Introduction
Colorectal cancer is the most common type of cancer
and the leading cause of cancer-related mortality worldwide
(1). There is a continuing need to
develop safe and effective anticancer drugs as current surgery and
chemotherapeutic options appear to be inadequate in curing or
controlling colorectal cancer (2).
Previously, plant-derived drugs have been important
in cancer therapy due to their low toxicity and high efficacy.
Numerous natural bioactive compositions found in medicinal herbs
are considered to be potential anticancer agents (3–5).
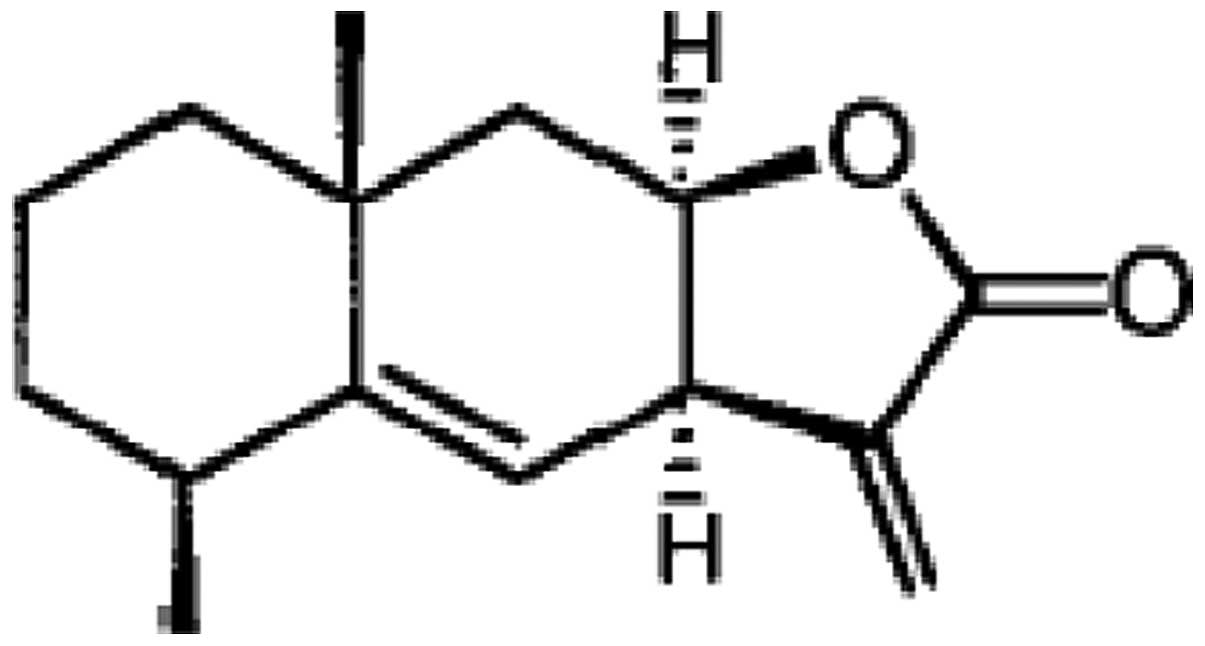
Alantolactone (Fig. 1), a
sesquiterpene lactone, is the active component of Inula
helenium (Compositae), a traditional Chinese herbal medicine
which has strong anthelmintic and antibacterial activities
(6) and possesses inhibitory
activities against human gastric adenocarcinoma MK-1 cells, human
uterine carcinoma HeLa cells and mouse melanoma B16F10 cells
(7). In addition, alantolactone
induces apoptosis in Jurkat leukemia T cells (8) and human chronic myelogenous leukemia
K562 cells (9). It has previously
been observed that alantolactone inhibits HCT-8 cell proliferation
(10). However, its antitumor
effect on colorectal cancer cells and the underlying mechanisms
involved have not been fully characterized.
Apoptosis is a programmed cell death process that
regulates normal development and homeostasis in organisms. A
contributing factor to the survival of tumor cells is loss of
apoptotic control, thus, numerous anticancer agents enhance cancer
cell apoptosis to control cancer development (11,12).
Apoptosis is characterized by distinct morphological changes,
including membrane blebbing, cell shrinkage, loss of mitochondrial
membrane potential (MMP), chromatin condensation and DNA
fragmentation (12,13). At the biochemical level, apoptosis
is mediated by the activation of a class of cysteine proteases
known as caspases. In mammalian cells, caspase activation mainly
occurs via death receptor activation or mitochondrial membrane
depolarization (14).
Mitochondrial-dependent apoptosis is regulated principally by the
Bcl-2 protein family. In response to apoptotic signals, Bax, a
proapoptotic member of the Bcl-2 family, is redistributed from the
cytosol to the mitochondria (3).
The ratio of expression of Bax protein and Bcl-2 protein ultimately
determines cell death or survival in the mitochondrial apoptotic
pathway (15).
Reactive oxygen species (ROS) play a key role in
mitochondria-mediated apoptosis. ROS are the byproducts of aerobic
respiration and primarily arise from the mitochondria (16,17).
At low concentrations, ROS has been identified as a second
messenger in signaling pathways. However, high levels of ROS in the
mitochondria may cause mitochondrial membrane depolarization, the
release of mitochondrial factors and triggering of caspase cascades
(18). Under normal conditions,
the majority of anticancer drugs target mitochondrial function,
producing ROS (19–22). Therefore, ROS is important in
mitochondria-mediated apoptosis.
