Introduction
Lactoferrin (Lf) is an 80 kDa, iron-binding
glycoprotein, which is expressed abundantly in the exocrine
secretions of mammals, particularly in milk and fluids of the
digestive tract (1). Lf is also
released by mucosal epithelia and neutrophils during inflammation
(1).
Lf plays a direct antimicrobial role when present in
mucosal membrane secretions of the epithelia and provides innate or
mucosal immune defense by limiting the proliferation and adhesion
of microbes and/or by microbicidal targeting. These properties are
predominantly associated with the ability of Lf to sequester iron
in biological fluids or in the destabilization of the membranes of
microorganisms (2).
Iron-independent microbicidal activities, which
require direct interaction between Lf and structural components of
the surface of microbes, have also been demonstrated (1,2).
In vitro and in vivo studies have shown that Lf may
modulate adaptive and innate immune responses and thus protects the
host against viral infections and other complex conditions,
including septic shock (1,2).
Macrophages are important elements that provide
innate immune defense against infection (3). During the course of an infection,
macrophages are among the first cells in the major organs to be
exposed to microbial challenges and are major producers of
interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α)
following infection (3).
Ulcerative colitis (UC) is a non-specific chronic
inflammatory disease of the intestinal tract (4). Although the definitive causes of UC
remain unclear, the major symptoms that characterize the disease
include abdominal pain, diarrhea, presence of occult blood and
mucus (5). Primary therapy of UC
with various agents, including salazosulfamide, glucocorticoids and
immunodepressant probiotics, has been attempted, but usually
results in the poor treatment compliance of patients and increases
rates of relapse of UC (6).
It was previously shown that gastrointestinal
bacteria may be involved in the pathogenesis of UC (6). Thus, Lf has been increasingly
considered as a therapeutic option in UC, due in part to Lf
exhibiting multiple bioactivities. These bioactivites include
restoration of the intestinal microbiota, anti-inflammatory
properties and adjusting the intestinal immune response.
Previously, Lf was observed to improve symptoms of UC in a rat
model (7). In the current study, a
model of DSS-induced colitis was used to study the therapeutic
effect and mechanism of action of Lf in experimental UC.
Materials and methods
Lf
Lf was obtained from Tatua Co-operative Dairy
Company, Limited (Tatua, New Zealand). To deplete iron, citric acid
was added to the Lf solution and adjusted to pH 2.1 to obtain a
final iron saturation of 7%.
(NH4)2Fe(SO4)2 and
HCO3− were added to the Lf solution to obtain
a 100% iron-saturated Lf solution, as previously described
(8).
Reagents
Dextran sulphate sodium (DSS) was provided as a
36–50 kDa reagent by MP Biomedicals (Santa Ana, CA, USA). All
reagents required for quantitative (q)PCR analysis were obtained
from Promega Corporation (Madison, WI, USA).
Experimental design
Equal numbers of male ten-week-old BALB/c mice, with
a mean weight of 22.0±2.0 g, were obtained from Vital River
Laboratory Animal Technology Co., Ltd. (Beijing, China). Mice were
housed in clean filter-top cages under standard conditions of
50±10% humidity and an equal 12-h dark/light cycle and fed with
standard mouse chow for 7 days. Mice were randomly divided into the
following 4 groups: i) Normal, normal diet in the absence of any
special treatment; ii) model, permitted to drink 2.5% DSS for
modeling on days 15–21, plus normal saline (100 μl/10 g) by gavage
on days 1–28; iii) apo-Lf, 2.5% DSS for modeling on days 15–21,
plus apo-Lf (100 mg/kg/d) by gavage on days 1–28; and iv) holo-Lf,
2.5% DSS for modeling on days 15–21, plus holo-Lf (100 mg/kg/d) by
gavage on days 1–28. On day 28, all mice were sacrificed.
To reflect the general conditions of the mice,
disease activity index (DAI) scores were determined by an
investigator blinded to the protocol. The extent of loss in body
weight, fecal character, fecal occult blood or hematochezia was
assessed as previously described (9). On day 28 following treatment, the
colon was removed in the region that spanned the colo-cecal
junction to the anus. The length of the resected tissue was
measured, rinsed with 5 ml 0.01 mol/l PBS (pH 7.4) to remove fecal
remnants and dissected longitudinally at the mesenteric attachment.
