Introduction
Obesity is becoming increasingly prevalent, leading
to reduced life expectancy and increased health problems (1). High body mass index (BMI) is
associated with various diseases, including cardiovascular,
obstructive sleep apnea, diabetes mellitus type 2, specific types
of cancer and osteoarthritis (2).
Bone metabolism is also abnormal in obesity (3) and positive and negative factors have
been identified to be associated with bone health status (4–6). A
number of previous studies have revealed that obesity stimulates
bone formation by inhibiting apoptosis. A recent study demonstrated
that high BMI prevents normal bone fracture and osteoporotic
fracture in various age groups and genders (7). By contrast, it has also been reported
that osteoporosis occurs in obese individuals (8). These inconsistencies may be due to
the use of different animal models in each study.
Adipose tissue, a large endocrine organ, secretes a
number of hormones that affect bone metabolism. Adipocytes in the
bone marrow microenvironment differentiate from bone marrow
mesenchymal cells and secrete cytokines which affect osteoblast and
osteoclast levels in bone marrow. The effect of adipocytes on
osteoblasts/osteoclasts remains controversial.
The aim of the present study was to identify the
function of adipocytes in the bone marrow microenvironment. To
analyze the biological foundations of bone metastasis in obesity, a
high-fat diet was selected to establish a mouse model of obesity
(9). The results indicate that
adipocytes accumulate in the bone marrow environment and affect
bone turnover and osteoblast/osteoclast differentiation.
Materials and methods
Chemicals and proteins
Recombinant murine receptor activator of nuclear
factor κB ligand (RANKL) was purchased from R&D Systems
(Minneapolis, MN, USA). Antibodies were purchased from Cell
Signaling Technology, Inc. (Danvers, MA, USA) and Abcam (Cambridge,
UK). All other chemicals were from Sigma-Aldrich (St. Louis, MO,
USA). Osteoprotegerin (OPG), RANKL and RANK ELISA kits were
obtained from R&D Systems. Alkaline phosphatase (ALP) and
tartrate-resistant acid phosphatase (TRAP) staining kits were
purchased from Sigma-Aldrich.
Animals
Male C57BL/6 mice were purchased from Beijing HFK
Bio-technology Co., Ltd. (Beijing, China) at 7–8 weeks old. Animals
were randomly divided into 2 groups. One group was housed in a 12-h
dark/light cycle and fed high-fat food and water ad libitum,
the other group was fed normal food (control). All procedures were
approved by the Animal Care and Use Committee, Tongji Medical
College (Wuhan, China)
Animals were sacrificed by CO2 inhalation
and cervical dislocation 12 weeks later. Weights of bilateral
inguinal fat and the right tibiae were recorded. The right tibiae
were analyzed using micro-computed tomography (mCT). The left
tibiae were embedded undecalcified in methylmethacrylate and
analyzed by histomorphometry.
mCT analysis
mCT analysis was performed using a Scanco μCT50
(Scanco Medical AG, Bassersdorf, Switzerland). Scans were performed
using the following instrument settings: E, 70 KVp; I, 110 μA,
increment, 10 μm; threshold value, 289. Parameters of the tibia
were computed by the software in μCT50.
Cell culture
The murine preosteoblastic cell line, MC3T3-E1, was
purchased from the American Type Culture Collection (ATCC;
Manassas, VA, USA). Murine bone marrow stromal cells (BMSCs) were
purchased from Cyagen (Guangzhou, China). RAW 264.7 murine
pre-osteoclastic cells were purchased from ATCC. The cells were
cultured in α-MEM (Hyclone Laboratories, Inc., Logan, UT, USA)
supplemented with 100 U/ml penicillin, 100 μg/ml streptomycin and
10% FCS (Gibco-BRL, Carlsbad, CA, USA) at 37ºC in a humidified
atmosphere of 5% CO2. The medium was changed every 3
days.