The aim of the present study was to explore the
cytotoxic activity of alantolactone on human RKO colon cancer cells
and its underlying mechanisms, through an analysis of the
ROS-mediated mitochondrial pathway, accumulation of intracellular
ROS and the disruption of MMP. In addition, the expression of
Bcl-2, Bax and activated caspase-3 and -9 was determined.
Materials and methods
Cell lines
RKO were cultured in RPMI-1640 medium with 10% fetal
bovine serum (TBD Science, Tianjin, China), 100 U/ml penicillin and
100 μg/ml streptomycin, at 37°C and 5% CO2.
Antibodies and reagents
Antibodies against Bax and Bcl-2 were purchased from
Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA) and antibodies
against cleaved caspase-3 and -9 were purchased from Cell Signaling
Technology Inc., (Beverly, MA, USA). A mouse monoclonal antibody
against GAPDH was purchased from KangCheng Bio-tech (Shanghai,
China). DAPI was purchased from the Beyotime Institute of
Biotechnology (Shanghai, China). DCFH-DA, N-acetylcysteine (NAC)
and DiOC6(3) were purchased from Sigma-Aldrich (St.
Louis, MO, USA).
Cell proliferation analysis
Effect of alantolactone on RKO cell viability was
determined by MTT assay. Cells (1×104 cells/well) were
plated in 96-well plates for 24 h and treated with 1.25, 2.5, 5 and
10 μg/ml alantolactone in the presence of 3% serum. Following 1, 2,
3 and 4 day treatment, 20 μl MTT(5 mg/ml; Sigma-Aldrich) was added
to each well for an additional 4-h incubation. The experiment was
performed as described previously (23).
Nuclei staining
RKO cells were treated with 0, 2.5 and 5 μg/ml
alantolactone. The experiment was performed as described previously
(24). Analyses were performed in
triplicate and a minimum of 100 cells/field and at least four
fields in each well were counted.
TUNEL assay
TUNEL assay was performed using an Apoptosis
Detection kit (Roche Diagnostics GmbH, Steinheim, Germany). Cells
were treated with 2.5 μg/ml alantolactone for 24 h prior to being
harvested. The cells were then washed with PBS, fixed with 3.7%
paraformaldehyde for 10 min at room temperature and stained with a
TUNEL solution for 30 min at 37°C, followed by DAPI staining. The
cells were imaged using an inverted fluorescence microscopy
(Olympus BX50, Tokyo, Japan).
Protein extraction and western
blotting
Following alantolactone treatment for 24 h, the
cells were harvested and treated with lysis buffer (Beyotime
Institute of Biotechnology). Western blotting was performed as
described previously (24).
Plasmid transfection
Subcellular localization vector was used to label
mitochondria (mito-GFP, signal peptide sequence of cytochrome
c oxidase VII (25), was
inserted upstream of GFP between the HindIII and
EcoRI restriction sites in the pcDNA3 vector). The plasmids
were transfected using Lipofectamine™ 2000 (Invitrogen Life
Technologies, Carlsbad, CA, USA) according to the manufacturer’s
instructions.
Detection of ROS levels
RKO cells were treated with 2.5 μg/ml alantolactone
for 24 h. Cells were washed twice with PBS and incubated with
DCFH-DA (final concentration, 40 μM) for 30 min in the dark at
37°C. Fluorescent images were immediately captured under an
inverted fluorescence microscope (Olympus BX50).
Determination of MMP
RKO cells were treated with alantolactone for 12 h,
washed and incubated with DiOC6(3) (50 nM final) in PBS
for 30 min in the dark at 37°C. The cells were washed with PBS
twice and the fluorescence was immediately measured with FLUOstar
OPTIMA (BMG LABTECH GmbH, Ortenberg, Germany) using 507 nm EX and
529 nm EM filter settings.
Statistical analysis
All the presented data and results were confirmed in
at least three independent experiments. Statistical comparisons
were made using Student’s t-test. *P<0.05 and
**P<0.01 were considered to indicate statistically
significant differences. Error bars denote the standard deviation
(SD).
Results
Alantolactone inhibits cell proliferation
in RKO cells
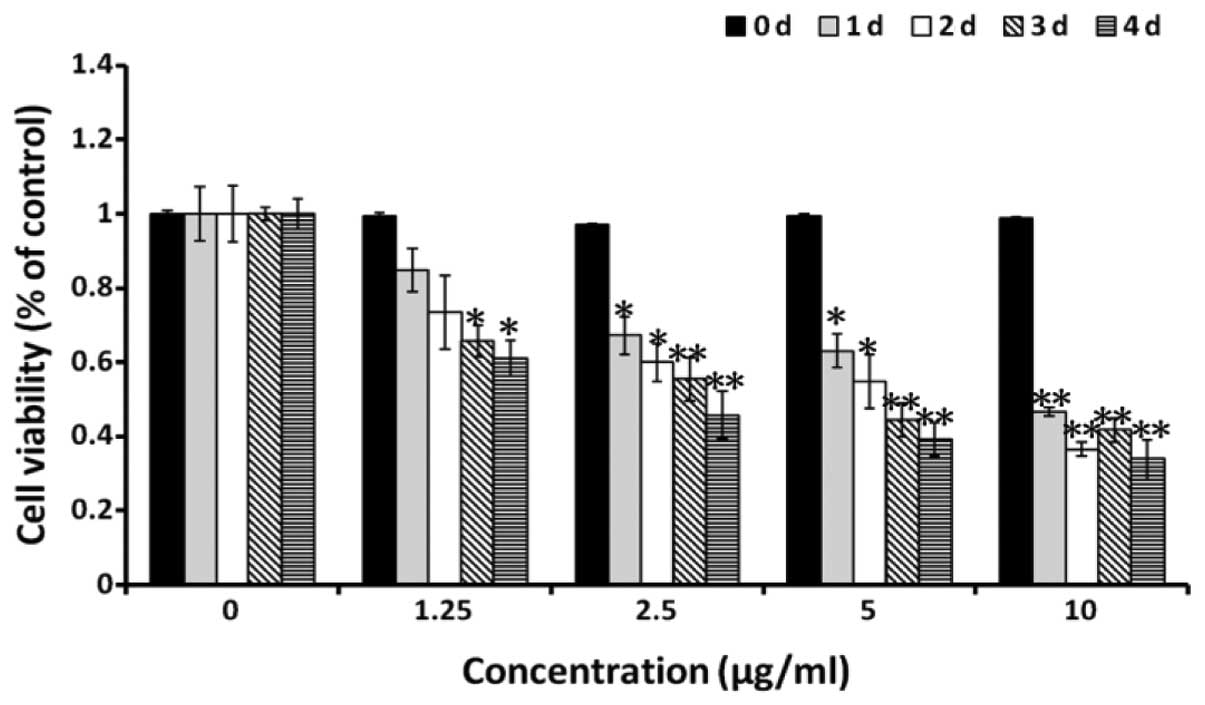
An MTT assay was used to determine the effect of
alantolactone on cell viability under various concentrations of
alantolactone (1.25, 2.5, 5 and 10 μg/ml) and following 1, 2, 3 and
4 days of treatment. Alantolactone was found to inhibit RKO cell
viability in a dose- and time-dependent manner (Fig. 2A). Thus, it was hypothesized that
alantolactone is capable of markedly inhibiting colon cancer
proliferation.
