Introduction
Thrombus is the main cause of atherothrombotic
disease, which itself is the primary cause of increased morbidity
worldwide. Therefore, inhibiting platelet function is a promising
approach for preventing thrombosis (1,2).
Injury to the blood vessel wall triggers rapid platelet activation
and platelet plug formation, followed by blood coagulation and the
formation of fibrin-containing thrombins that occlude the site of
injury. These events limit vital blood loss at the site of injured
tissue, but may also block narrow or diseased vessels, leading to
ischemia and/or tearing of vital organs (3,4).
Furthermore, platelet aggregation and subsequent thrombus formation
in coronary and cerebral arteries may cause myocardial infarction
and stroke, respectively. Platelets are necessary in thrombus
formation in injured blood vessels (5). Thus, excessive platelet aggregation
produces a pathological thrombus and plays a significant role in
the initiation and pathogenesis of atherothrombotic diseases.
A thrombus is composed of fibrin, which is produced
from its precursor, fibrinogen, by thrombin. An inappropriate
fibrin clot must be eliminated rapidly by fibrinolysis in order to
maintain homeostasis (6).
Dissolution of a fibrin clot is dependent on the action of plasmin,
a serine protease that is activated by a tissue plasminogen
activator. In clinical therapy, thrombolytic agents (fibrinolytic
enzymes), such as tissue plasminogen activator and urokinase,
convert inactive plasminogen to active plasmin, allowing
fibrinolysis to occur (7).
Antiplatelet agents, including aspirin,
thienopyridines and platelet glycoprotein (GP)IIb/IIIa receptor
inhibitors, have been extensively researched and developed as
potential therapies for the treatment and prevention of
cardiovascular disease. However, these reagents have several
clinical disadvantages including gastrointestinal side-effects and
hemorrhage (8–11).
The medicinal plant Ulmus macrocarpa Hance
(Ulmaceae) is a deciduous tree, widely distributed in Korea
(12). The stem and root bark of
Ulmus macrocarpa (Hance) have been used as an oriental
traditional medicine for the treatment of edema, mastitis, gastric
cancer, and inflammation. It has been reported that Ulmus
macrocarpa Hance, of the Ulmaceae family, exhibits marked
anti-oxidative activity on lipid peroxidation and an inhibitory
effect on endogenous nitric oxide (NO)-induced apoptotic cell death
(13). However, the antiplatelet
and anticoagulant effects of Ulmus macrocarpa have not been
reported previously. In this study, the inhibitory activities of
Ulmus macrocarpa extract (UME) on platelet aggregation in
vitro and ex vivo were examined. The fibrinolytic
effects of UME were also investigated. The antithrombotic activity
of UME in a ferric chloride (FeCl3)-induced arterial
thrombosis rat model was also investigated.
Materials and methods
Materials and animals
Collagen and adenosine 5′-diphosphate (ADP) were
purchased from Chrono-Log Co. (Havertown, PA, USA). Aspirin,
fibrinogen, thrombin, plasmin and DMSO were purchased from
Sigma-Aldrich (St. Louis, MO, USA). Thromboplastin and calcium
chloride were purchased from Instrumentation Laboratory Co. (Milan,
Italy). Other chemicals were of analytical grade. The standard
marker compounds, (+)-catechin and (−)-epicatechin were purchased
from Sigma-Aldrich (purity ≥98%). HPLC-grade reagents, methanol and
water were obtained from J.T. Baker (Phillipsburg, NJ, USA).
Male Sprague-Dawely rats weighing between 240–250 g
were obtained from Orient Co. (Seoul, Korea) and maintained in a
standard laboratory animal facility with free access to feed and
water and were allowed to acclimatize for at least two weeks prior
to the start of the study. The animal studies were carried out in
accordance with the Korea Institute of Oriental Medicine Care
Committee Guidelines.
UME preparation
Ulmus macrocarpa was obtained as a dried herb
from JungDo Co. (Seoul, Korea) and was authenticated, based on its
microscopic and macroscopic characteristics, by the Classification
and Identification Committee of the Korea Institute of Oriental
Medicine. The 70% ethanol extract from Ulmus macrocarpa was
made according to the following procedure. The roots of the plant
were boiled twice at 70–80°C each time for 2 h. The filtrates were
collected, concentrated and evaporated to dryness at 40–50°C under
vacuum. The dosage of the extract was indicated in the powdered
form. The powder was dissolved in 20 or 50% DMSO (v/v, in PBS) for
the experiments.
HPLC analysis
HPLC analysis was performed on a Waters 2695 system
(Waters Co. Milford, MA, USA), consisting of a solvent delivery
unit, an online degasser, an autosampler and a photodiode array
detector. A Luna C18 column (250 ×4.6 mm; particle size 5 μm,
Phenomenex, Torrance, CA, USA) was used for analysis and the mobile
phase was water (A) and MeOH (B). The gradient flow was as follows:
(A)/(B) = 10/90 (0 min) → (A)/(B) = 40/60 (30 min) → (A)/(B) =
0/100 (32 min; hold for 5 min) → (A)/(B) = 10/90 (38 min; hold for
12 min). The flow rate was 1.0 ml/min, the injection volume was 10
μl and the detection wavelength was 280 nm.