For adipocyte differentiation, BMSCs were cultured
in α-MEM supplemented with 10% FCS, 100 U/ml penicillin and 100
mg/ml streptomycin in a 5% CO2 humidified atmosphere and
allowed to reach confluence. After 2 days of confluence, BMSCs were
induced by exposure to a differentiation medium containing 10.0
μg/ml insulin, 1 μM dexamethasone and 0.5 mM
3-iso-butyl-1-methylxanthine in 10% FBS-supplemented α-MEM for 48
h. Following incubation, the culture medium was changed to α-MEM
supplemented with 10% FCS, 10.0 μg/ml insulin for an additional 48
h. Cells were cultured in α-MEM supplemented with 10% FCS, 100 U/ml
penicillin and 100 mg/ml streptomycin for 4 days. The cells were
then harvested and used for co-culture and detected by Oil Red
staining (Fig. 2A).
Osteoblast differentiation
A Transwell co-culture system was used to analyze
osteoblast differentiation. MC3T3-E1 cells (5×106
cells/well) were cultured in 6-well plates (Corning, Tewksbury, MA,
USA) until 80% confluence was reached after 2 days. MC3T3-E1 cells
were single- and co-cultured. For co-culture, following successful
induction of adipocytes in the upper chambers of the transwell
plate, the upper chamber was inserted into the 6-well plate with
MC3T3-E1 cells. Following 24 h, the culture medium was replaced
with condition medium (α-MEM containing 10% FCS, 10 mM
β-glycerophosphate, 50 μg/ml L-ascorbic acid and 100 nM
dexamethasone) for 7 days. The medium was changed every 2 days. For
the last 24 h prior to harvesting, cells were grown in 2 ml
condition medium without FCS and the medium was collected for ELISA
analysis. Cells in the lower chambers were detected using ALP
staining and activities.
Osteoclast differentiation
For osteoclast differentiation studies,
pre-osteoclast RAW 264.7 cells were used. Pre-osteoclast
differentiation was performed as described for preosteoblast
differentiation, however, the condition medium contained 10% FCS
and 10 ng/ml RANKL supplemented α-MEM. Harvested cells were
analyzed by TRAP staining and activities.
OPG and RANKL ELISA
OPG and RANKL levels were analyzed in the osteoblast
differentiation medium using a commercially available ELISA kit
according to the manufacturer’s instructions. Each sample was
analyzed three times.
RNA isolation and real-time PCR
analysis
Total RNA was isolated from harvested cells using
TRIzol reagent (Invitrogen Life Technologies, Carlsbad, CA, USA).
Extracted RNA was transcribed into cDNA using a cDNA RT kit
(Invitrogen Life Technologies) according to the manufacturer’s
instructions. Gene expression analysis was performed using
real-time PCR (iQ5; Bio-Rad, Hercules, CA, USA) and normalized
against β-actin. In the present study, runt-related transcription
factor 2 (RUNX2), ALP, osteocalcin, OPG and RANKL expression in
osteoblasts and RANK, cathepsin K (CTSK) and TRAP expression in
osteoclasts was detected.
Western blot analysis
Total cell lysates were obtained by lysing cells in
RIPA buffer containing 50 mM Tris-HCl, 150 mM NaCl, 1% NP-40, 0,1%
SDS, 0,5% sodium deoxycholate, 2 mM sodium fluoride, 1 mM EDTA, 1
mM EGTA and protease inhibitor cocktail. Protein concentration was
determined using the bicinchoninic acid protein assay (Pierce
Biotechnology, Inc., Rockford, IL, USA). Proteins were separated by
SDS-PAGE, transferred to nitrocellulose, blocked and incubated with
primary antibodies. The membrane was washed and incubated with the
respective secondary antibodies conjugated with peroxidase. Protein
detection was performed using a chemiluminescence detection system
(Pierce Biotechnology, Inc.). RUNX2, bone alkaline phosphatase,
osteocalcin, OPG and RANKL in osteoblasts and RANK and CTSK protein
expression in osteoclasts was detected.
Statistical analysis
Data were presented as the mean ± SEM. Statistical
analysis was performed using the Student’s t-test. Experiments were
repeated three times. P<0.05 was considered to indicate a
statistically significant difference.
