Introduction
Influenza has resulted in large numbers of
vaccine-preventable deaths worldwide (1). For example, the Spanish influenza
pandemic in 1918 alone caused a large number of fatalities
(estimated up to 40 million) within a short period (2). Furthermore, with the highly
infectious H1N1-2009 strain and the highly virulent H5N1 and H7N9
strains of recent years, influenza virus infection remains a major
health threat to humankind (3,4).
An emerging strategy in antiviral discovery is to
exploit the host innate antiviral mechanisms to counter viral
infections. This approach, as it is independent of viral mutation,
allows universal targeting of the virus since there are generally
no direct interactions with viral proteins (5–8). An
example of such a factor is viperin, which is highly induced by
both type I and II interferons in response to lipopolysaccharide
and double-stranded RNA (9,10).
Viperin interacts with farnesyl pyrophosphate synthase (FPPS),
which is an enzyme that catalyzes the formation of farnesyl
pyrophosphate, an isoprenoid metabolic pathway intermediate.
Cholesterol formation is thus reduced, which subsequently disrupts
raft assembly and perturbs lipid raft formation (11–13).
Since influenza virus requires lipid rafts for its
replication, including viral entry, assembly and budding (14,15),
we therefore hypothesized that influenza virus is susceptible to
FPPS inhibition. An in vitro study demonstrated that the
ectopic expression of viperin successfully inhibits influenza virus
budding from cells, leading to reduced virus titers (11). FPPS is also inhibited by
nitrogen-containing bisphosphonates (N-bisphosphonates), a family
of FPPS inhibitors used clinically in the treatment of bone
diseases (16,17). These FPPS inhibitors act by
recognizing and binding to bisphosphonate-binding sites on the FPPS
enzyme, thereby inactivating it (18). Therefore, we hypothesized that
N-bisphosphonates prevent the formation of lipid rafts by mimicking
the mechanism of viperin.
The present study investigated a potential treatment
for influenza infection using disodium pamidronate, otherwise known
as Pamisol® (PAM), a clinically approved
N-bisphosphonate. The study tested the efficacy of this
commercially available FPPS inhibitor as an alternative to
recombinant viperin protein in influenza therapy. The rationale
underlying this study is to leverage on novel host antiviral
factors and responses to mitigate influenza infection.
Materials and methods
Virus and cell cultures
The influenza virus strain A/H1N1/WSN/1933 (WSN33;
American Type Culture Collection, Manassas, VA, USA) was employed.
MDCK cells (American Type Culture Collection) were cultured in
Eagle’s minimal essential medium with 10% fetal bovine serum (FBS).
HeLaM cells (American Type Culture Collection) were cultured in
Dulbecco’s modified Eagle’s medium (DMEM) with 10% FBS.
Drug treatment and animals
All experiments involving treatment of C57BL/6 mice
with PAM (Hospira, Inc., Lake Forest, IL, USA) were approved by the
Institutional Animal Care and Use Committee (IACUC), National
University of Singapore (protocol 050/11).
Infection and treatment of cells
Overnight cultures of 1×105 HeLaM cells
were infected with 1 μg/ml TPCK-trypsin-activated WSN33 virus at an
MOI of 0.5, and incubated at 37°C for 1 h. The viral inoculum was
replaced with 500 μl DMEM containing 100, 500 or 1,000 μM PAM, or
with DMEM containing PBS as control. Following incubation at 37°C
for 48 h, each cell culture supernatant was harvested for
determination of the viral titer. Quantification of the viral titer
was determined by a virus plaque assay, as previously described
(19).
Cell viability assay
Overnight cultures of 1×105 HeLaM cells
in 96-well plates were treated with DMEM containing 1,000 μM PAM or
PBS, while DMEM alone served as a control. Following incubation at
37°C for 48 h, 20 μl pre-mixed
3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium
inner salt (MTS) reagent (Promega, Madison, WI, USA) was added to
each well. After incubation at 37°C for 3 h, the absorbance
readings at 490 nm of each well were obtained using an ELISA plate
reader (Infinit M200; Tecan, Männedorf, Switzerland) to assess
whether the PAM treatment had detrimental effects.
