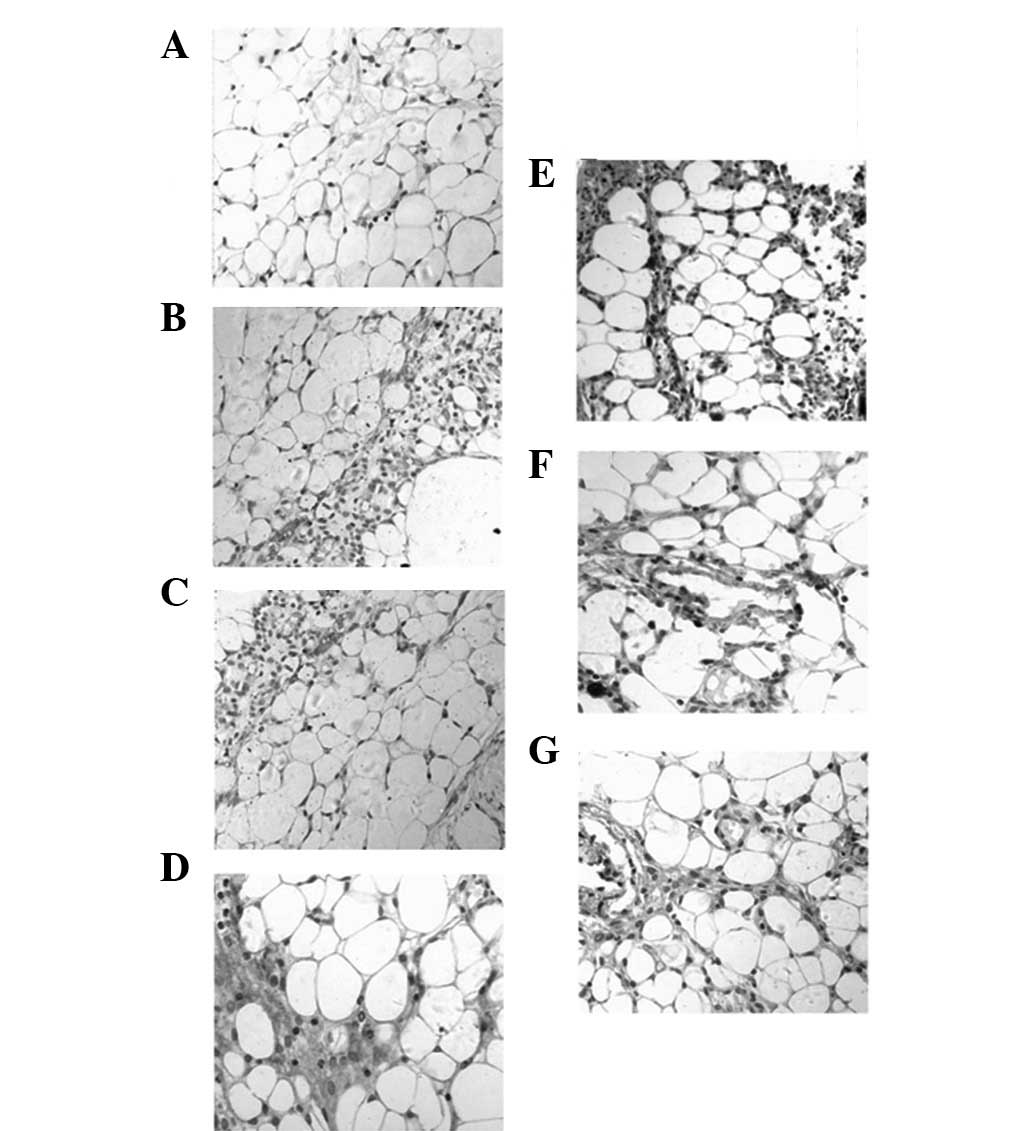
Ang-2 protein expression was observed in peritoneal capillary
endothelial cells and in the cytoplasm of the peritoneal
mesothelial cells using immunohistochemistry. Of all the groups,
staining in the dialyze group was the strongest, whilst it was
weakest in the dialyze + 0.50 Tie2 group (Fig. 2).