Results
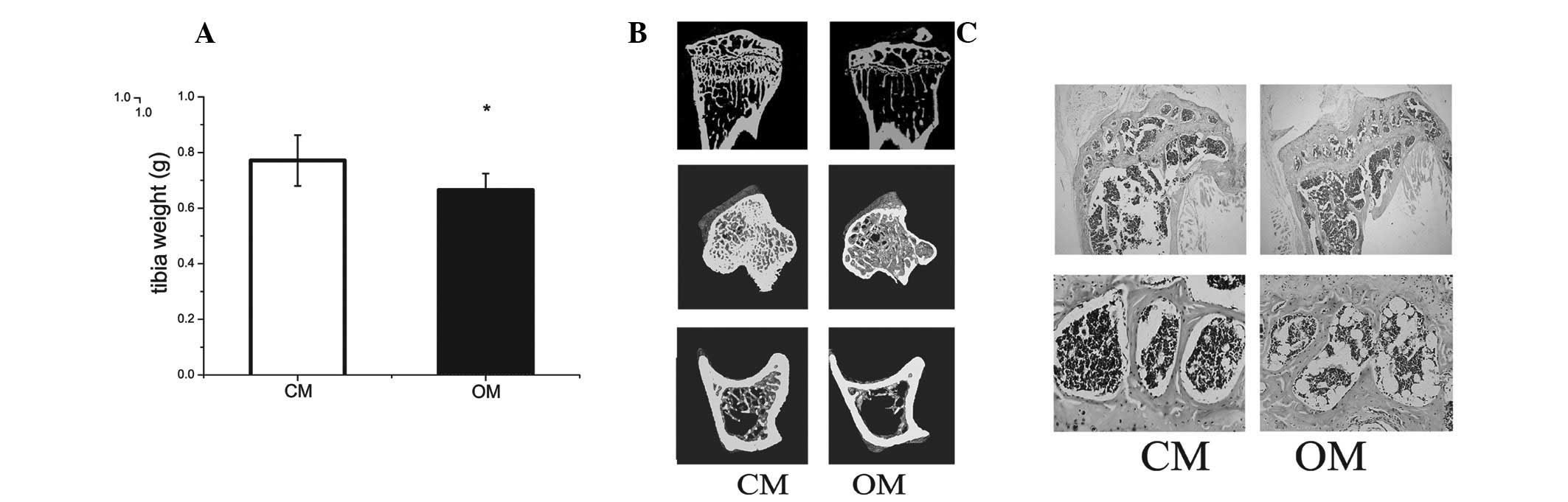
Obese mice have lower tibial bone mineral
density
Tibial weight in obese mice was lower than that of
the control (Fig. 1A). Micro-CT
revealed that bone mineralization density of tibiae in obese mice
was lower compared with the control (Fig. 1B and Table I).
 | Table IBMD of cortical bone mass in control
and obese mice. |
Table I
BMD of cortical bone mass in control
and obese mice.
| C57BL/6 (n=4) | Obese mice (n=4) | P-value |
|---|
| BMD (mg HA/ccm) | 1342.7±108.2 | 706.3±94.1a | 0.006 |
Histomorphometric analysis indicated an increased
number of adipocytes in the bone marrow of obese mice compared with
the control (Fig. 1C).
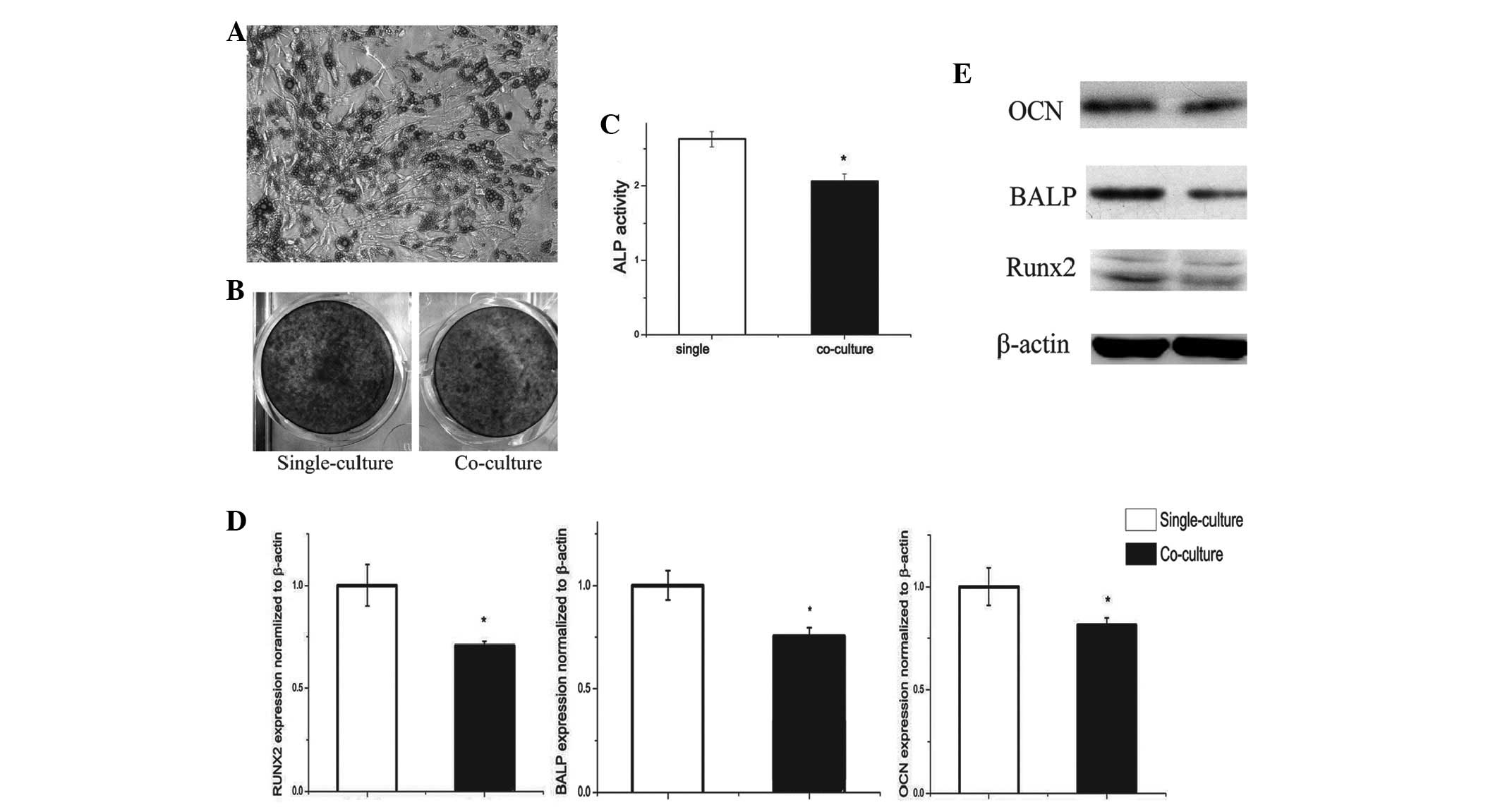
Differentiation of preosteoblasts is
decreased by co-culture with adipocytes
In the co-culture system, adipocytes from fully
differentiated BMSCs were observed to decrease mRNA and protein
expression of ALP (30%) and osteocalcin (OCN; 10%) in osteoblasts
at day 5 of differentiation (Fig.
2D).
BMSCs decreased mRNA levels of ALP and OCN in
osteoblasts at day 5 of differentiation. These results are
consistent with protein levels of these genes (Fig. 2E).
To determine whether adipocytes had any effect on
preosteoblast differentiation, ALP staining (Fig. 2B) and analysis of activity were
conducted. Co-culture for 7 days was found to significantly reduce
ALP activity by 34% compared with the single-culture group
(Fig. 2C).