Alantolactone induces apoptosis in RKO
cells
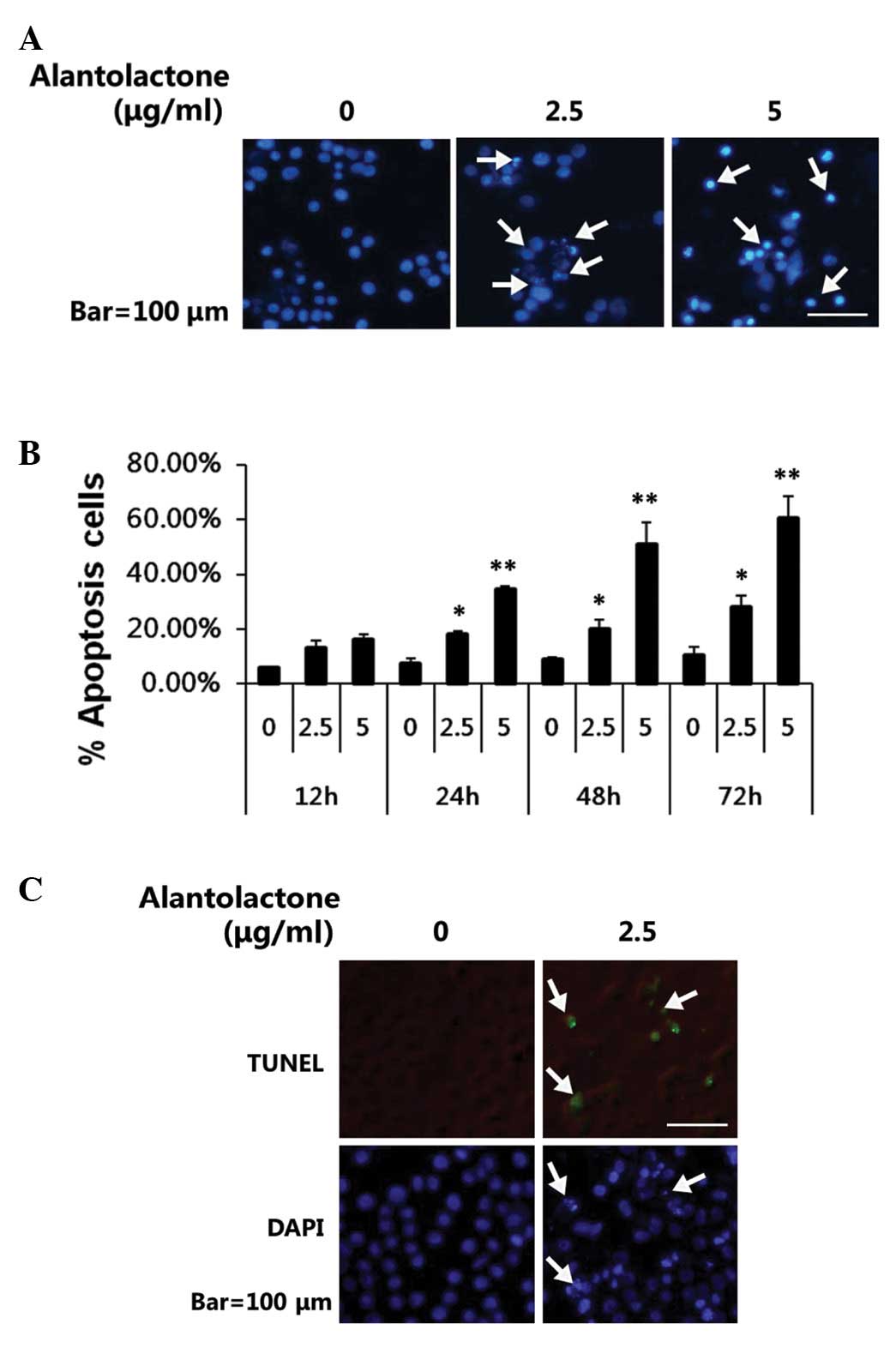
To test whether alantolactone-mediated growth
inhibition was due to induction of apoptosis, DAPI staining and
TUNEL assay were performed to analyze the effects of alantolactone
on apoptosis in RKO cells. DAPI staining revealed that the control
cells were round with homogeneous nuclei, by contrast,
alantolactone-treated cells showed condensed and fragmented nuclei
(arrows; Fig. 3A). In addition,
alantolactone was observed to induce apoptosis in a dose-and
time-dependent manner (Fig. 3B).