Next, 5 mm of the distal colon was removed and fixed for 48 h in
PBS buffered with 10% formalin. The tissue was then processed for
paraffin embedding, cut into 5-μm sections and stained with
hematoxylin and eosin. Other sections of the colon were preserved
in liquid nitrogen. The study was approved by the Ethics Committee
of the College of Veterinary Medicine, Inner Mongolia Agricultural
University.
Disease activity
The following parameters were evaluated by
statistical methods: Weight loss, fecal character, fecal occult
blood and hematochezia (Table I)
(9).
 | Table IDisease activity index score. |
Table I
Disease activity index score.
| Score | Weight loss (%) | Fecal character | Fecal bleeding |
|---|
| 0 | 0 | Normal | None |
| 1 | 1–5 | | |
| 2 | >5–10 | Loose | Hemoccult + |
| 3 | >10–15 | | |
| 4 | >15 | Watery | Bleeding
(visible) |
Histological scoring
Histological scores were given based on previously
described criteria (10), with
slight modifications. An extra score was added, which was denoted
as 3.5 and represented observation of total crypt loss, but with
evidence of retention to the surface epithelium. In addition, the
lamina propria and sub-mucosa showed a serious inflammatory
cellular infiltration in this group (Table II). Whole tissue specimens on
slides were evaluated at magnification ×200 using light microscopy.
Each microscopic field was evaluated and given a score. If more
than one score was present in any given field of view, the scores
were multiplied by the estimated percentage in the field and the
sum of those values was calculated. At the conclusion of this
evaluation process, the average score of the microscopic fields was
recorded and analyzed statistically. The grading score was based on
previously described criteria (10).
 | Table IIHistological scores for hematoxylin
and eosin-stained colon sections. |
Table II
Histological scores for hematoxylin
and eosin-stained colon sections.
| Score | Description |
|---|
| 0.0 | Normal colonic
mucosa |
| 1.0 | Shortening and loss
of the basal 1/3 of the crypts |
| 2.0 | Loss of the basal 2/3
of the crypts |
| 3.0 | Total crypt loss, but
with retention to the epithelial surface
Lamina propria and sub-mucosa showed a mild inflammatory cellular
infiltration |