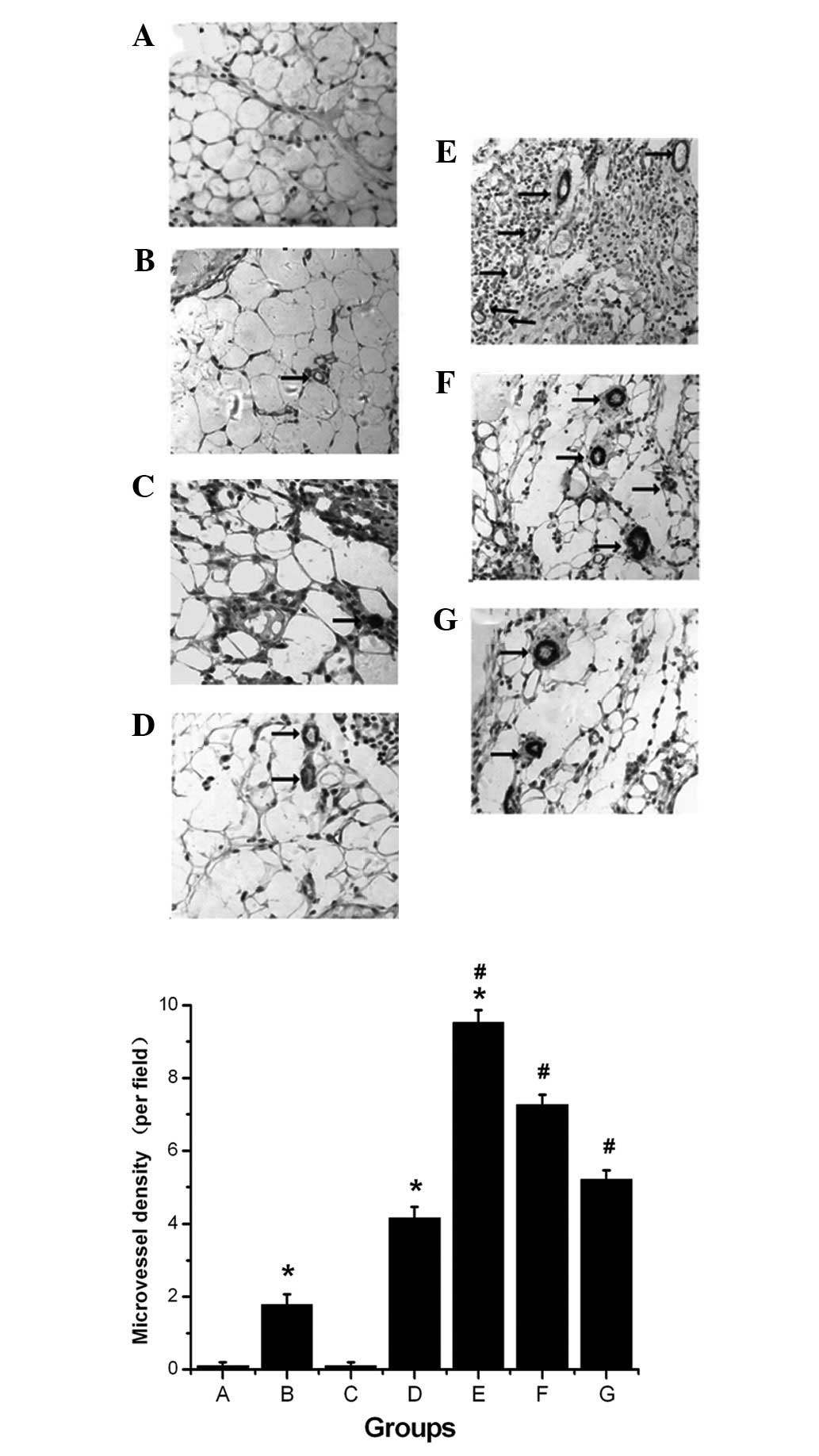
Effects of recombinant rat sTie2/Fc on
MVD
Few new microvessels were observed in the control
and sham surgery groups. However, there was a significant
difference in the MVD between control, uremic and dialyze groups
(1.78±0.29 vs. 4.16±0.31 vs. 9.53±0.33, respectively; P<0.05).
Moreover, there was a significant difference in the MVD between the
dialyze, dialyze + 0.25Tie2 and dialyze + 0.50Tie2 groups
(9.53±0.33 vs. 7.27±0.27 vs. 5.21±0.25, respectively; P<0.05;
Fig. 3).