A standard stock solution mixture of (+)-catechin
and (−)-epicatechin were prepared in 70% EtOH at a concentration of
1,000 μg/ml, respectively, and stored at 4°C. UME powder (500 mg)
was transferred to a 100 ml volumetric flask and dissolved up to
the 100 ml mark with methanol. The mixture was subsequently
sonicated for 30 min at room temperature and filtered through a 0.2
μm membrane filter. Calibration of the standard mixture was
prepared by serial dilution of the standard stock solution mixture
to yield concentrations of 10–1,000 μg/ml for (+)-catechin and
(−)-epicatechin. These standard component solutions at five
different concentrations were injected in triplicate. Calibration
curves were generated by plotting the peak areas vs. the
concentrations of the two components.
Arterial thrombus formation in vivo
Arterial thrombus formation in vivo was
investigated as previously described (14). UME was orally administered on a
daily basis at doses of 300 and 600 mg/kg for 3 days. The rats were
fasted overnight, and then orally administered the extract or the
1% carboxymethylcellulose (CMC) solution as a vehicle. The rats
were i.p. anaesthetized with 60 mg/kg pentobarbital sodium salt and
placed on a heat source. A segment of the right carotid artery was
isolated and dissected free of the vagus nerve and surrounding
tissues. Aortic blood flow was measured with a Doppler velocimeter
(ADInstruments, Colorado Springs, CO, USA). Arterial thrombus
formation was induced by wrapping a 2 mm2 Whatmanno no.
1 filter paper saturated with 50% FeCl3 around the
carotid artery near the probe for 10 min. The time required for
occlusion was measured for up to 60 min. An occlusion time of 60
min was assigned for vessels that did not occlude within 60
min.
Platelet aggregation and coagulation
times ex vivo
Ex vivo platelet aggregation was investigated
as previously described (14). The
rats (n=7) were orally administered UME at a dose of 300 mg/kg for
3 days. Platelet-rich plasma (PRP) was obtained by centrifuging the
blood sample at 180 × g for 10 min, and platelet poor plasma (PPP)
was obtained by centrifuging the PRP at 2,100 × g for 10 min
continuously. PRP was adjusted to 4×108 platelets/ml
with PPP. Platelet aggregation was measured with an aggregometer
(Chrono-Log Co.), and collagen (5 μg/ml) and ADP (5 μM) were used
as aggregation stimulators. The plasma-activated partial
thromboplastin time (APTT) and prothrombin time (PT) were measured
with an Automated Coagulation Laboratory 7000 Instrument
(Instrumentation Laboratory) as previously described (14). PPP was incubated at 37°C for 7 min,
and subsequently 100 μl of incubated plasma was mixed with 50 μl of
cephalin in the process plate. Coagulation was initiated by the
addition of CaCl2 plus 100 μl thromboplastin or 100 μl
polybrene for the APTT and PT assays, respectively.
Platelet preparation and aggregation in
vitro
PRP was obtained by centrifuging the blood samples
at 180 × g for 10 min, and PPP was obtained by centrifuging the PRP
at 2,100 × g for 10 min. PRP was adjusted to 4×108
platelets/ml with PPP. The number of platelets was counted using a
Coulter Counter (Coulter Electronics, Inc., Hialeah, FL, USA).
Evaluating platelet aggregation was undertaken by
optical platelet aggregometry in an aggregometer (Chrono-Log Co.).
Aggregation was recorded as the percentage change in light
transmission; the baseline value was set using PRP and the maximal
transmission using PPP. They were incubated at 37°C for 4 min in
the aggregometer. Following incubation, platelet aggregation was
induced by the addition of collagen (5 μg/ml) or ADP (5 μM). The
inhibition of platelet aggregation for each sample was expressed as
the percentage decrease in the maximal transmittance compared with
the stimulated control.
Fibrinolytic activity assay in vitro
Fibrinolytic activity was evaluated according to the
method of Astrup and Mullertz with minor modifications (15). The fibrin plate was prepared by
mixing 0.6% human fibrinogen solution (Sigma-Aldrich) in 1X PBS (pH
7.4) with 50 NIH units of human thrombin. In order to speed up the
clotting process, the plates were incubated at 37°C for 30 min.
Plates were prepared fresh each time. UME (250, 500 and 1,000
mg/ml), standard solutions (plasmin) and control solutions were
placed separately onto membrane discs in the fibrin plate. We used
20% DMSO in PBS as a control. The plates were incubated at 37°C for
15 h. The diameters of the transparent rings were measured with
calipers (Mitutoyo Co., Tokyo, Japan), and subsequently the
fibrinolysis area (mm2) was calculated. The mean
diameter of the hydrolyzed clear zone was measured and volume of
lysis caused by each sample was calculated.