| 3.5 | Total crypt loss, but
with retention to the epithelial surface
Lamina propria and submucosa showed a serious inflammatory cellular
infiltration |
| 4.0 | Erosions and marked
cellular infiltration |
Myeloperoxidase (MPO) activity
MPO activity was measured as an indicator of
neutrophil accumulation in colonic mucosa as previously described
(11).
qPCR
All primers (Table
III) were designed using Primer Premier 5.0 software (Premier
Biosoft, Palo Alto, CA, USA) and the primers were manufactured by
Shinegene Molecular Biotechnology Co., Ltd. (Shanghai, China). The
annealing temperatures ranged between 58 and 53°C for 45 min and
amplification was performed using 30 cycles for each gene of
interest.
 | Table IIIPCR primers and products. |
Table III
PCR primers and products.
| Primer sequences | Product size, bp |
|---|
| IL-1β | F:
AGCCCATCCTCTGTGACTCATG
R: GCTGATGTACCAGTTGGGGAAC | 422 |
| TNF-α | F:
GGCAGGTCTACTTTGGAGTCATTGC
R: CATTCGAGGCTCCAGTGAAATTCGG | 299 |
| GAPDH | F:
ACCACAGTCCATGCCATCAC
R: TCCACCACCCTGTTGCTGTA | 440 |
Statistical analysis
Statistical analysis was performed using SPSS
version 13.0 (SPSS, Inc., Chicago, IL, USA) software program for
Windows. Data are presented as the mean ± SD. Data sets were
analyzed by one-way analysis of variance (ANOVA) and Fisher’s
protected LSD post-hoc test. Those values with a significant
difference were further analyzed by the Student-Newman-Keuls test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
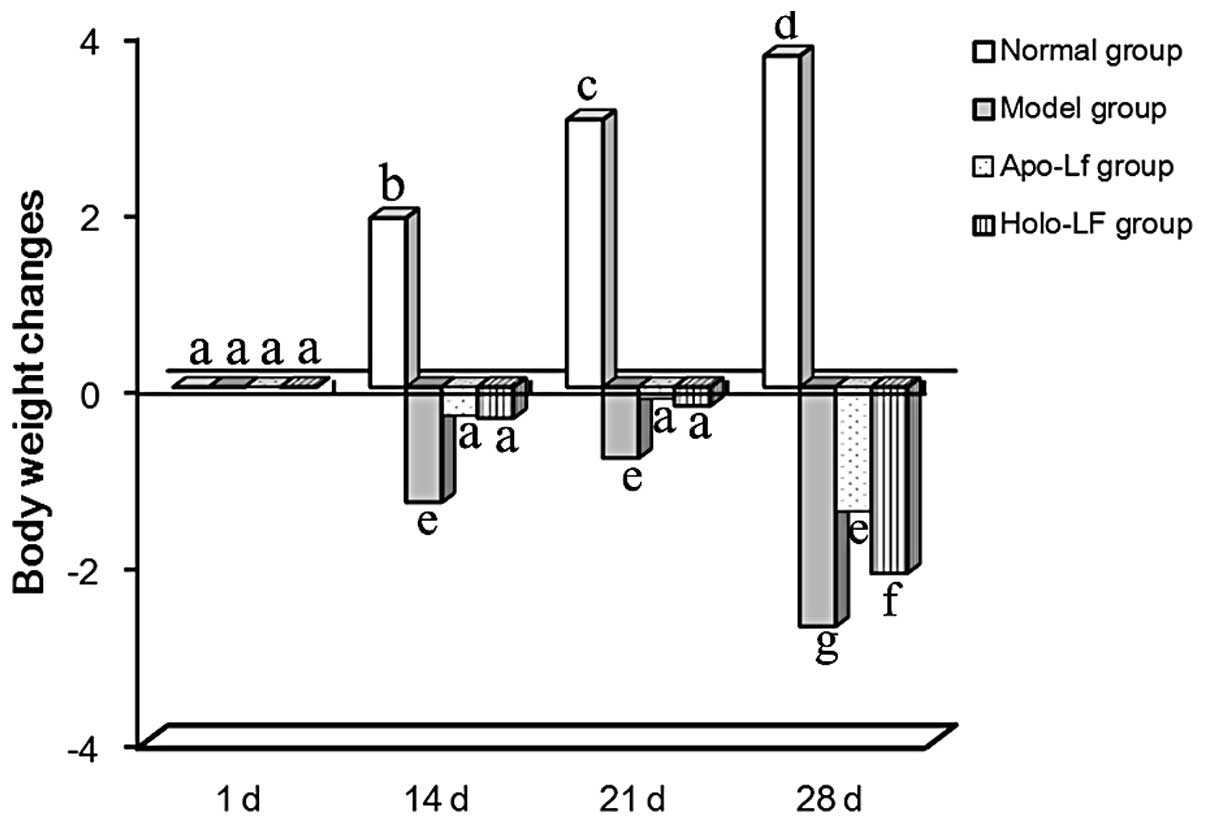
Weight loss
Among the 4 groups of mice on day 1, no difference
in body weight was observed (as determined by ANOVA). However, body
weight increased gradually in the normal group. Mice in the model
group showed weight loss over 1–14 days due to the gavage treatment
received and a significant weight loss was observed from day 15 due
to double stimulation by treatment with DSS and gavage. Treatment
with holo-Lf and apo-Lf was observed to inhibit weight loss
(Fig. 1).
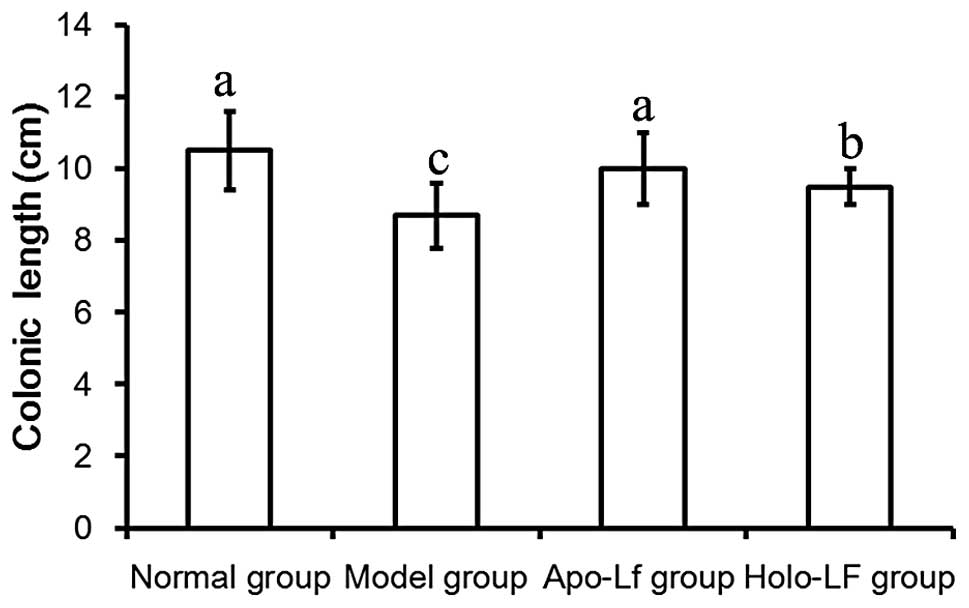
Length of the colon
Length of the colon in the model group was
significantly decreased compared with the normal group (P<0.05;
Fig. 2). These observations
indicate that Lf was capable of preventing shortening of the
colon.