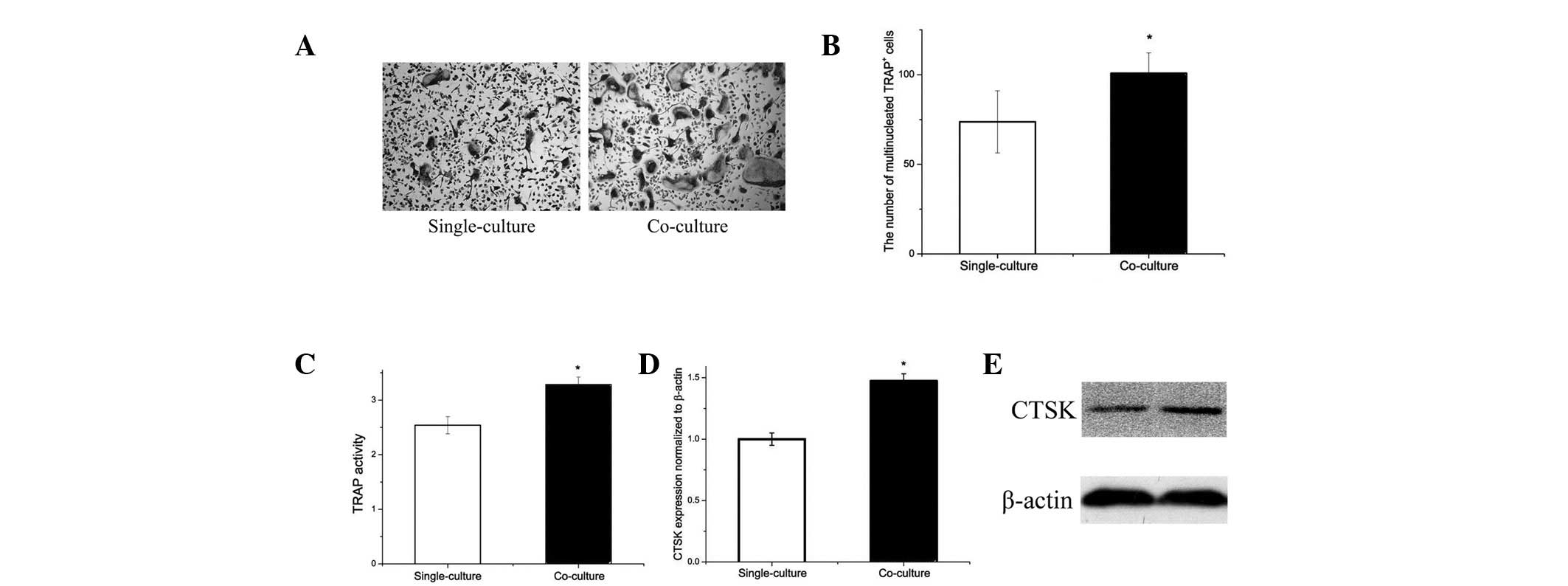
Differentiation of pre-osteoclasts is
increased by co-culture with adipocytes
Since obese mice exhibit accumulation of adipocytes
in the bone marrow microenvironment, the effect of adipocytes on
osteoclastogenesis and osteoclast-specific gene expression by
RANKL-induced osteoclast differentiation was analyzed. Following
co-culture with adipocytes, osteoclastogenesis was increased
compared with that of single-cultured samples (Fig. 3A). The number of multinucleated
TRAP-positive cells was increased by 35% (Fig. 3b) and the TRAP activity was 40%
higher in the co-culture compared with single-culture (Fig. 3C).
Gene expression analysis revealed that adipocytes
affect CTSK expression (Fig. 3D).
CTSK protein expression in osteoclasts was increased by co-culture
with adipocytes (Fig. 3E). The
results indicate that adipocytes may increase osteoclastogenesis
in vitro.