Statistical analysis
Experimental results were expressed as the means ±
SD. Differences between the groups were used for multiple
comparisons followed by an unpaired Student’s t-test. P<0.05 was
considered to indicate a statistically significant difference.
Results
HPLC analysis
We employed HPLC to confirm and quantify the
standard contents of UME, such as catechin and epicatechin, for
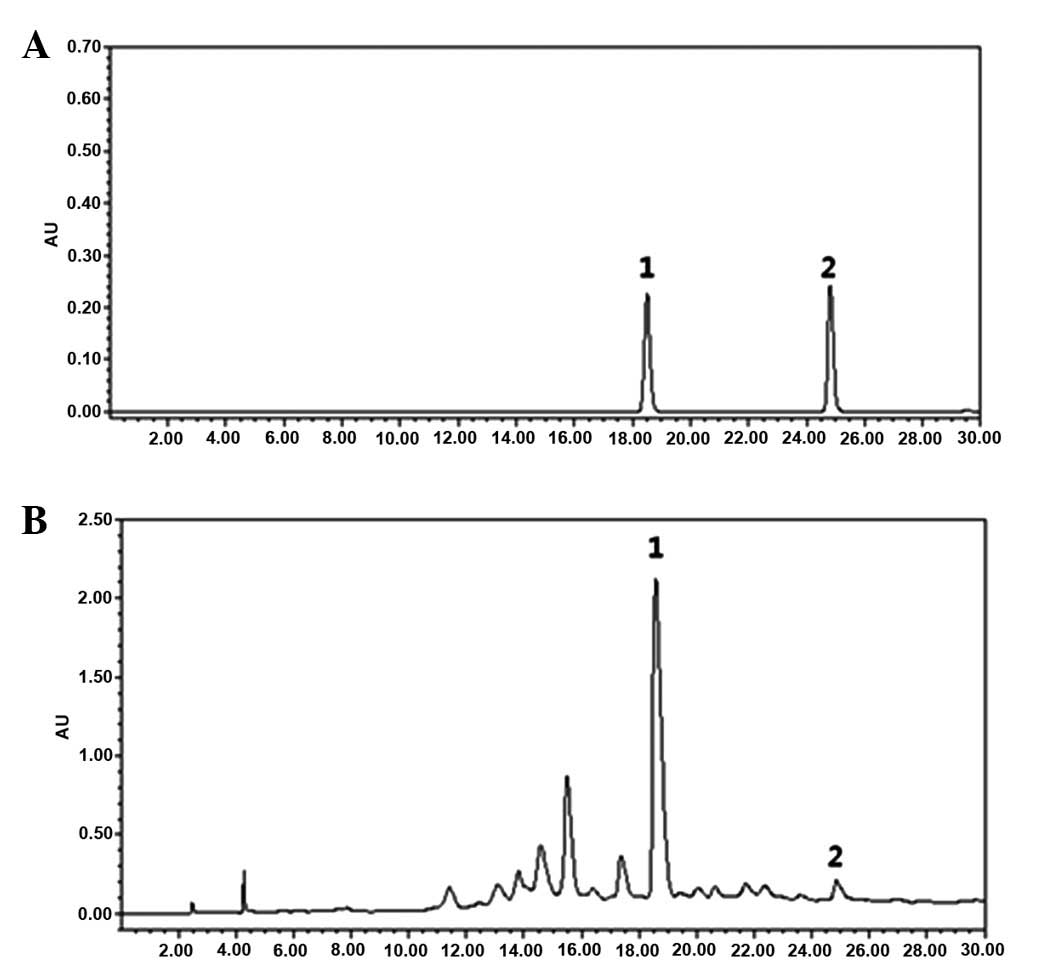
standardization and quality control. The representative
chromatogram at 280 nm reveals that UME contained two marker
compounds, catechin and epicatechin whose peaks were retained at
18.55 and at 24.83 min, respectively (Fig. 1). Correlation coefficients were
better than 0.999 for all standard components. The limits of
detection (LOD) and quantification (LOQ) for both components were
1.5 and 5.0 μg/ml, respectively. The catechin and epicatechin
levels in UME were quantified as 1.397 and 0.069%,
respectively.