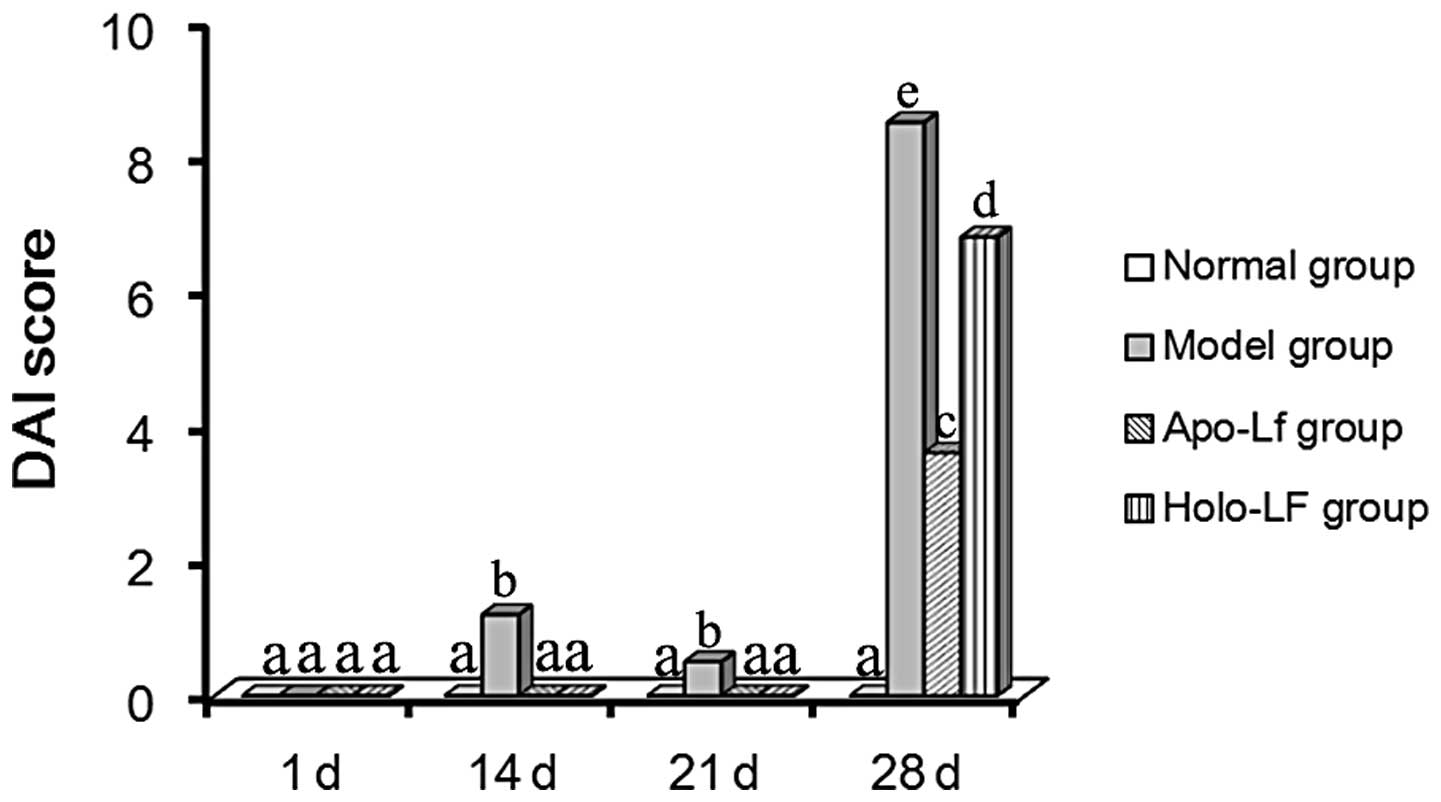
DAI scores
Weight loss, fecal character, fecal occult blood and
hematochezia were evaluated individually as previously described
(12). The highest DAI score was
observed in the model group (Fig.