Cholera toxin subunit-B (CTxB) staining
for lipid rafts
Overnight cultures of 1×105 HeLaM cells
were infected with 1 μg/ml TPCK-trypsin-activated WSN33 virus at an
MOI of 0.5. Following incubation at 37°C for 1 h, the viral
inoculum was replaced with DMEM containing 1,000 μM PAM, or with
DMEM containing PBS as control. Following incubation at 37°C for 24
or 48 h, the cells were trypsinized, washed and resuspended in
chilled DMEM with 10% FBS. The cells were centrifuged at 1,200 × g
for 5 min and resuspended in 2 ml of 1 μg/ml CTxB fluorescent
conjugate (Molecular Probes, Camarillo, CA, USA) in complete
medium, and incubated at 4°C for 10 min. The cells were then washed
and fixed in 4% formaldehyde at 4°C for 15 min, and washed again.
The cells were cyto-centrifuged onto microscopy slides at 600 × g
for 5 min and exposed to mounting medium with DAPI (Vector
Laboratories, Burlingame, CA, USA). Using a fluorescence microscope
(BX60; Olympus, Tokyo, Japan), 10 random fields were selected for
each slide and the mean percentage of CTxB-positive cells was
calculated for analysis of the lipid raft expression levels in the
cells.
In vivo treatment with an FPPS inhibitor
(PAM)
Ten-week old C57BL/6 mice were divided into four
groups and weighed prior to the experiments. For the prophylactic
(PRO) treatment regime, mice were anesthetized with a mixture of
7.5 mg ketamine and 0.1 mg medetomidine, and given a 50 μl dose of
5 mg/kg PAM via the intratracheal route one day prior to infection
(day -1). The day after the first treatment (day 0), a second 50 μl
dose of 5 mg/kg PAM pre-mixed with a lethal dose of WSN33 virus
(1,000 pfu) was administered via the intratracheal route. For the
post-exposure prophylaxis (PEP) regime, mice were infected with
1,000 pfu of WSN33 virus and then subjected to a 50-μl dose of 5
mg/kg PAM on days 0, 1 and 2. Infected mice were monitored daily
for their weight and clinical condition, and were promptly
euthanized in a CO2 chamber when they reached the end
point of either 30% weight loss, moribund condition or at day 14 in
compliance with IACUC guidelines. Following euthanasia, the lungs
of the mice were harvested for further processing as previously
described (19), where 10% of the
total lung mass was used for virologic and molecular analyses,
while the remaining 90% was for histopathology.
Histopathological staining of murine
lungs
The remaining 90% of each lung was fixed in 4%
formalin, and stained with hematoxylin and eosin (H&E) as
previously described (19). The
scoring of lung damage was performed by an experienced pulmonary
histopathologist (J. E. Seet), based on a modified scoring system
(20), i.e. lung damge score =
percentage significant lung damage × [alveolar hemorrhage +
2(alveolar inflitrates) + 3(fibrin) + alveolar septal congestion].
Each of the four criteria was scored on a scale of 0–3. The scoring
was based on the worst-affected areas of the lungs. The percentages
of the significantly damaged regions (defined as at least two
criteria with a maximum score of 3, with the whole lung considered
as 100%) were determined.
Quantitative PCR (qPCR) and statistical
analyses
RNA extraction and qPCR were performed as previously
described (19). Statistical
analyses were performed using SPSS software, version 19 (SPSS,
Inc., Chicago, IL, USA) and GraphPad Prism, version 5 (GraphPad
Software, Inc., La Jolla, CA, USA), via Student’s t-test for
comparison of means, and Kaplan-Meier log rank survival analysis
for survival data. Results were represented as the mean ± standard
deviation. P<0.05 was considered to indicate a statistically
significant difference.