Results obtained from TUNEL assay (Fig. 3C) suggested that alantolactone may
significantly induce apoptosis in RKO cells.
Alantolactone causes the generation of
ROS
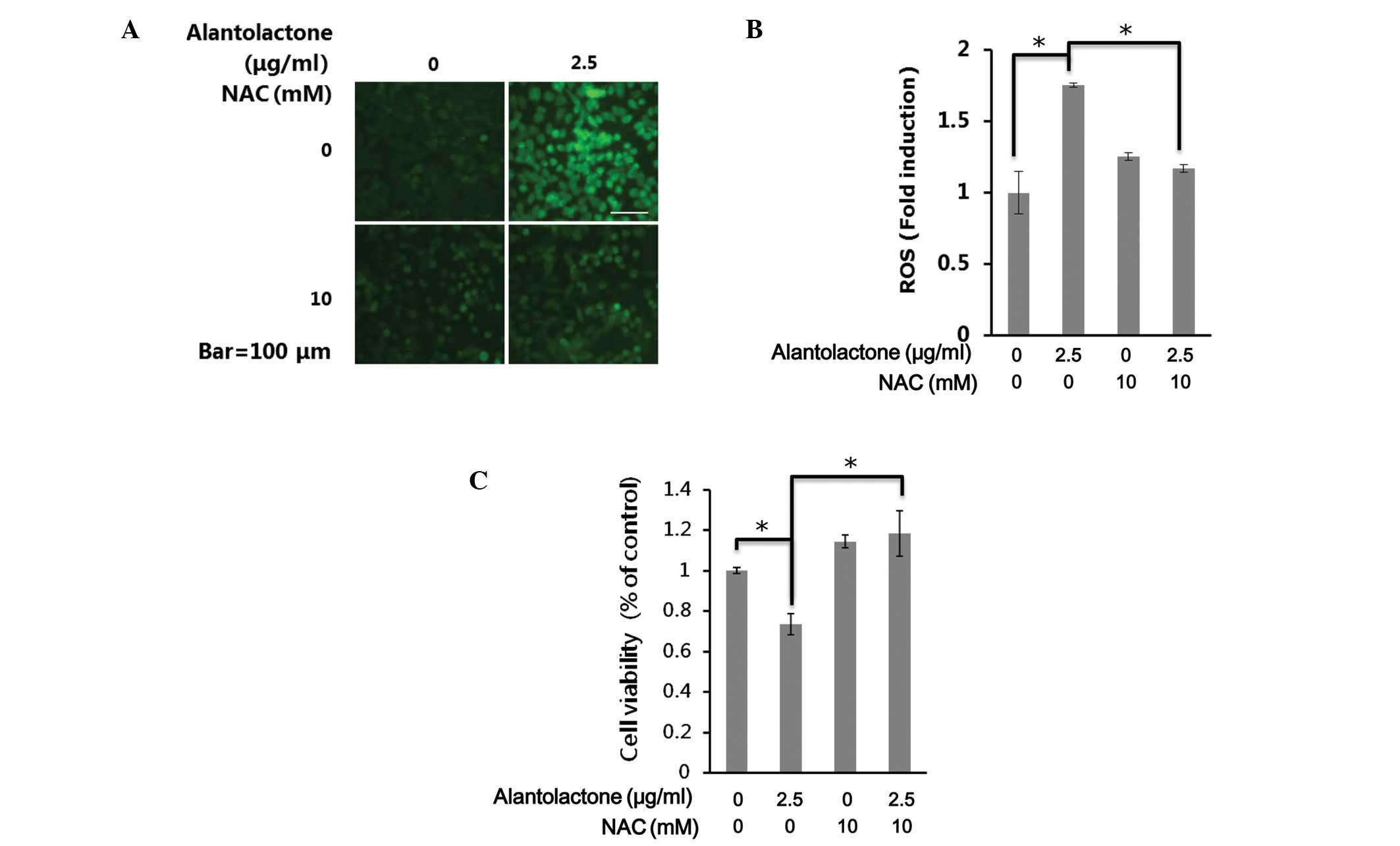
As ROS generation is important in apoptosis, the
effect of alantolactone on the generation of ROS was investigated.
Cells were exposed to alantolactone (2.5 μg/ml) for 24 h and
analyzed for the accumulation of ROS by fluorescence microscopy
following staining with DCFH-DA. As shown in Fig. 4A, treatment with alantolactone
resulted in a significant increase in intracellular ROS in RKO
cells. NAC is a potent antioxidant that may inhibit oxidative
stress by directly scavenging ROS and replenishing GSH (26). The decrease of intracellular ROS by
NAC was also observed following DCFH-DA staining (Fig. 4A).
To investigate the role of ROS in
alantolactone-induced apoptosis in RKO cells, cell death was
measured following treatment of alantolactone only or with NAC. As
shown in Fig. 4B, alantolactone
(2.5 μg/ml) increased cell death, whereas removing intracellular
ROS by NAC significantly inhibited alantolactone-induced cell death
(Fig. 4C). These results indicate
that alantolactone may induce cell apoptosis by the generation of
ROS in RKO cells.