3). Lf also promoted a beneficial effect in experimental
colitis. Low DAI scores were observed in the Lf groups (Fig. 3).
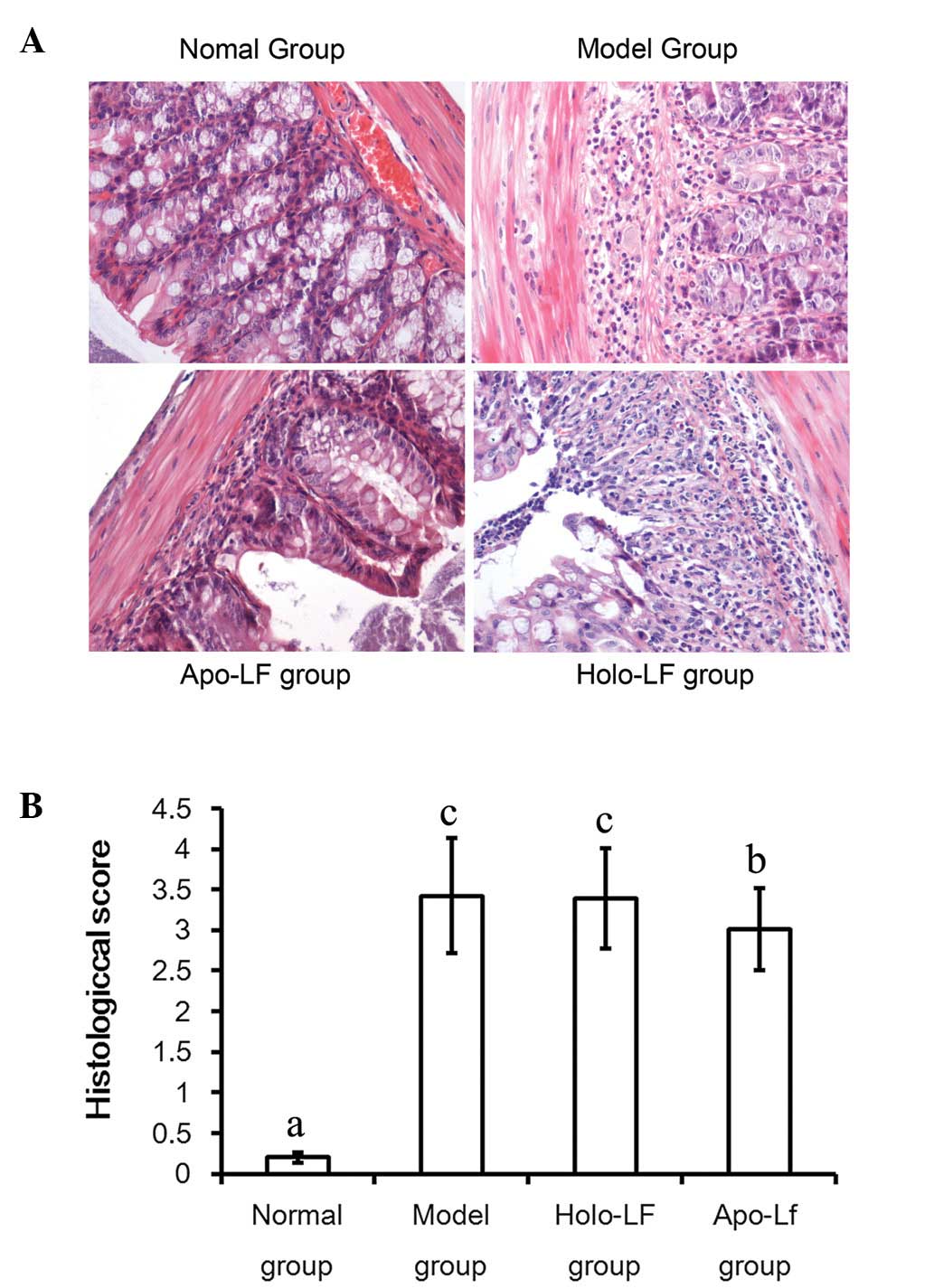
Histological scores
Histological changes in mice of the 4 groups were
evaluated individually as previously described (10). The highest score was observed in
mice of the model group and a lower score was observed in the Lf
treatment groups when compared with the model group; this was
particularly true in the context of mice in the apo-Lf treatment
group (Fig. 4A and B).