Effect of UME on arterial thrombus
formation in vivo
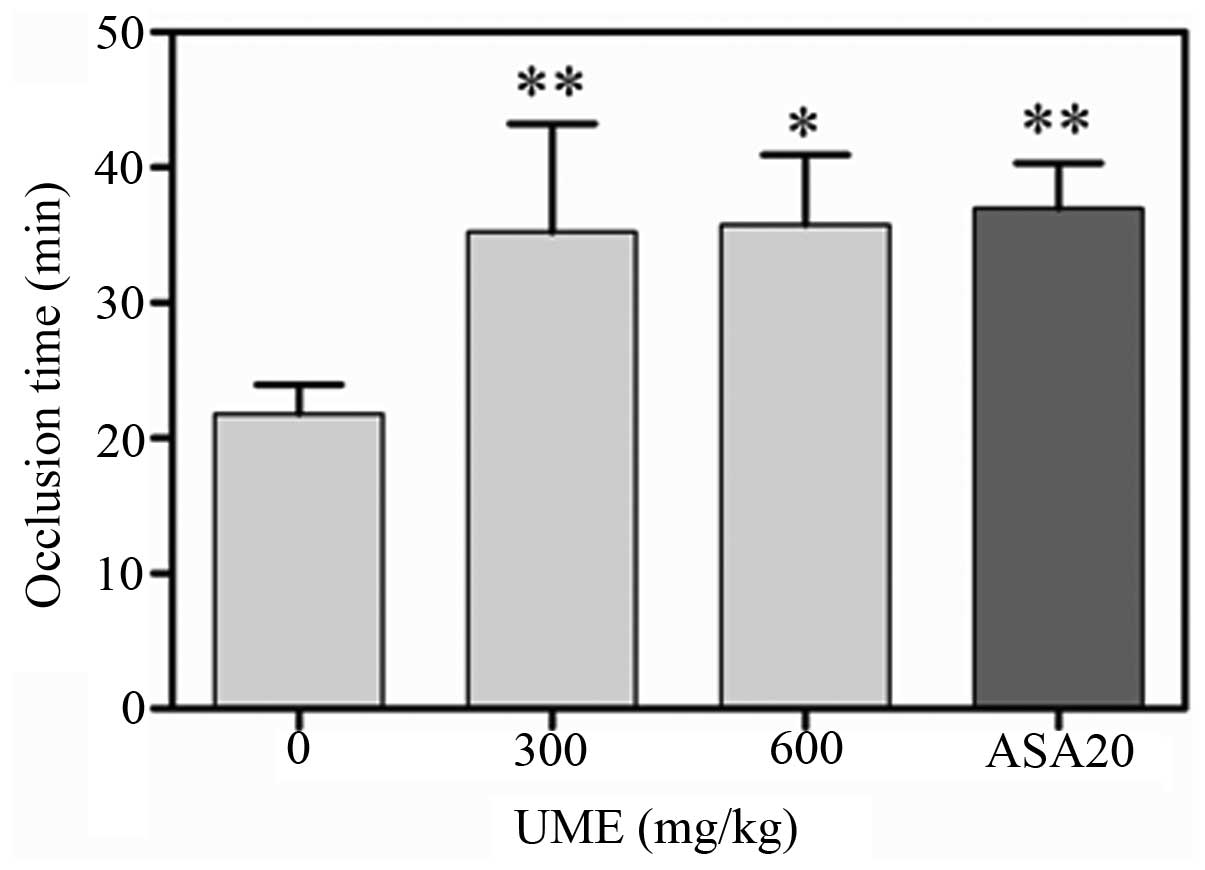
The effect of UME on arterial thrombus formation
in vivo was evaluated by using the FeCl3-induced
rat carotid artery injury model. Following the application of 50%
FeCl3, injured vessels of the control group occluded
within 21.0 ± 5.7 min (n=7). Oral UME treatment for 3 days
significantly extended the occlusion times to 13.4 ± 8.0 (n =7) and
13.9 ± 13.6 min (n =7) for doses of 300 and 600 mg/kg, respectively
(Fig. 2).
Effect of UME on platelet aggregation and
coagulation times ex vivo
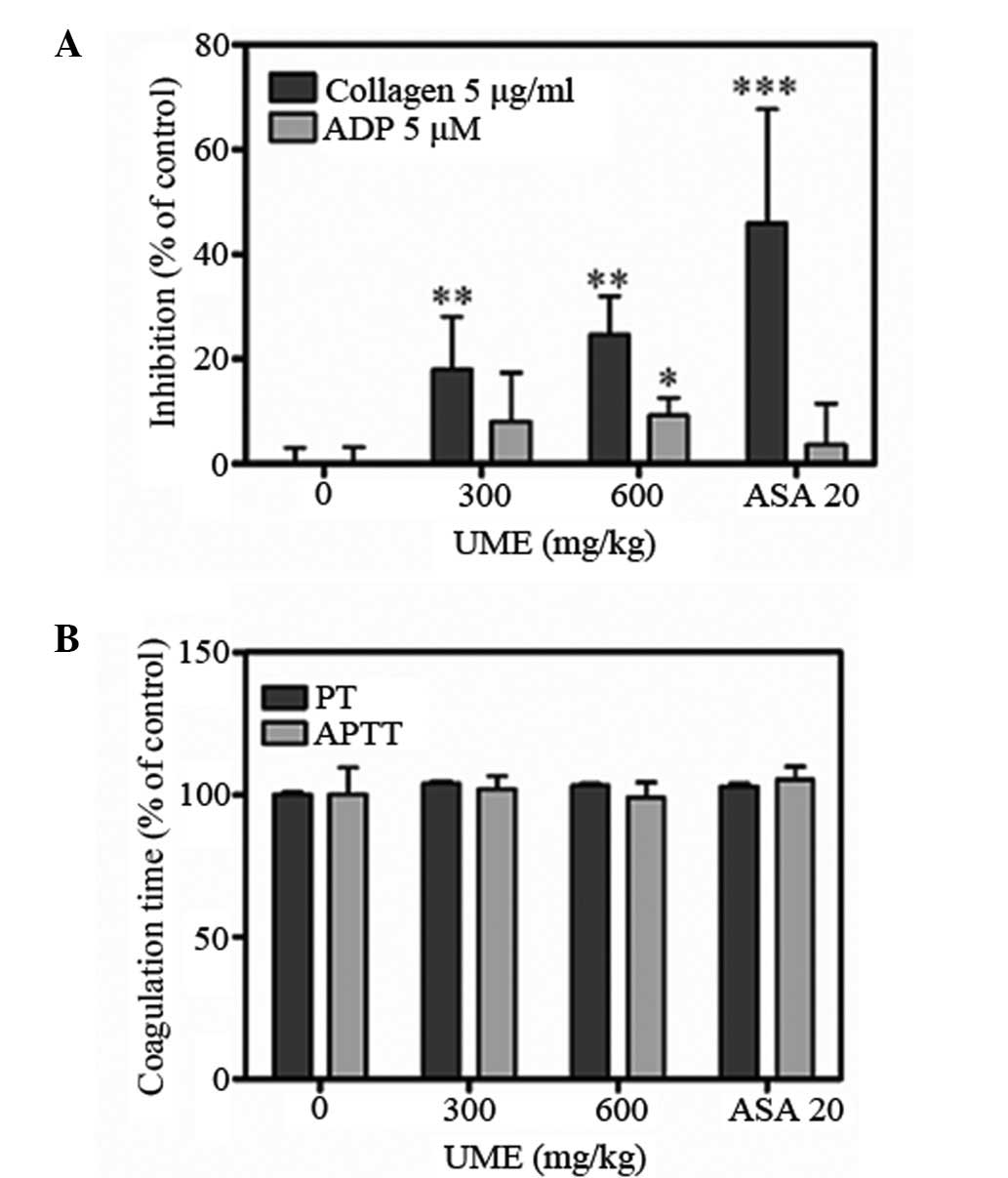
UME significantly inhibited ADP- and
collagen-induced platelet aggregations ex vivo (Fig. 3A). Collagen- and ADP-stimulated
aggregations were inhibited by 18.0±10.0 and 8.0±9.4% at 300mg/kg,
and 24.7±7.3 and 9.3±3.3% at 600mg/kg, respectively (n=7). The APTT
and PT of the control group were 17.5±1.7 and 14.7±10.0 sec,
respectively (Fig. 3B). UME
treatment did not significantly alter APTT and PT, which were
17.8±0.8 and 15.2±0.9 sec at 300mg/kg, and 17.3±0.9 and 15.1±0.7
sec at 600 mg/kg, respectively. These data indicate that UME
significantly inhibits platelet aggregation but does not affect the
coagulation system. Aspirin (20 mg/kg), was used as a positive
control and completely blocked collagen-induced aggregation.
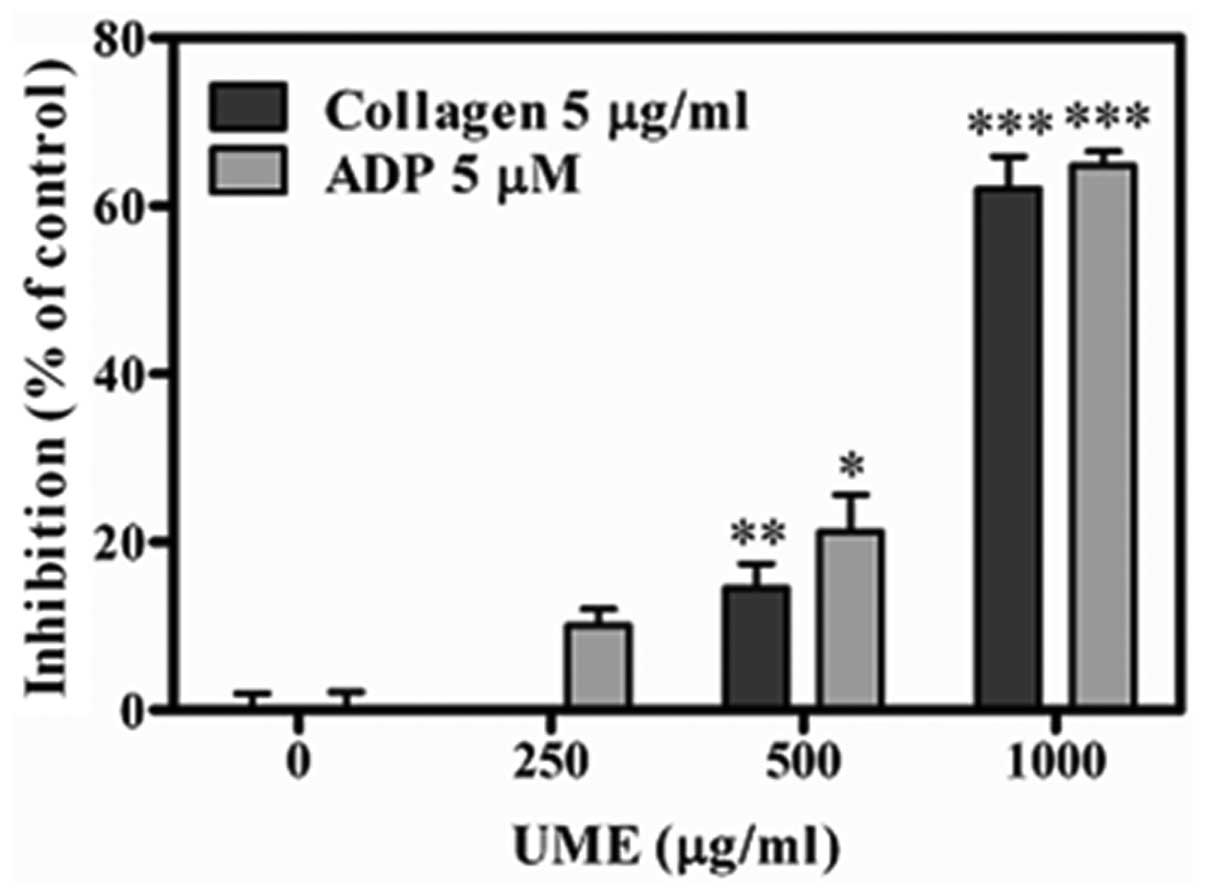