Alantolactone induces apoptosis in the
mitochondrial pathway
Mitochondria are a major source of ROS and excessive
ROS accumulation leads to oxidative stress and ultimately apoptosis
(27). To analyze whether
mitochondria are involved in alantolactone-induced apoptosis, the
changes of MMP using DiOC6(3) staining were analyzed.
Alantolactone resulted in marked depolarization in mitochondria
(Fig. 5A). In addition,
mitochondria usually undergo marked morphological changes during
the early stages of apoptosis (28). To visualize the effect of
alantolactone on mitochondrial morphology, mitochondrially-targeted
green fluorescent protein (mito-GFP) plasmids were transfected into
RKO cells. As shown in Fig. 5B,
mito-GFP expressing RKO cells revealed a markedly fragmented
mitochondrial phenotype when treated with alantolactone, suggesting
that depolarization in mitochondria and its fragmented phenotype
are involved in alantolactone-induced apoptosis in RKO cells.
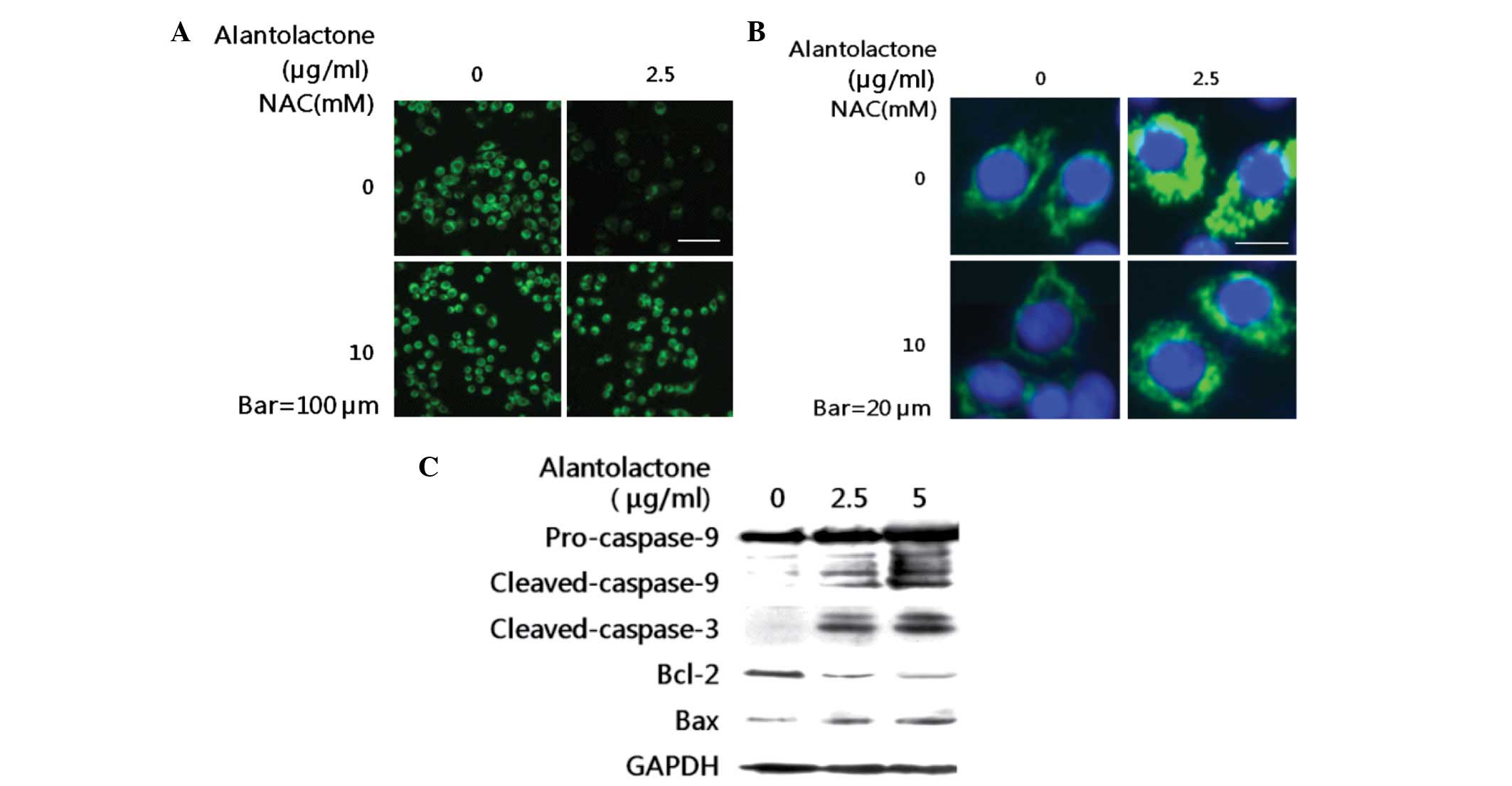 | Figure 5Effect of alantolactone on
mitochondrial membrane potential, mitochondria injury and caspase
activation. (A) RKO cells were treated with/without NAC (10 mM) 2 h
prior to treatment with 2.5 μg/ml alantolactone. Following 12 h
alantolactone treatment, cells were incubated with
DiOC6(3) for 30 min, MMP levels were detected by
fluorescence microscopy. (B) RKO cells were transfected with a
mitochondrially targeted green fluorescent protein (mito-GFP)
plasmid to visualize mitochondrial networks. Following 24 h, cells
were treated with/without NAC (10 mM) 2 h prior to treatment with
2.5 μg/ml alantolactone. Following 12 h treatment, the fragmented
mitochondrial phenotype was detected by fluorescence microscopy.