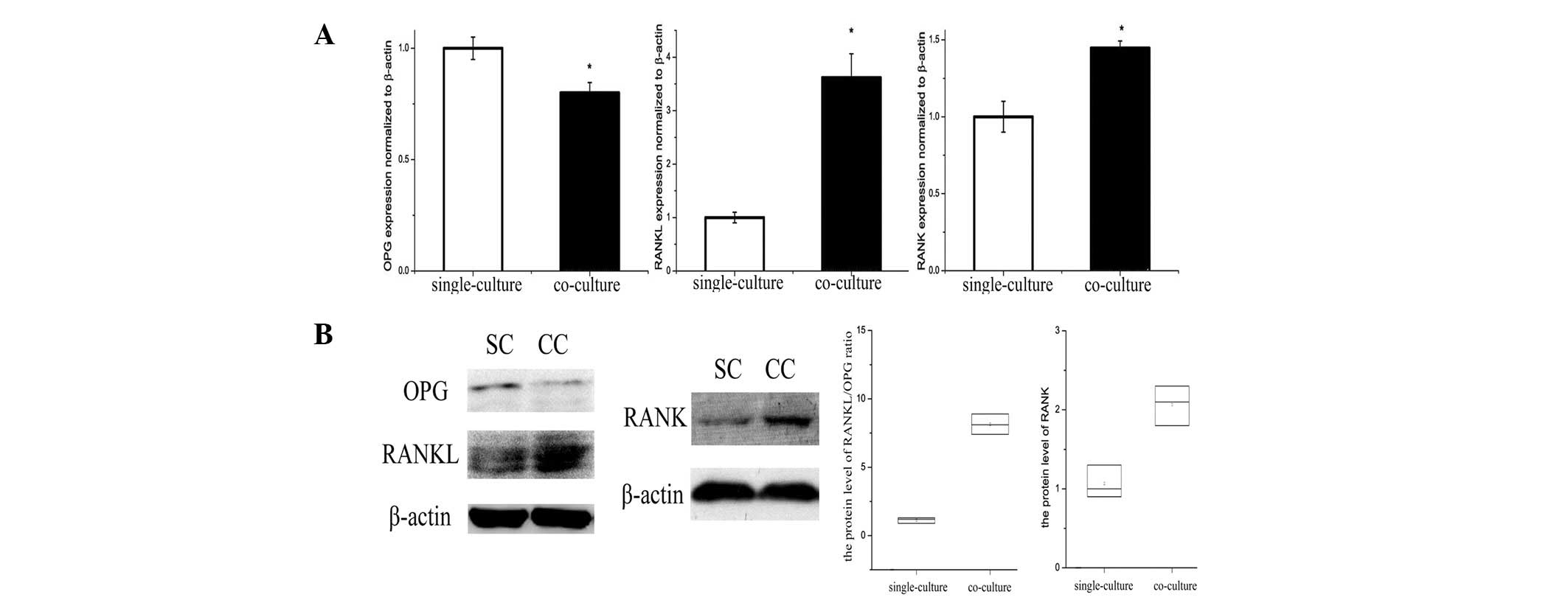
OPG/RANKL/RANK trail levels in
osteoblasts and osteoclasts are altered by co-culture with
adipocytes
OPG/RANKL/RANK is an essential mechanism for
regulation of bone metabolism, and was affected by adipocytes in
the bone marrow microenvironment. An increased RANKL/OPG ratio
secreted by co-cultured osteoblasts compared with single-cultured
was identified by ELISA analysis of cell culture medium (Table II). In addition, RANKL/OPG protein
expression in osteoblasts was decreased in the co-culture group
(Fig. 4A and B). RANK gene and
protein expression in osteoclasts was increased by co-culture with
adipocytes (Fig. 4A and B).
 | Table IIOPG and RANKL levels in the culture
medium of the osteoblastogenesis assay, detected by ELISA. |
Table II
OPG and RANKL levels in the culture
medium of the osteoblastogenesis assay, detected by ELISA.
| Single-culture
(pg/ml, n=6) | Co-culture (pg/ml,
n=6) | P-value |
|---|
| OPG | 1342.7±208.2 | 706.3±94.1a | 0.004 |
| RANKL | 1439.7±143.5 | 5471.2±432.5a | 0.009 |
Discussion
The present study investigated the effect of
adipocytes on preosteoblasts and preosteoclasts in the bone marrow
microenvironment. Results demonstrate that i) bone mineral density
(BMD) was significantly reduced in high-fat diet mice, ii)
pre-osteoblast function was suppressed, iii) differentiation of
pre-osteoclast was enhanced and iv) the RANKL/OPG ratio secreted by
pre-osteoblasts was increased and RANK expression in
pre-osteoclasts was increased by co-culture with adipocytes.
Adults with a high BMI have been previously reported
to be associated with low BMD (10). Obesity is being increasingly
recognized as a negative factor of bone metastasis in humans
(11). In this study, high-fat
diet mice exhibited high body weights. However, trabecular and
cortical bone densities in the tibia were low. In addition, the
number of adipocytes in bone marrow of high-fat diet mice was found
to be significantly higher than that of the control. By contrast, a
number of previous studies have observed that increased BMI is a
positive factor on femur cortical bone mass due to its effect on
leptin signaling (12). However,
an increasing number of studies have indicated that obesity in
female mice is accompanied by bone loss (8,13–15).