Results
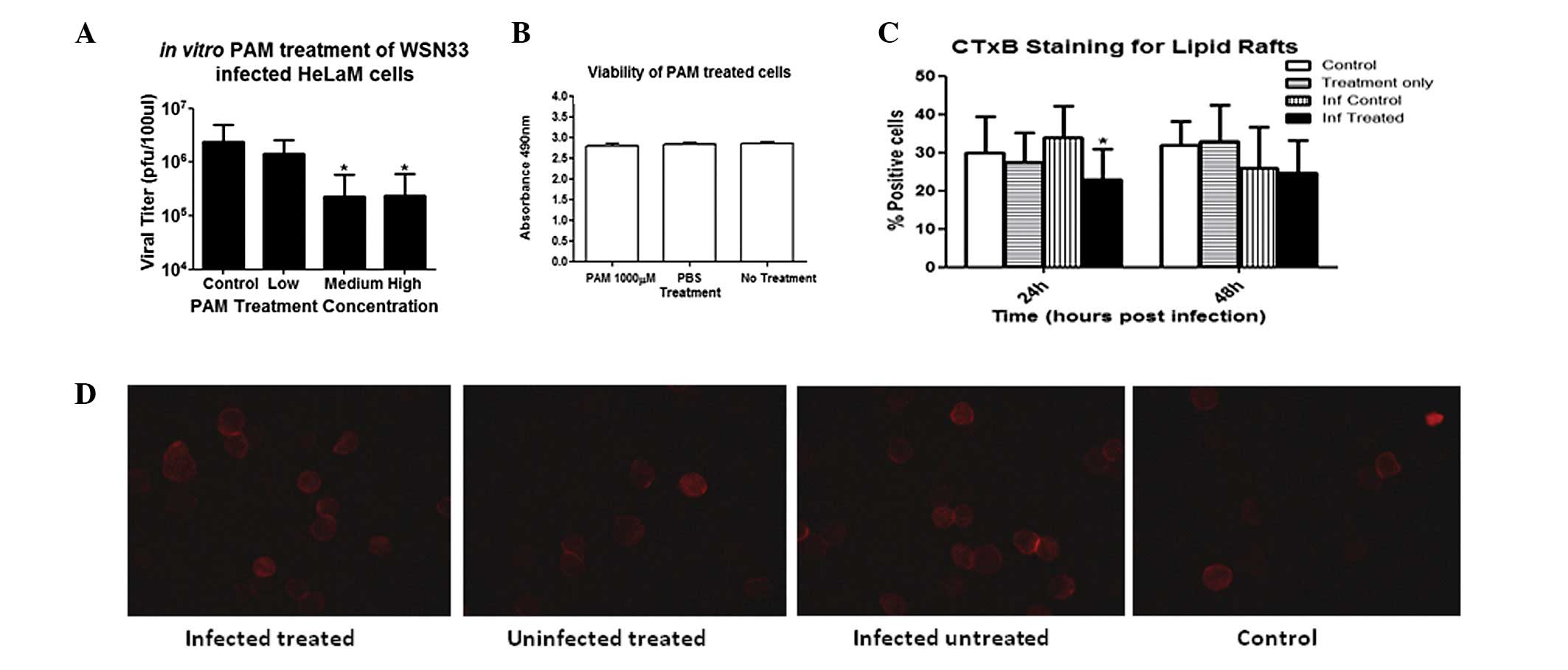
PAM treatment reduces viral titer and has
no detrimental effect on cell viability in vitro
Treatment of infected HeLaM cells with PAM at 500
and 1,000 μM in vitro consistently reduced viral titers by
~1 log compared with the titer in untreated infected control cells
(Fig. 1A), suggesting a mechanism
through FPPS inhibition. However, there was no further reduction in
the viral titer of >1 log at PAM concentrations of >500 μM.
Using the MTS assay, no significant differences were observed
between the cell viability upon PAM treatment and the viability in
PBS-treated or untreated controls (Fig. 1B), indicating that PAM treatment
did not cause deleterious effects in the cells in vitro.
PAM treatment reduces lipid raft
formation in HeLaM cells
To ascertain whether the reduction in viral titer
was due to lipid raft reduction by PAM treatment, staining for CTxB
protein in cells treated with 1,000 μM PAM in comparison with
untreated cells was conducted. As shown in Fig. 1C and D, there was a reduction in
the number of CTxB-positive cells in the infected and PAM-treated
group compared with that in the infected but untreated group at 24
h post-infection, whereas no significant difference was observed
between the two groups at 48 h post-infection. There were also no
differences between uninfected cells subjected to different
treatments at all time-points. These findings suggest that PAM
treatment did not reduce existing lipid rafts, but prevented the
increased lipid raft production required for influenza virus
budding, which culminated in the decreased viral titer. The
promising finding that PAM-mediated reduction of lipid raft
production inhibited influenza replication in vitro
warranted further investigations in vivo.