Effect of UME on platelet aggregation in
vitro
UME significantly inhibited collagen-(5 μg/ml), and
ADP (5 μM)-induced platelet aggregations in vitro in a
concentration-dependent manner, with IC50 values of
915.7±4.6 and 833.3±2.5 μg/ml, respectively (Fig. 4). This result supports the
anti-platelet effect on platelet aggregation ex vivo.
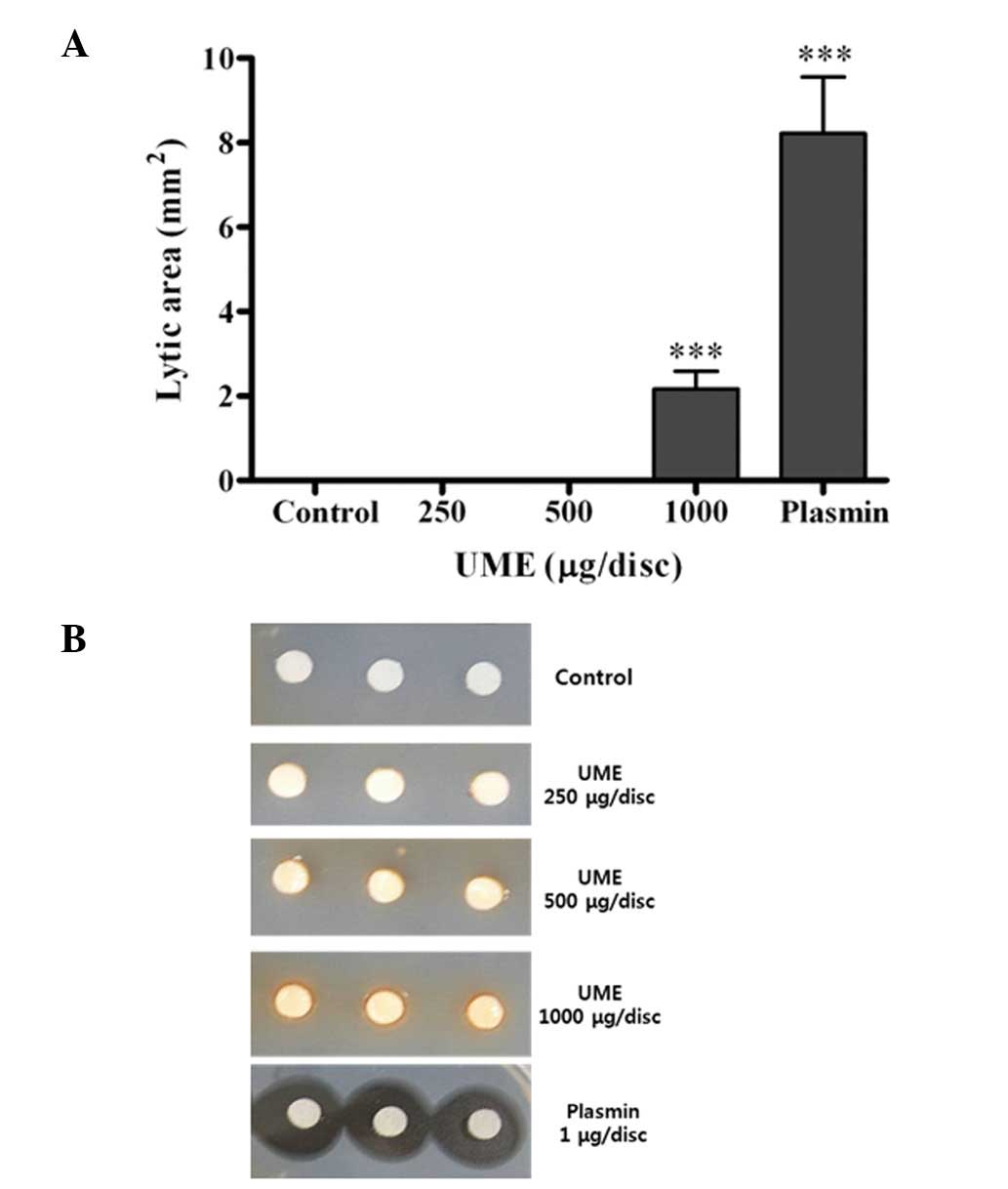
Effect of UME on fibrinolytic activity in
vitro
In the fibrin plate, fibrinolysis was increased by
treatment with UME in a dose-dependent manner (Fig. 5). UME induced fibrinolysis at 1,000
μg/ml, compared with the control group (2.2±0.4 vs. 0.0±0.0
mm2), respectively.
Discussion
Blood flow disturbances at sites of atherosclerotic
plaque rupture promote platelet activation and arterial thrombus
formation (16,17). In the present study, UME
significantly inhibited thrombus formation in rat carotid arteries
in vivo and platelet aggregation ex vivo, but did not
affect coagulation times such as PT and APTT. Therefore, UME has
the potential to prevent thrombotic or cardiovascular diseases via
antiplatelet, rather than anticoagulation activity.
Thus, we investigated the activities of UME as an
antiplatelet and thrombolytic agent. The results indicated that
Ulmus macrocarpa may have the potential to prevent
thrombotic and cardiovascular diseases, which may be due to its
antiplatelet and fibrinolytic effects. Inappropriate thrombus