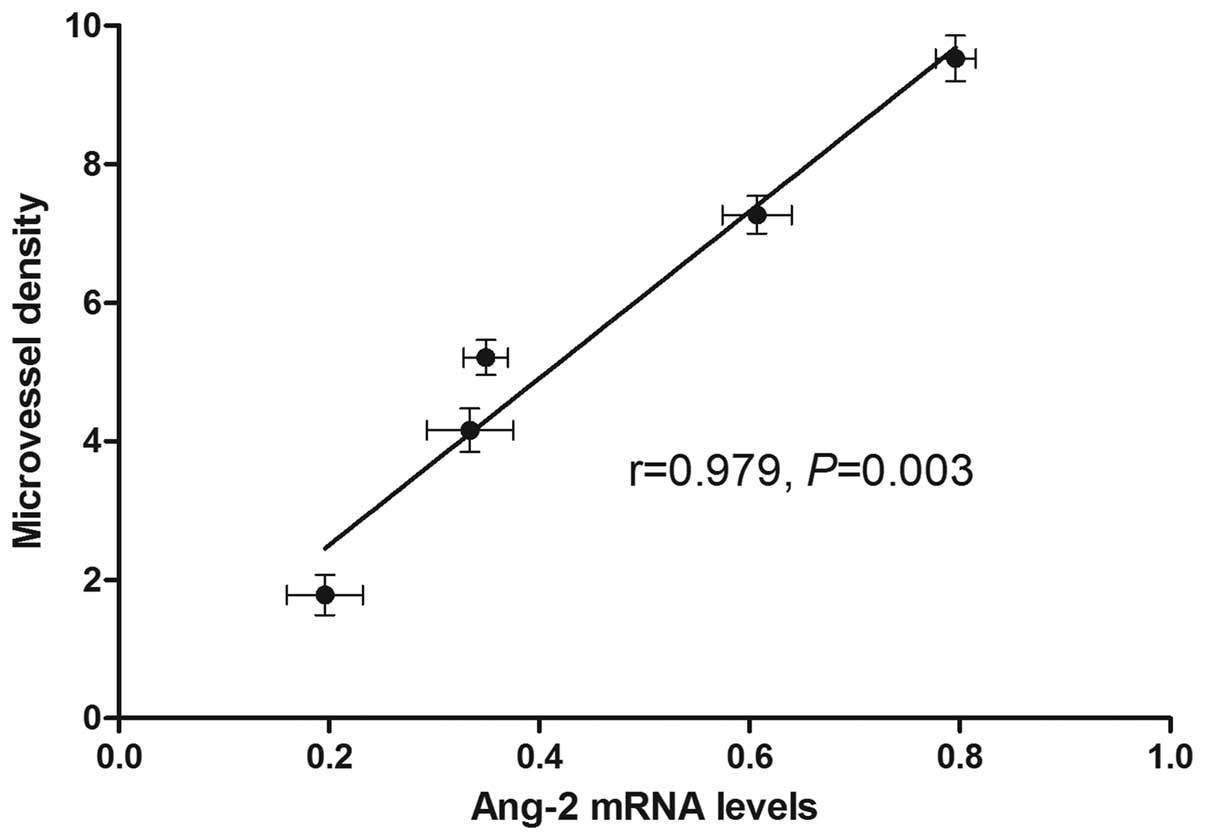
Spearman results
A significant positive correlation was observed
between Ang-2 mRNA expression and MVD (r=0.9790; P=0.003; Fig. 4).
Discussion
UFF is one of the main reasons that long-term PD
patients choose to discontinue their treatment (20). Peritoneal angiogenesis is
considered to be the most probable mechanism leading to UFF
(1). Increases in peritoneal
angiogenesis lead to an increase in peritoneal vascular surface
area, which accelerates the transport of small soluble molecules;
this causes rapid disappearance of the osmotic gradient, thus
inducing the loss of the peritoneal UF capacity. These events may
predict not only technical failure, but also patient mortality
(21,22). Studies have revealed that
anti-angiogenic treatments, such as angiostatin, slow the process
of peritoneal angiogenesis, which is a more effective method of
protecting the peritoneal membrane function than directly
inhibiting peritoneal fibrosis (23). As with any disease, early
intervention, diagnosis and treatment may improve the outcome for
PD patients.
In 1996, Hanahan and Folkman (24) proposed a hypothesis on the balance
of the angiogenesis switch, where angiogenesis is regulated by an
equilibrium between inducers and inhibitors. Once the balance is
broken, the endothelial cells are activated and promote
neoangiogenesis. Other studies have demonstrated that in a uremic
and high-glucose environment, the levels of certain inducers
(including the fibroblast growth factor and VEGF) were raised in
the peritoneum and positively correlated with angiogenesis
(24–27). Inhibitors (including
thrombospondin-1, 16 kD prolactin, interferon α/β, platelet
factor-4 and angiostatin) are able to inhibit peritoneal
neoangiogensis by downregulating the expression of inducer factors
in the peritoneum (24,27).