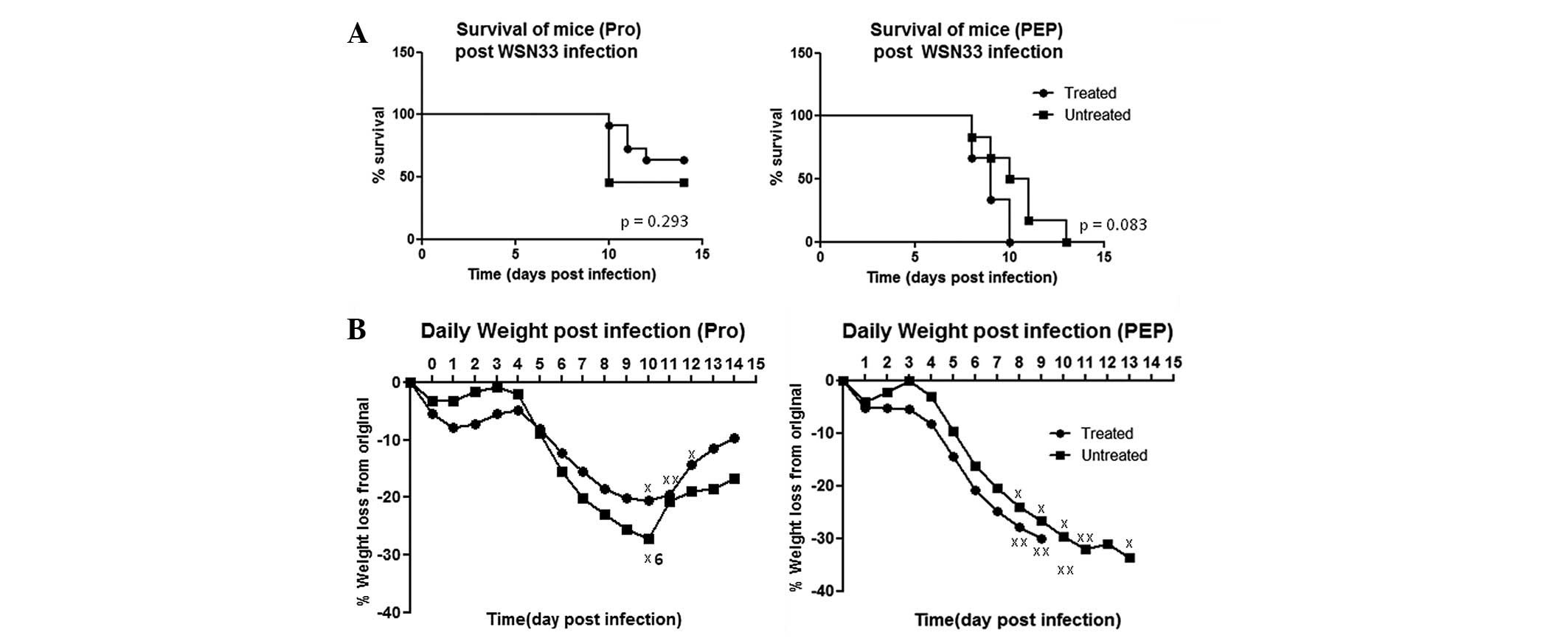
PAM treatment does not confer protection
against influenza challenge in mice
To assess the efficacy of PAM treatment in
vivo, infected mice were subjected to PRO and PEP regimes.
Compared with PAM treatment studies of other infections (21), the PAM dosage was decreased to take
into account direct delivery into the lungs. More frequent dosing
was conducted in the PEP regime to achieve a higher PAM
concentration in the animals than in the animals treated with the
PRO regime.
Despite the PAM treatment of mice subjected to
lethal influenza challenge, there were no significant differences
in the Kaplan-Meier survival analyses (Fig. 2A), with mortality rates of 36.3%
for the treated mice versus 54.5% for the untreated mice in the PRO
regime; and 100% for the treated mice versus 100% for the untreated
mice in the PEP regime. Similar weight loss patterns were observed
with both regimes across all days (Fig. 2B). The higher mortality rates with
the PEP regime may be attributed to the intratracheal treatment
procedures following infection of the mice. Therefore, PAM
treatment may not be efficacious in protecting against influenza
challenge in vivo, in contrast to the in vitro
observations.