This may be due to the role of adipose tissue as an endocrine
organ, secreting pro-inflammatory cytokines, whose effects damage
the trabecular bone (16).
Therefore, the use of different mouse models in prevous studies may
explain these inconsistencies.
Adipocytes derived from BMSCs undergo crosstalk with
osteoblasts. In the present study, osteoblast expression of RUNX2,
osteocalcin and ALP mRNA and protein was decreased in the late
stages of differentiation following co-culture with adipocytes. It
has previously been reported that the adipocyte-secreted hormone,
adiponectin, may negatively modulate osteoblast function through
adiponectin- receptor I-mediated changes in PPAR-γ expression
(17). The osteogenic function of
osteoblasts was attenuated by adipocytes.
In this study, an osteoclastogenesis system was used
to analyze RANKL-induced RAW 264.7 cells differentiated to
osteoclastic cells exhibiting TRAP-positive staining (18). Osteoclastogenesis was found to be
significantly increased by co-culture with adipocytes. However, the
mechanism remains elusive. Oshima et al(19) demonstrated that adiponectin induced
by cytokines secreted by adipocytes directly suppresses
bone-resorption activity by inhibiting osteoclastogenesis. However,
observations of Goto et al(20) demonstrate that primary human bone
marrow adipocytes promote osteoclast differentiation and
activities. Leptin, an adipocyte-derived cytokine, has been found
to function as a negative regulator of bone mass in a mouse model
(21).
The OPG/RANKL/RANK system in the bone
microenvironment is an essential mechanism for regulation of bone
metabolism. RANKL combined with RANK is associated with
osteoclastogenesis through the c-Jun N-terminal kinase, nuclear
factor κB and protein kinase B-mediated signaling pathways, which
promote bone resorption. By contrast, RANKL combined with OPG
inhibits osteoclast differentiation, which impairs bone resorption.
The ratio of RANKL/RANK and RANKL/OPG regulates the bone
microenvironment. If the balance between these ratios is disturbed,
bone metabolism derangement is likely to occur (22).
The results of this study indicate that the
OPG/RANKL ratio secreted by osteoblasts was decreased by adipocyte
stimulation. As a result of reduced OPG/RANKL ratio,
osteoclastogenesis was increased and was shown to be consistent
with CTSK staining of bone sections from our animal model. In
addition, osteoclast RANK expression was upregulated. Following
this, adipocyte-secreted cytokines affected the OPG/RANKL/RANK
trail system, resulting in a marked increase in osteoclast
differentiation. Halade et al(16) reported that OPG secretion decreased
and RANKL expression increased in osteoblasts stimulated with
adipocyte-secreted factors. By contrast, Goto et al(20) demonstrated that adipocyte-secreted
cytokines upregulated RANKL mRNA expression in osteoblasts,
consistent with osteoclast differentiation in vitro. These
observations are in agreement with those of this study and
demonstrate that adipocytes increase osteoclastogenesis via the
OPG/RANKL/RANK system in the bone microenvironment.
In conclusion, the present in vivo study has
demonstrated that obesity caused by a high-fat diet results in
significant bone resorption by increasing osteoclast and decreasing
osteoblast functions. In addition, adipocyte-secreted cytokines
were observed to attenuate osteoblast function and significantly
increase osteoclastogenesis. The balance of the OPG/RANKL/RANK
system was disrupted by adipocyte-derived cytokines. The results
indicate that obesity is associated with decreases in bone density
partly due to increased osteoclastogenesis.
Acknowledgements
The present study was supported by a research grant
from the National Natural Sciences Research Program of China (no.
81070691).