(C) RKO cells were incubated with 0, 2.5 and 5 μg/ml alantolactone
for 24 h. Cell lysates were prepared and subjected to SDS-PAGE.
Pro-caspase-9, cleaved-caspase-9, cleaved-caspase-3, Bcl-2 and Bax
were determined by western blotting using specific antibodies. NAC,
N-acetylcysteine. |
Caspase activation is generally considered to be a
key hallmark of apoptosis (14).
Mitochondria are involved in a variety of key events leading to
apoptosis, including the release of caspase activators, the
production of ROS and participation in regulation of proapoptotic
and antiapoptotic Bcl-2 family proteins (15). A decrease in mitochondrial membrane
potential disrupts the outer mitochondrial membrane, followed by
the release of cytochrome c, activation of caspase-3,
caspase-9 and subsequent apoptosis. The effect of alantolactone on
the activation of caspase-3 and -9, which are crucial initiators
and effectors, respectively, was investigated. Alantolactone
activated caspase-3 and -9, following decreased procaspase-9 levels
(Fig. 5C). To investigate the cell
mechanism underlying alantolactone-induced apoptosis in RKO cells,
Bcl-2 and Bax expression was analyzed. The expression levels of
Bcl-2 decreased and Bax expression increased in a dose-dependent
manner (Fig. 5C). These results
indicate that alantolactone induced RKO cell apoptosis-involved
proteins from the Bcl-2 family and caspase activation.
Discussion
Considerable attention has been focused on
identifying a naturally occurring bioactive composition capable of
inhibiting, retarding or reversing the carcinogenic process,
particularly the development of colorectal cancer. In the colon,
the elimination of transformed cells via apoptosis induction is
considered to be a crucial step for the treatment of colorectal
cancer (29).
In the present study, alantolactone was observed to
be cytotoxic in vitro and to induce apoptosis in colorectal
cancer RKO cells. To study the mechanism of alantolactone-induced
apoptosis, the effects of alantolactone on ROS generation and MMP
changes were investigated. The results showed that the accumulation
of ROS was detected when treated with alantolactone (Fig. 4). To identify the role of ROS in
alantolactone-induced apoptosis, NAC was used as a ROS scavenger,
which may protect cells against oxidative damage by reacting with
H2O2 as a direct antioxidant and increasing
the cytoplasmic reserve of glutathione (30). If ROS production mediates
alantolactone-induced cell death, we hypothesize that NAC is able
to inhibit alantolactone-induced cell death. In the present study,
NAC was observed to block intracellular ROS generation and further
suppress alantolactone-induced apoptosis in RKO cells.
Mitochondria are a major source of ROS production
and excessive ROS accumulation may lead to oxidative stress, induce
decreased MMP and promote mitochondria-dependent apoptosis
(27). Observations of the current
study indicate that alantolactone causes marked depolarization in
mitochondria. In addition, mitochondria undergo marked
morphological changes during alantolactone-induced apoptosis. Thus,
these results suggest that alantolactone-induced apoptosis is
involved in ROS generation and mitochondrial dysfunction.
Mitochondria play vital roles in apoptosis induced
by caspase-dependent and -independent pathways. Caspase-3 is a key
apoptotic executive caspase and is activated by proteolytic
cleavage by caspase-8 and -9 (31,32).
In the current study, caspase-3 and -9 activation was observed to
be involved in alantolactone-induced apoptosis, as indicated by
decreased pro-caspase-9 and increased cleavage of caspase-3.
The Bcl-2 protein family, whose members may be
antiapoptotic or proapoptotic, regulates cell death by controlling
mitochondrial membrane permeability during apoptosis (33,34).
Bcl-2 is a potent antiapoptotic factor, whereas Bax, an antagonist
of Bcl-2, is inserted into the outer membrane of the mitochondria,
allowing for the release of cytochrome c and initiating
apoptosis. It is therefore suggested that Bcl-2 family proteins may
be involved in apoptosis induced by alantolactone. The present
study showed that Bcl-2 decreased and Bax increased in
alantolactone-induced apoptosis. These results indicate that
treatment with alantolactone leads to a shift from an antiapoptotic
to a proapoptotic state, resulting in the activation of capsase-3.
Thus, alantolactone induces mitochondria-dependent apoptotic
pathways in RKO cells, which involves the suppression of Bcl-2,
elevation of Bax and activation of caspase-9 and -3.