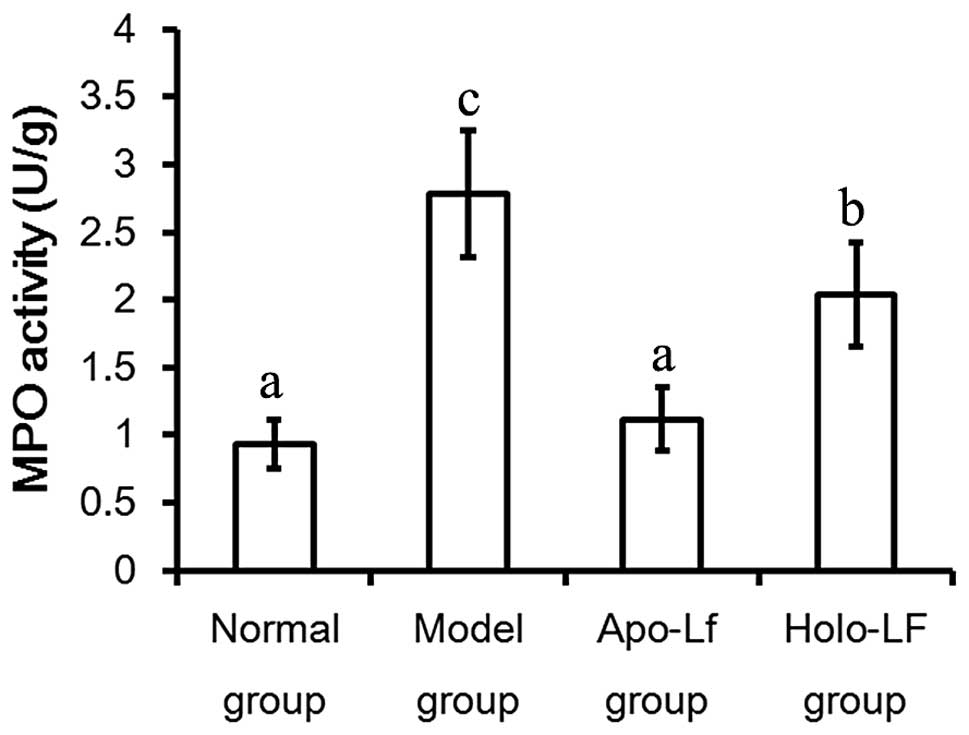
MPO activity
Maximal MPO activity was observed to be 2.78±0.39
U/g in the model group, which was significantly higher than that
noted in the control group (0.93±0.13 U/g) (P<0.05). MPO
activities also decreased following Lf treatment (Fig. 5).
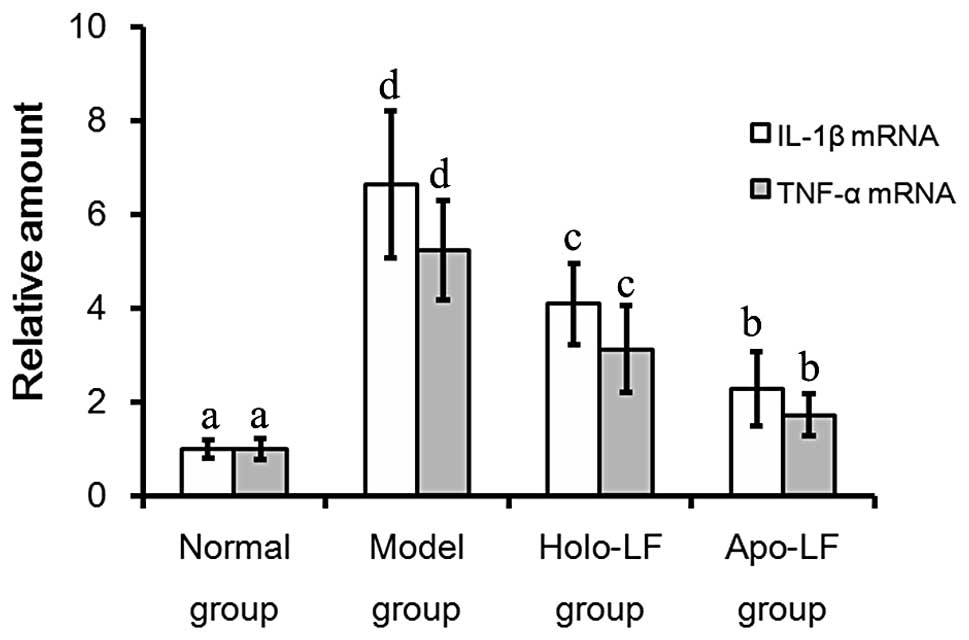
qPCR analysis
IL-1β mRNA levels were observed to be lowest in the
normal group and at the highest levels in the model group (Fig. 6). In addition, levels of IL-1β mRNA
were observed to be lower in the Lf treatment groups than in the
model group (2.27±0.79 vs. 6.63±1.57; P<0.05). TNF-α mRNA levels
were lowest in the normal group and highest in the model group
(Fig. 6). In addition, TNF-α mRNA
levels were observed to be lower in the Lf treatment group compared
with the model group (1.73±0.46 vs. 5.23± 1.05; P<0.05; Fig. 6).