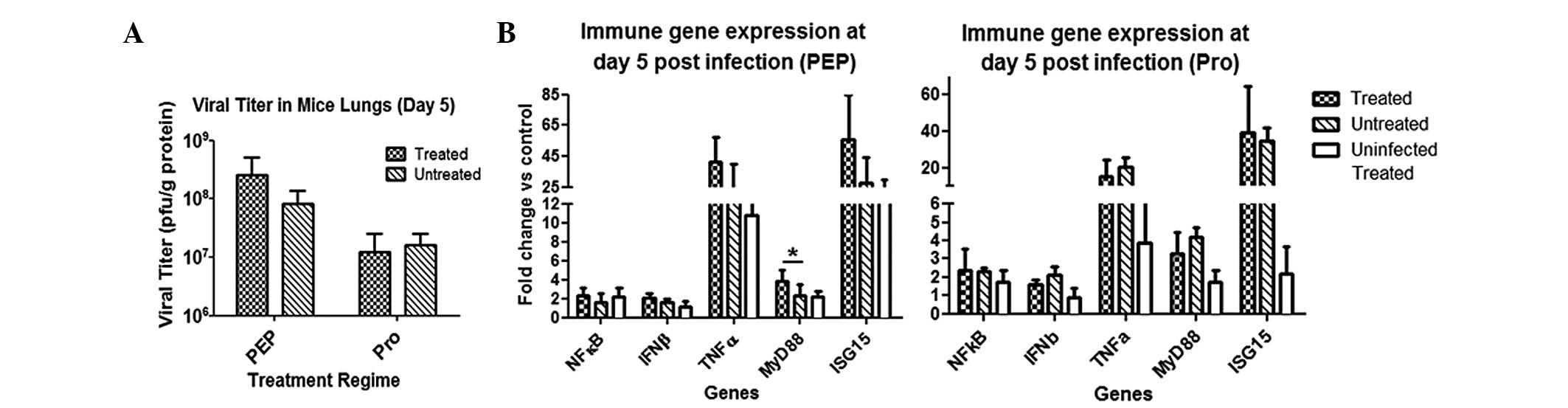
PAM treatment does not significantly
affect viral titer or immune gene expression in infected lungs
There were no observable differences in survival
between PAM-treated and untreated mice post-influenza challenge.
Therefore, it was investigated if there were any differences at the
tissue and molecular levels in the animals. The viral titer in the
lungs at day five post-infection remained unchanged following PAM
treatment using either treatment regime (Fig. 3A). In contrast to the in
vitro studies, PAM treatment failed to achieve a reduction in
the viral titer in vivo, implying that its antiviral
mechanism may not be useful in an in vivo setting.
qPCR analyses demonstrated that there were virtually
no changes in the immune gene expression profiles between the
treated and untreated mice infected with influenza virus (Fig. 3B). However, the only significant
difference was elevated MyD88 expression levels in the PEP
treatment group. Overall, PAM treatment in vivo did not
cause major changes in the immune gene expression of infected
lungs.
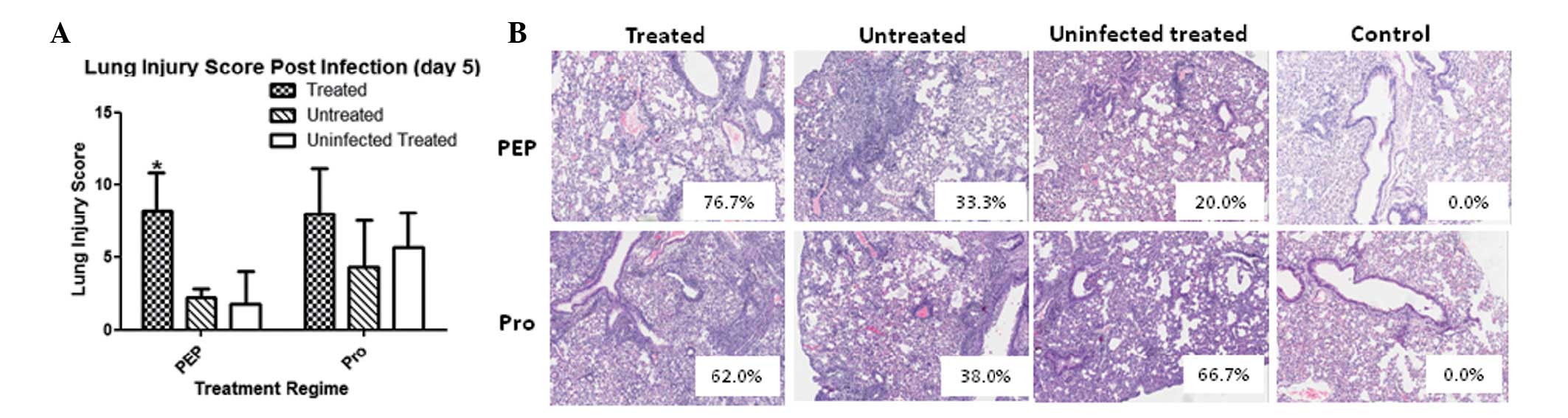
Histopathological studies of lung sections involving
semi-quantitative scoring revealed that the lung injury scores were
not significantly different between the infected groups in the PRO
regime. Notably however, the PEP regime resulted in a significantly
higher lung injury score in the PAM-treated group (Fig. 4A). The increased lung injury was
largely attributed to the more pronounced and widespread areas of
damage in the lungs (Fig. 4B). It
was hypothesized that one mechanism of PAM treatment may be
enhancement of the permeability of the lung vasculature, allowing
the inflammation to be more diffuse in the lungs. Hence, the in
vivo mechanism of PAM necessitates further investigation prior
to exploitation as a potential therapy against infections that
utilize lipid rafts, such as influenza.