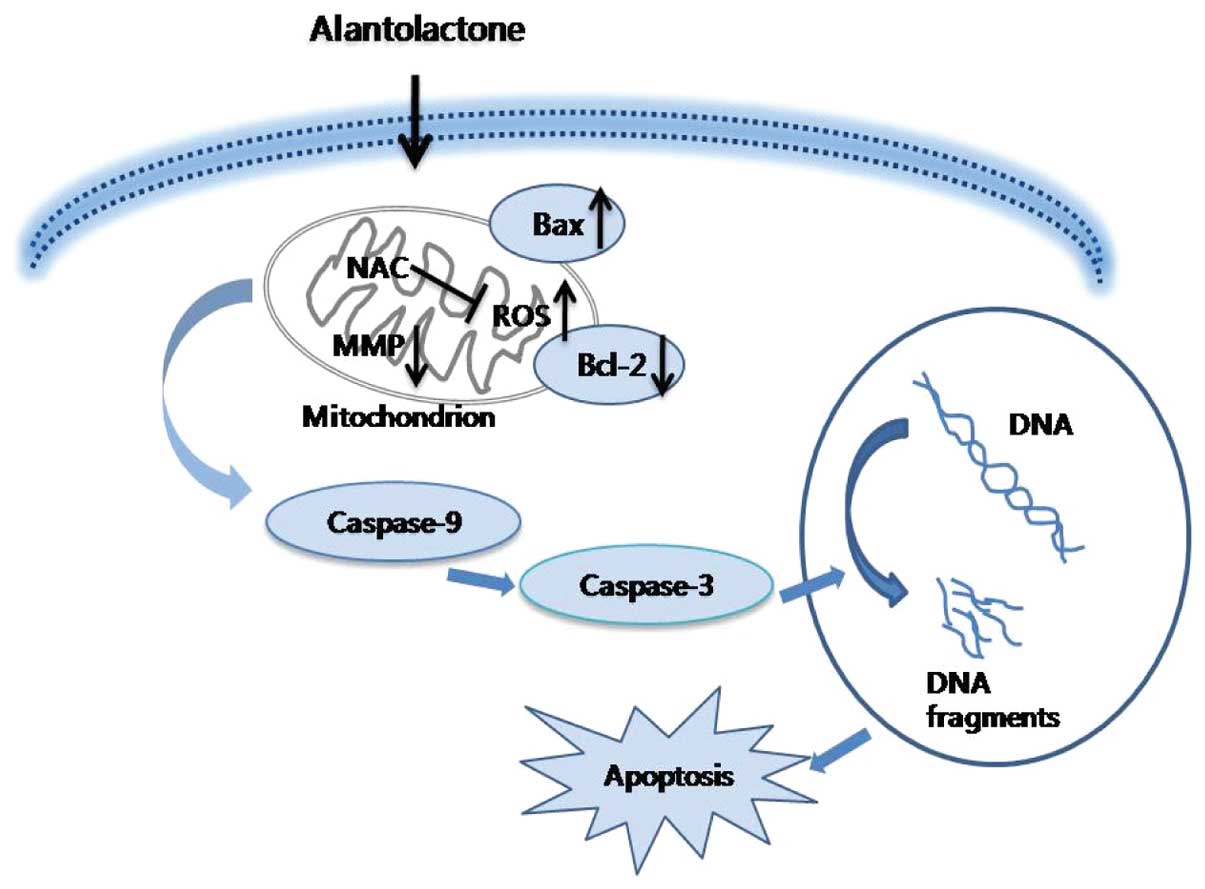
In summary, the current study is the first to
demonstrate that alantolactone inhibits cell proliferation and
induces apoptosis via a ROS-mediated mitochondrial dependent
pathway in RKO cells. Downregulation of Bcl-2, upregulation of Bax
and activation of caspase-3 and -9 are involved in this process
(Fig. 6). Therefore, alantolactone
is an attractive agent for human colon cancer research and may
become a potent chemotherapeutic agent in colon cancer.
Acknowledgements
This study was supported by grants from the
Fundamental Research Funds for the Central Universities (no.
11QNJJ021), National Natural Science Foundation of China (nos.
30873409, 30670220 and 31070318), the Fundamental Research Funds
for the Central Universities (no. 11QNJJ021), Cultivation Fund of
the Scientific and Technical Innovation Project of Northeast Normal
University (no. NENU-STB07008), Administration of Traditional
Chinese Medicine of Jilin Province (nos. 2010pt068 and 2011-zd17)
and the Research Foundation of Jilin Provincial Science and
Technology Development (nos. 20100911, 200805131 and
201201065).
References
|
1
|
Jemal A, Murray T, Ward E, et al: Cancer
statistics, 2005. CA Cancer J Clin. 55:10–30. 2005. View Article : Google Scholar
|
|
2
|
Gustin DM and Brenner DE: Chemoprevention
of colon cancer: current status and future prospects. Cancer
Metastasis Rev. 21:323–348. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fauzi AN, Norazmi MN and Yaacob NS:
Tualang honey induces apoptosis and disrupts the mitochondrial
membrane potential of human breast and cervical cancer cell lines.
Food Chem Toxicol. 49:871–878. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hsu HF, Houng JY, Kuo CF, Tsao N and Wu
YC: Glossogin, a novel phenylpropanoid from Glossogyne
tenuifolia, induced apoptosis in A549 lung cancer cells. Food
Chem Toxicol. 46:3785–3791. 2008.PubMed/NCBI
|
|
5
|
Luo M, Liu X, Zu Y, et al: Cajanol, a
novel anticancer agent from Pigeonpea [Cajanus cajan (L.)
Millsp] roots, induces apoptosis in human breast cancer cells
through a ROS-mediated mitochondrial pathway. Chem Biol Interact.
188:151–160. 2010.PubMed/NCBI
|
|
6
|
Cantrell CL, Abate L, Fronczek FR, et al:
Antimycobacterial eudesmanolides from Inula helenium and
Rudbeckia subtomentosa. Planta Med. 65:351–355. 1999.
View Article : Google Scholar
|
|
7
|
Konishi T, Shimada Y, Nagao T, Okabe H and
Konoshima T: Antiproliferative sesquiterpene lactones from the
roots of Inula helenium. Biol Pharm Bull. 25:1370–1372.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dirsch VM, Stuppner H and Vollmar AM:
Cytotoxic sesquiterpene lactones mediate their death-inducing
effect in leukemia T cells by triggering apoptosis. Planta Med.