Discussion
UC is a form of intestinal inflammation,
characterized by the diffuse infiltration of inflammatory cells and
multiple ulcer formations in the colon. However, the causes and
pathogenesis of UC remain unclear (13). In the current study, 2.5% DSS
solution was administered to BALB/c mice in drinking water for
seven days to establish a mouse model of DSS-induced colitis. This
condition resembles human UC in the context of disease
manifestations and pathological features (14).
Typical symptoms of colitis, including diarrhea,
fecal occult blood and hematochezia, were observed in the colitis
mice. In addition, intestinal histology reactions, including severe
mucosal defects, hemorrhage, destruction of the glands and crypts,
large and deep ulcer formation and massive inflammatory cell
infiltrates were observed under light microscopy. Episodes of
diarrhea, presence of fecal occult blood and hematochezia were
observed to be markedly improved following oral treatment with Lf.
Notably, intestinal mucosal inflammation and the shortening of the
colon were significantly improved following oral Lf treatment. The
DAI value and histology scores were significantly lower in the Lf
treatment group when compared with the control group (P<0.05),
indicating that Lf may therapeutically target and improve the
symptoms of DSS-induced colitis.
A previous study showed that altered intestinal
microenvironment and intestinal immunology are important in the
pathogenesis of UC (15).
Cytokines, including IL-1β and TNF-α, are not only important
mediators of the immune response, but have been the focus of
studies into the pathogenesis of UC (16). During the acute phase of
DSS-induced colitis, the massive infiltrates of inflammatory cells
secreted high levels of inflammatory cytokines that provoked
intestinal mucosal injury and exacerbated the intestinal
inflammatory reaction (12). The
current study showed that mRNA levels of IL-1β and TNF-α in the
intestinal mucosa of DSS-induced mice were significantly increased.
These cytokines may be significant in the pathogenesis of UC and
exacerbate mucosal inflammation and cellular apoptosis (17,18).
In the current study, mRNA levels of IL-1β and TNF-α
in colonic tissues were quantified by qPCR, which showed that the
two cytokines were elevated in the DSS-induced UC model. In
addition, oral Lf treatment decreased mRNA levels of IL-1β and
TNF-α, indicating that Lf may have an anti-inflammatory effect,
thus preventing UC formation and ameliorating inflammation by
inhibiting the synthesis of inflammatory cytokines. The current
study also showed that apo-Lf improved anti-inflammatory effects
when compared with holo-Lf. This indicated that the
anti-inflammatory effects of Lf may be associated with the
saturation state of iron in Lf, which is similar to its reported
microbicidal effect (19,20).
LPS is produced by gram-negative bacteria and is one
of the major factors leading to a marked and uncontrollable
inflammatory response (21). The
occurrence of colitis was hypothesized to be inhibited by apo-Lf
through a mechanism dependent on restoration of homeostasis of
intestinal bacterial flora (data not shown). Therefore, apo-Lf may
reinforce the suppression of the inflammatory responses by
inhibiting cytokine and LPS production. Further studies must be
performed to investigate the effects of apo-Lf on the treatment of
UC.
Acknowledgements
The authors thank Mr Zhang Ming (Key Laboratory of
Functional Dairy, Beijing, China). This study was financed partly
by the Inner Mongolia Yili Industrial Group Co., Ltd. (Hohhot,
China).
References
|
1
|
Legrand D, Elass E, Carpentier M and
Mazurier J: Lactoferrin: a modulator of immune and inflammatory
responses. Cell Mol Life Sci. 62:2549–2559. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Valenti P and Antonini G: Lactoferrin: an
important host defence against microbial and viral attack. Cell Mol
Life Sci. 62:2576–2587. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mogensen SC and Virelizier JL: The
interferon-macrophage alliance. Interferon. 8:55–84.