formation is associated with a failure in homeostasis, and the
major elements involved are coagulation factors, platelets and
blood vessels (18).
We examined the in vivo antithrombotic effect
of UME in a FeCl3-mediated artery thrombosis in a rat
model, composed of fibrin, activated platelets and entrapped
erythrocytes. This type of thrombus is found in the coronary
arteries following sudden mortality and acute myocardial infarction
(19,20). In this model, FeCl3
induces oxidative injury and exposes the subendothelial matrix.
Platelets interact with the matrix via GPIb-V-IX and α2bβ3 on the
platelet membrane, and collagen and vWF in the matrix. Glycoprotein
VI binding to collagen is required for platelet activation, and
activated platelets undergo calcium mobilization and the release of
ADP and TXA2 to accelerate platelet recruitment and
activation for thrombus formation (21). Thus, platelet aggregation
contributes to thrombus formation. UME significantly increased the
occlusion time in a dose-dependent manner (Fig. 2), which indirectly indicated that
UME may inhibit thrombus formation in vivo. The exact
mechanism by which thrombus formation was triggered in this model
is unclear, but it has been demonstrated that the morphology of the
thrombi is similar to those found in humans (22). Therefore, we examined whether UME
affected platelet activity and plasma coagulation using ex
vivo platelet aggregation and coagulation assays. UME
significantly and dose-dependently inhibited collagen- and
ADP-induced platelet aggregation but did not affect plasma
coagulation times (Fig. 3). Thus,
the antithrombotic effect of UME in the artery thrombosis model may
result from its antiplatelet rather than its anticoagulation
activity (23–25).
UME was found to exhibit antiplatelet aggregation
activity, induced by ADP and collagen (Fig. 4). This supports the theory that UME
has an anti-platelet effect on platelet aggregation ex vivo.
Furthermore, we investigated the fibrinolytic activity of UME using
plasmin as a positive control. Plasmin was converted from
plasminogen, whereupon plasmin degraded the fibrin network in clots
(26). In this study, UME
dose-dependently increased fibrinolysis in the fibrinolytic assay
(Fig. 5).
In conclusion, we demonstrated that UME
significantly inhibits artery thrombus formation in vivo,
which may be due to its antiplatelet activity, and also
demonstrated potential as a clot-dissolving agent for thrombolytic
therapy. The beneficial effect of UME on the cardiovascular system
may be due to its modulation of platelet activation.
Acknowledgements
This study was supported by the ‘Discovery of Herbal
Medicine for the Prevention of Prehypertension’ project (K13202)
from the Ministry of Education, Science and Technology of
Korea.