67:557–559. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lawrence NJ, McGown AT, Nduka J, Hadfield
JA and Pritchard RG: Cytotoxic Michael-type amine adducts of
alpha-methylene lactones alantolactone and isoalantolactone. Bioorg
Med Chem Lett. 11:429–431. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shi Y, Bao YL, Wu Y, et al: Alantolactone
inhibits cell proliferation by interrupting the interaction between
Cripto-1 and activin receptor type II A in activin signaling
pathway. J Biomol Screen. 16:525–535. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bellamy CO, Malcomson RD, Harrison DJ and
Wyllie AH: Cell death in health and disease: the biology and
regulation of apoptosis. Semin Cancer Biol. 6:3–16. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Reed JC: Apoptosis-regulating proteins as
targets for drug discovery. Trends Mol Med. 7:314–319. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kaufmann SH, Kottke TJ, Martins LM,
Henzing AJ and Earnshaw WC: Analysis of caspase activation during
apoptosis. Curr Protoc Cell Biol. Chapter 18(Unit 18):
22001.PubMed/NCBI
|
|
14
|
Danial NN and Korsmeyer SJ: Cell death:
critical control points. Cell. 116:205–219. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Murphy KM, Ranganathan V, Farnsworth ML,
Kavallaris M and Lock RB: Bcl-2 inhibits Bax translocation from
cytosol to mitochondria during drug-induced apoptosis of human
tumor cells. Cell Death Differ. 7:102–111. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chatterjee S, Kundu S and Bhattacharyya A:
Mechanism of cadmium induced apoptosis in the immunocyte. Toxicol
Lett. 177:83–89. 2008. View Article : Google Scholar
|
|
17
|
Pathak N and Khandelwal S: Role of
oxidative stress and apoptosis in cadmium induced thymic atrophy
and splenomegaly in mice. Toxicol Lett. 169:95–108. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Martindale JL and Holbrook NJ: Cellular
response to oxidative stress: signaling for suicide and survival. J
Cell Physiol. 192:1–15. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhou YJ, Zhang SP, Liu CW and Cai YQ: The
protection of selenium on ROS mediated-apoptosis by mitochondria
dysfunction in cadmium-induced LLC-PK(1) cells. Toxicol In Vitro.
23:288–294. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang JS, Chen GW, Hsia TC, et al: Diallyl
disulfide induces apoptosis in human colon cancer cell line (COLO
205) through the induction of reactive oxygen species, endoplasmic
reticulum stress, caspases casade and mitochondrial-dependent
pathways. Food Chem Toxicol. 47:171–179. 2009. View Article : Google Scholar
|
|
21
|
Wei A, Zhou D, Xiong C, Cai Y and Ruan J:
A novel non-aromatic B-ring flavonoid: isolation, structure
elucidation and its induction of apoptosis in human colon HT-29
tumor cell via the reactive oxygen species-mitochondrial
dysfunction and MAPK activation. Food Chem Toxicol. 49:2445–2452.
2011. View Article : Google Scholar
|
|
22
|
Wang L, Xu ML, Hu JH, Rasmussen SK and
Wang MH: Codonopsis lanceolata extract induces G0/G1 arrest
and apoptosis in human colon tumor HT-29 cells - involvement of ROS
generation and polyamine depletion. Food Chem Toxicol. 49:149–154.
2011. View Article : Google Scholar
|
|
23
|
Zhou L, Bao YL, Zhang Y, et al: Knockdown
of TSP50 inhibits cell proliferation and induces apoptosis in P19
cells. IUBMB Life. 62:825–832. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang Y, Zhou L, Bao YL, et al: Butyrate
induces cell apoptosis through activation of JNK MAP kinase pathway
in human colon cancer RKO cells. Chem Biol Interact. 185:174–181.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rizzuto R, Nakase H, Darras B, et al: A
gene specifying subunit VIII of human cytochrome c oxidase is
localized to chromosome 11 and is expressed in both muscle and
non-muscle tissues. J Biol Chem. 264:10595–10600. 1989.PubMed/NCBI
|
|
26
|
Zafarullah M, Li WQ, Sylvester J and Ahmad
M: Molecular mechanisms of N-acetylcysteine actions. Cell Mol Life
Sci. 60:6–20. 2003. View Article : Google Scholar
|
|
27
|
Rego AC and Oliveira CR: Mitochondrial
dysfunction and reactive oxygen species in excitotoxicity and
apoptosis: implications for the pathogenesis of neurodegenerative
diseases. Neurochem Res. 28:1563–1574. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shen Z, Shen J, Chen M, Li Q and Hong C:
Morphological changes of mitochondria in apoptosis of esophageal
carcinoma cells induced by As(2)O(3). Zhonghua Bing Li Xue Za Zhi.
29:200–203. 2000.(In Chinese).
|
|
29
|
Gryfe R, Swallow C, Bapat B, et al:
Molecular biology of colorectal cancer. Curr Probl Cancer.
21:233–300. 1997. View Article : Google Scholar
|
|
30
|
Sadowska AM, Manuel Y-Keenoy B and De
Backer WA: Antioxidant and anti-inflammatory efficacy of NAC in the
treatment of COPD: discordant in vitro and in vivo dose-effects: a
review. Pulm Pharmacol Ther. 20:9–22. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Budihardjo I, Oliver H, Lutter M, Luo X
and Wang X: Biochemical pathways of caspase activation during
apoptosis. Annu Rev Cell Dev Biol. 15:269–290. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kroemer G and Martin SJ:
Caspase-independent cell death. Nat Med. 11:725–730. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Adams JM and Cory S: The Bcl-2 protein
family: arbiters of cell survival. Science. 281:1322–1326. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yin XM: Signal transduction mediated by
Bid, a pro-death Bcl-2 family proteins, connects the death receptor
and mitochondria apoptosis pathways. Cell Res. 10:161–167. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nomura M, Shimizu S, Ito T, et al:
Apoptotic cytosol facilitates Bax translocation to mitochondria
that involves cytosolic factor regulated by Bcl-2. Cancer Res.
59:5542–5548. 1999.PubMed/NCBI
|