It has been demonstrated that the Ang/Tie pathway is
an inducer factor in vasculogenesis and angiogenesis, but little is
known about its involvement in peritoneal angiogenesis. One study
confirmed increased angiogenesis and fibrosis in the uremic rat
model under PD, and suggested that this increase was due to
increased Ang-2 levels associated with decreased Tie2, indicating
that this pathway may be targeted in order to preserve the
peritoneum (9). The present study
revealed that in a uremic and high-glucose environment, Ang-2 mRNA
and protein expression increased in peritoneal tissues, indicating
that upregulation of Ang-2 was the likely cause of peritoneal
angiogenesis. Furthermore, we observed a positive correlation
between Ang-2 and peritoneal angiogenesis.
A previous study (27) reported that soluble receptors for
angiogenic growth factors, which were used as endogenous ligand
antagonists, may be useful in inhibiting signal transduction, thus
influencing the biological action of ligands. Studies showed that
the Ang/Tie2 system was involved in cancer tissue and retinal
neovascularization, and that the use of a sTie-2/Fc effectively
inhibited angiogenesis (28,29).
Results of the present study are consistent with a previous study
(9) demonstrating that the
Ang/Tie2 system is upregulated in a uremic rat model treated with
PD. Results suggest that inhibiting this pathway may be an
effective method of preventing peritoneal angiogenesis, allowing PD
patients to continue with their treatment and improving survival
rates. This study demonstrated that sTie-2/Fc may be used to
effectively inhibit peritoneal angiogenesis. VEGF and VEGF receptor
inhibitors have been approved for angiogenesis inhibition in a
number of studies, but their use alone is unable to completely
prevent angiogenesis (28,30). As VEGF is also upregulated in
uremic patients under PD (31),
the combined use of a VEGF inhibitor and sTie-2/Fc may be an
effective method to reduce angiogenesis in PD patients (28).
In conclusion, results from this study suggest that
targeting Ang-2 using a soluble Tie-2 receptor may be an effective
method for preventing peritoneal angiogenesis in uremic patients
undergoing PD. This study also provides an experimental basis for
the development of a future clinical treatment using sTie-2/Fc.
References
|
1
|
van Westrhenen R, Zweers MM, Kunne C, de
Waart DR, van der Wal AC and Krediet RT: A pyruvate-buffered
dialysis fluid induces less peritoneal angiogenesis and fibrosis
than a conventional solution. Perit Dial Int. 28:487–496.
2008.PubMed/NCBI
|
|
2
|
Saxena R: Pathogenesis and treatment of
peritoneal membrane failure. Pediatr Nephrol. 23:695–703. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ramsauer M and D’Amore PA: Getting Tie(2)d
up in angiogenesis. J Clin Invest. 110:1615–1617. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Koh GY, Kim I, Kwak HJ, Yun MJ and Leem
JC: Biomedical significance of endothelial cell specific growth
factor, angiopoietin. Exp Mol Med. 34:1–11. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Guo P, Imanishi Y, Cackowski FC, et al:
Up-regulation of angiopoietin-2, matrix metalloprotease-2, membrane
type 1 metalloprotease, and laminin 5 gamma 2 correlates with the
invasiveness of human glioma. Am J Pathol. 166:877–890. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Reiss Y: Angiopoietins. Recent Results
Cancer Res. 180:3–13. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shin HY, Kim JH, Phi JH, et al: Endogenous
neurogenesis and neovascularization in the neocortex of the rat
after focal cerebral ischemia. J Neurosci Res. 86:356–367. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mandriota SJ and Pepper MS: Regulation of
angiopoietin-2 mRNA levels in bovine microvascular endothelial
cells by cytokines and hypoxia. Circ Res. 83:852–859. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yuan J, Fang W, Lin A, Ni Z and Qian J:
Angiopoietin-2/Tie2 signaling involved in TNF-α induced peritoneal
angiogenesis. Int J Artif Organs. 35:655–662. 2012.PubMed/NCBI
|
|
10
|
Yuan J, Fang W, Ni Z, et al: Peritoneal
morphologic changes in a peritoneal dialysis rat model correlate
with angiopoietin/Tie-2. Pediatr Nephrol. 24:163–170. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lin P, Polverini P, Dewhirst M, Shan S,
Rao PS and Peters K: Inhibition of tumor angiogenesis using a
soluble receptor establishes a role for Tie2 in pathologic vascular
growth. J Clin Invest. 100:2072–2078. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhao ZZ, Cao Y, Liu ZS, et al: Effects of
recombinant human endostatin on peritoneal angiogenesis in
peritoneal dialysis rats. Nephrology (Carlton). 16:599–606. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gao D, Zhao ZZ, Liang XH, Li Y, Cao Y and
Liu ZS: Effect of peritoneal dialysis on expression of vascular
endothelial growth factor, basic fibroblast growth factor and
endostatin of the peritoneum in peritoneal dialysis patients.