References
|
1
|
Pepine CJ: Residual risk for secondary
ischemic events in patients with atherothrombotic disease:
opportunity for future improvements in patient care. Ann Med.
42:19–35. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Varon D and Spectre G: Antiplatelet
agents. Hematology Am Soc Hematol Educ Program. 267–272. 2009.
View Article : Google Scholar
|
|
3
|
Aronow WS: Management of peripheral
arterial disease of the lower extremities in elderly patients. J
Gerontol A Biol Sci Med Sci. 59:172–177. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
McNicol A and Israels SJ: Platelets and
anti-platelet therapy. J Pharmacol Sci. 93:381–396. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Stoyioglou A and Jaff MR: Medical
treatment of peripheral arterial disease: a comprehensive review. J
Vasc Interv Radiol. 15:1197–1207. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Medved L and Nieuwenhuizen W: Molecular
mechanisms of initiation of fibrinolysis by fibrin. Thromb Haemost.
89:409–419. 2003.PubMed/NCBI
|
|
7
|
Collen D and Lijnen HR: Thrombolytic
agents. Thromb Haemost. 93:627–630. 2005.PubMed/NCBI
|
|
8
|
Rezkalla SH and Benz M: Antiplatelet
therapy from clinical trials to clinical practice. Clin Med Res.
1:101–104. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gum PA, Kottke-Marchant K, Welsh PA, White
J and Topol EJ: A prospective, blinded determination of the natural
history of aspirin resistance among stable patients with
cardiovascular disease. J Am Coll Cardiol. 41:961–965. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bhatt DL and Topol EJ: Scientific and
therapeutic advances in antiplatelet therapy. Nat Rev Drug Discov.
2:15–28. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Eisert WG: How to get from antiplatelet to
antithrombotic treatment. Am J Ther. 8:443–449. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim KW, Park JS, Kim KS, et al: Inhibition
of Ulmus davidiana Planch (Ulmaceae) on bone resorption
mediated by processing of cathepsin K in cultured mouse
osteoclasts. Phytother Res. 22:511–517. 2008.
|
|
13
|
Jun CD, Pae HO, Kim YC, et al: Inhibition
of nitric oxide synthesis by butanol fraction of the methanol
extract of Ulmus davidiana in murine macrophages. J
Ethnopharmacol. 62:129–135. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jin YR, Ryu CK, Moon CK, Cho MR and Yun
YP: Inhibitory effects of J78, a newly synthesized
1,4-naphthoquinone derivative, on experimental thrombosis and
platelet aggregation. Pharmacology. 70:195–200. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Astrup T and Mullertz S: The fibrin plate
method for estimating fibrinolytic activity. Arch Biochem Biophys.
40:346–351. 1952. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chou J, Mackman N, Merrill-Skoloff G,
Pedersen B, Furie BC and Furie B: Hematopoietic cell-derived
microparticle tissue factor contributes to fibrin formation during
thrombus propagation. Blood. 104:3190–3197. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Steinhubl SR and Moliterno DJ: The role of
the platelet in the pathogenesis of atherothrombosis. Am J
Cardiovasc Drugs. 5:399–408. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Azevedo AP, Farias JC, Costa GC, et al:
Anti-thrombotic effect of chronic oral treatment with Orbignya
phalerata Mart. J Ethnopharmacol. 111:155–159. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kurz KD, Main BW and Sandusky GE: Rat
model of arterial thrombosis induced by ferric chloride. Thromb
Res. 60:269–280. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lockyer S and Kambayashi J: Demonstration
of flow and platelet dependency in a ferric chloride-induced model
of thrombosis. J Cardiovasc Pharmacol. 33:718–725. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Furie B and Furie BC: Thrombus formation
in vivo. J Clin Invest. 115:3355–3362. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Farrehi PM, Ozaki CK, Carmeliet P and Fay
WP: Regulation of arterial thrombolysis by plasminogen activator
inhibitor-1 in mice. Circulation. 97:1002–1008. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cui X, Sakaguchi T, Shirai Y and
Hatakeyama K: Orally administered Panax ginseng extract
decreases platelet adhesiveness in 66% hepatectomized rats. Am J
Chin Med. 27:251–256. 1999.
|
|
24
|
Park HJ, Rhee MH, Park KM, Nam KY and Park
KH: Effect of non-saponin fraction from Panax ginseng on
cGMP and thromboxane A2 in human platelet aggregation. J
Ethnopharmacol. 49:157–162. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yun YP, Do JH, Ko SR, et al: Effects of
Korean red ginseng and its mixed prescription on the high molecular
weight dextran-induced blood stasis in rats and human platelet
aggregation. J Ethnopharmacol. 77:259–264. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Baruah DB, Dash RN, Chaudhari MR and Kadam
SS: Plasminogen activators: a comparison. Vascul Pharmacol. 44:1–9.
2006. View Article : Google Scholar : PubMed/NCBI
|