Abbreviations:
|
TRAP
|
tartrate-resistant acid
phosphatase
|
|
RANK
|
receptor activator of nuclear factor
κB
|
|
RANKL
|
receptor activator of nuclear factor
κB ligand
|
|
OPG
|
osteoprotegerin
|
|
CTSK
|
cathepsin K
|
References
|
1
|
Bahia L, Coutinho ES, Barufaldi LA, et al:
The costs of overweight and obesity-related diseases in the
Brazilian public health system: cross-sectional study. BMC Public
Health. 12:4402012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mathus-Vliegen EM: Obesity and the
elderly. J Clin Gastroenterol. 46:533–544. 2012. View Article : Google Scholar
|
|
3
|
Cao JJ: Effects of obesity on bone
metabolism. J Orthop Surg Res. 6:302011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Halade GV, Rahman MM, Williams PJ and
Fernandes G: High fat diet-induced animal model of age-associated
obesity and osteoporosis. J Nutr Biochem. 21:1162–1169. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bredella MA, Torriani M, Ghomi RH, et al:
Vertebral bone marrow fat is positively associated with visceral
fat and inversely associated with IGF-1 in obese women. Obesity
(Silver Spring). 19:49–53. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dimitri P, Bishop N, Walsh JS and Eastell
R: Obesity is a risk factor for fracture in children but is
protective against fracture in adults: a paradox. Bone. 50:457–466.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Prieto-Alhambra D, Premaor MO, Fina Aviles
F, et al: The association between fracture and obesity is
site-dependent: a population-based study in postmenopausal women. J
Bone Miner Res. 27:294–300. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kawai M, de Paula FJ and Rosen CJ: New
insights into osteoporosis: the bone-fat connection. J Intern Med.
272:317–329. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Aguila MB, Pinheiro Ada R, Parente LB and
Mandarim-de-Lacerda CA: Dietary effect of different high-fat diet
on rat liver stereology. Liver Int. 23:363–370. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Compston JE, Watts NB, Chapurlat R, et al:
Obesity is not protective against fracture in postmenopausal women:
GLOW. Am J Med. 124:1043–1050. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shapses SA and Sukumar D: Bone metabolism
in obesity and weight loss. Annu Rev Nutr. 32:287–309. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Thomas T and Burguera B: Is leptin the
link between fat and bone mass? J Bone Miner Res. 17:1563–1169.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Verzeletti GN, Gaio EJ, Linhares DS and
Rösing CK: Effect of obesity on alveolar bone loss in experimental
periodontitis in Wistar rats. J Appl Oral Sci. 20:218–221. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dytfeld J, Ignaszak-Szczepaniak M, Gowin
E, et al: Influence of lean and fat mass on bone mineral density
(BMD) in postmenopausal women with osteoporosis. Arch Gerontol
Geriatr. 53:e237–e242. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen JR, Lazarenko OP, Wu X, et al:
Obesity reduces bone density associated with activation of PPARγ
and suppression of Wnt/β-catenin in rapidly growing male rats. PLoS
One. 5:e137042010.PubMed/NCBI
|
|
16
|
Halade GV, El Jamali A, Williams PJ, et
al: Obesity-mediated inflammatory microenvironment stimulates
osteoclastogenesis and bone loss in mice. Exp Gerontol. 46:43–52.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Savopoulos C, Dokos C, Kaiafa G and
Hatzitolios A: Adipogenesis and osteoblastogenesis:
trans-differentiation in the pathophysiology of bone disorders.
Hippokratia. 15:18–21. 2011.PubMed/NCBI
|
|
18
|
Hirotani H, Tuohy NA, Woo JT, et al: The
calcineurin/nuclear factor of activated T cells signaling pathway
regulates osteoclastogenesis in RAW264.7 cells. J Biol Chem.
279:13984–13992. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Oshima K, Nampei A, Matsuda M, et al:
Adiponectin increases bone mass by suppressing osteoclast and
activating osteoblast. Biochem Biophys Res Commun. 331:520–526.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Goto H, Osaki M, Fukushima T, et al: Human
bone marrow adipocytes support dexamethasone-induced osteoclast
differentiation and function through RANKL expression. Biomed Res.
32:37–44. 2011. View Article : Google Scholar
|
|
21
|
Fujita Y, Watanabe K and Maki K: Serum
leptin levels negatively correlate with trabecular bone mineral
density in high-fat diet-induced obesity mice. J Musculoskelet
Neuronal Interact. 12:84–94. 2012.PubMed/NCBI
|
|
22
|
Tyrovola JB, Spyropoulos MN, Makou M and
Perrea D: Root resorption and the OPG/RANKL/RANK system: a mini
review. J Oral Sci. 50:367–376. 2008. View Article : Google Scholar : PubMed/NCBI
|