Nephrology (Carlton). 16:736–742. 2011. View Article : Google Scholar
|
|
14
|
Ghosh SS, Krieg RJ, Sica DA, Wang R,
Fakhry I and Gehr T: Cardiac hypertrophy in neonatal nephrectomized
rats: the role of the sympathetic nervous system. Pediatr Nephrol.
24:367–377. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang XD and Qian JQ: An improved animal
model of peritoneal dialysis mimicked human peritoneal dialysis.
Chin J Blood Purif. 4:326–328. 2005.
|
|
16
|
Bunone G, Vigneri P, Mariani L, et al:
Expression of angiogenesis stimulators and inhibitors in human
thyroid tumors and correlation with clinical pathological features.
Am J Pathol. 155:1967–1976. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Deng MY, Wang H, Ward GB, Beckham TR and
McKenna TS: Comparison of six RNA extraction methods for the
detection of classical swine fever virus by real-time and
conventional reverse transcription-PCR. J Vet Diagn Invest.
17:574–578. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sambrook J and Russell DW: Molecular
Cloning: A Laboratory Manual. 3rd edition. CSHL Press; Cold Spring
Harbor, NY: 2001
|
|
19
|
Weidner N: Intratumor microvessel density
as a prognostic factor in cancer. Am J Pathol. 147:9–19.
1995.PubMed/NCBI
|
|
20
|
Chaudhary K: Peritoneal dialysis drop-out:
causes and prevention strategies. Int J Nephrol. 2011:4346082011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Noh H, Kim JS, Han KH, et al: Oxidative
stress during peritoneal dialysis: implications in functional and
structural changes in the membrane. Kidney Int. 69:2022–2028. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Parikova A, Smit W, Struijk DG and Krediet
RT: Analysis of fluid transport pathways and their determinants in
peritoneal dialysis patients with ultrafiltration failure. Kidney
Int. 70:1988–1994. 2006.PubMed/NCBI
|
|
23
|
Margetts PJ, Gyorffy S, Kolb M, et al:
Antiangiogenic and antifibrotic gene therapy in a chronic infusion
model of peritoneal dialysis in rats. J Am Soc Nephrol. 13:721–728.
2002.PubMed/NCBI
|
|
24
|
Hanahan D and Folkman J: Patterns and
emerging mechanisms of the angiogenic switch during tumorigenesis.
Cell. 86:353–364. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhao ZZ, Liu ZS and Liang XH: Expression
and significance of aquaporin and basic fibroblast growth factor in
human peritoneal mesothelial cells. Chin J Nephrol. 24:669–670.
2008.
|
|
26
|
Zhao ZZ, Cao Y and Liu ZS: The effects of
recombinant human edostatin on peritoneul angiogenesis in
peritoneal dialysis rats. Chin J Nephrol. 29:791–795. 2010.
|
|
27
|
Donnelly R and Yeung JM: Therapeutic
angiogenesis: a step forward in intermittent claudication. Lancet.
359:2048–2050. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Takagi H, Koyama S, Seike H, et al:
Potential role of the angiopoietin/tie2 system in ischemia-induced
retinal neovascularization. Invest Ophthalmol Vis Sci. 44:393–402.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang H and Liu ZL: Suppression of
experimental choroidal neovascularization by soluble Tie2 fusion
protein. Int J Ophthalmol. 8:272–274. 2008.
|
|
30
|
Shibuya M: Vascular endothelial growth
factor-dependent and -independent regulation of angiogenesis. BMB
Rep. 41:278–286. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Selgas R, del Peso G, Bajo MA, et al:
Vascular endothelial growth factor (VEGF) levels in peritoneal
dialysis effluent. J Nephrol. 14:270–274. 2001.PubMed/NCBI
|