1987.PubMed/NCBI
|
|
4
|
Jiang XL and Cui HF: An analysis of 10218
ulcerative colitis cases in China. World J Gastroenterol.
8:158–161. 2002.PubMed/NCBI
|
|
5
|
Carvalho R and Hyams JS: Diagnosis and
management of inflammatory bowel disease in children. Semin Pediatr
Surg. 16:164–171. 2007. View Article : Google Scholar
|
|
6
|
Shanahan F: Crohn’s disease. Lancet.
359:62–69. 2002.
|
|
7
|
Togawa J, Nagase H, Tanaka K, et al:
Lactoferrin reduces colitis in rats via modulation of the immune
system and correction of cytokine imbalance. Am J Physiol
Gastrointest Liver Physiol. 283:G187–G195. 2002. View Article : Google Scholar
|
|
8
|
Lu RR: Isolation, purification and
bioactive functions of lactoferrin (unpublished PhD thesis).
Jiangnan University; 2003, (In Chinese).
|
|
9
|
Hamamoto N, Maemura K, Hirata I, Murano M,
Sasaki S and Katsu K: Inhibition of dextran sulphate sodium
(DSS)-induced colitis in mice by intracolonically administered
antibodies against adhesion molecules (endothelial leucocyte
adhesion molecule-1 (ELAM-1) or intercellular adhesion molecule-1
(ICAM-1)). Clin Exp Immunol. 117:462–468. 1999. View Article : Google Scholar
|
|
10
|
Cooper HS, Murthy SN, Shah RS and
Sedergran DJ: Clinicopathologic study of dextran sulfate sodium
experimental murine colitis. Lab Invest. 69:238–249.
1993.PubMed/NCBI
|
|
11
|
Fabia R, Ar’Rajab A, Johansson ML, et al:
The effect of exogenous administration of Lactobacillus
reuteri R2LC and oat fiber on acetic acid-induced colitis in
the rat. Scand J Gastroenterol. 28:155–162. 1993.PubMed/NCBI
|
|
12
|
Egger B, Bajaj-Elliott M, MacDonald TT,
Inglin R, Eysselein VE and Büchler MW: Characterisation of acute
murine dextran sodium sulphate colitis: cytokine profile and dose
dependency. Digestion. 62:240–248. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang SZ, Zhao XH and Zhang DC: Cellular
and molecular immunopathogenesis of ulcerative colitis. Cell Mol
Immunol. 3:35–40. 2006.PubMed/NCBI
|
|
14
|
Yan Y, Kolachala V, Dalmasso G, et al:
Temporal and spatial analysis of clinical and molecular parameters
in dextran sodium sulfate induced colitis. PLoS One. 4:e60732009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Beagley KW and Elson CO: Cells and
cytokines in mucosal immunity and inflammation. Gastroenterol Clin
North Am. 21:347–366. 1992.PubMed/NCBI
|
|
16
|
Ishiguro Y: Mucosal proinflammatory
cytokine production correlates with endoscopic activity of
ulcerative colitis. J Gastroenterol. 34:66–74. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang QY, Chen CL, Wang JD, Lai ZS, Ma Q
and Zhang YL: Expression of pro-inflammation cytokines and
activation of nuclear factor kappab in the intestinal mucosa of
mice with ulcerative colitis. Di Yi Jun Yi Da Xue Xue Bao.
23:1202–1205. 2003.(In Chinese).
|
|
18
|
Rachmilewitz D, Karmeli F, Shteingart S,
Lee J, Takabayashi K and Raz E: Immunostimulatory oligonucleotides
inhibit colonic proinflammatory cytokine production in ulcerative
colitis. Inflamm Bowel Dis. 12:339–345. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim WS, Tanaka T, Kumura H and Shimazaki
K: Lactoferrin-binding proteins in Bifidobacterium bifidum.
Biochem Cell Biol. 80:91–94. 2002. View
Article : Google Scholar
|
|
20
|
Tomita M, Wakabayashi H, Yamauchi K,
Teraguchi S and Hayasawa H: Bovine lactoferrin and lactoferricin
derived from milk: production and applications. Biochem Cell Biol.
80:109–112. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cheng XY and Zhang LL: The relation of
fulminant hepatitis with Endotoxin and its receptors and
inflammatory cytokines. Jiangxi Medical Journal. 41:114–117.
2006.(In Chinese).
|