Discussion
The major motivation of this study was to assess
whether a small molecule compound could be effectively utilized in
the treatment of influenza for greater cost effectiveness and ease
of delivery compared with recombinant proteins such as viperin.
Therefore, the present study evaluated the efficacy of a known
chemical inhibitor of FPPS based on the hypothesis that its effects
may be similar to those of viperin in controlling influenza
infection.
Notably, the in vitro experiments revealed a
reduction of lipid raft formation in response to the drug treatment
of infected cells, whereas the lipid rafts of uninfected cells were
not affected by the treatment. This suggests that PAM prevented an
increase in lipid raft production by interfering with FPPS
enzymatic activity. The kinetics of CTxB staining further suggest
that the significant reduction in viral titer may be attributed to
delayed lipid raft production following the treatment of infected
cells compared with that in the untreated control cells. Further
reduction was not observed with increasing PAM dosage, possibly due
to the complex influenza virus budding process, i.e. budding stages
where lipid rafts promote bud initiation and growth but inhibit bud
excision and release (14). This
finding thus suggests the potential use of conventional FFPS
inhibitors to inhibit pathogens that require lipid raft formation
as part of their life cycles (21).
By contrast, however, the in vivo results of
the present study were not congruent with the hypothesis, implying
that the direct delivery of PAM into the lungs may create a
suboptimal environment for influenza virus replication. The
prophylactic and therapeutic regimens that were tested did not
significantly alter the susceptibility of mice to the infection,
nor did they decrease viral titers in the lungs; however, they
unexpectedly increased lung injury in the mice.
These in vivo findings appear to be similar
to those in the viperin knockout mouse model (19), where despite viperin efficacy in
vitro against influenza infection, the importance of the
protein and its mechanism appears to be negated by other factors
in vivo, as demonstrated by the equal susceptibility of
wild-type and knockout mice to influenza infection. In the present
study, similar results were obtained by mimicking the mechanism of
viperin instead of using the knockout phenotype. The effects of the
intervention appeared to have been negated by the more complex
scenario in vivo, where even repeated doses of PAM did not
produce the desired effect.
One possible explanation is that the treatment
interferes with cholesterol synthesis by epithelial and endothelial
cells in the lungs, thus affecting their membrane integrity and
causing greater ‘leakiness’, allowing the infection site to be more
extensive. This was demonstrated by the more diffuse extent of lung
injury in the PAM-treated mice. Such damage may arise from the
leakage from the vasculature into the lungs, culminating in cell
death.
Another possible explanation may be the drug’s
ability to activate γδ T-cells in an acute phase response in
infected mice, which aggravates the inflammation (22). PAM confers a protective effect
through the activation of γδ T-cells when administered via the
intraperitoneal route in a humanized mouse model (23). The greater magnitude of
inflammation arising from the direct activation of γδ T-cells in
the lungs may augment lung damage, as observed by the more diffused
damage and higher lung injury score in the PAM-treated mice in the
present study.
In conclusion, although PAM effectively diminished
influenza virus titers in vitro, this molecular inhibitor of
FPPS lacked efficacy in alleviating influenza-induced pulmonary
damage in vivo. Nevertheless, this study indicated the
potential of a clinically approved FPPS inhibitor as a strategy for
controlling influenza infection, by targeting lipid rafts which are
required for the viral budding and release process. The
administration of this drug may be further refined to allow its
optimal retention in infected tissues, or PAM may be tested in
other infections that are more susceptible to FPPS inhibition, such
as West Nile virus (24).
Acknowledgements
The authors thank H.M. Shen, Y. Shi, Connie Foo,
S.H. Lau, N. Li, A.N. Moorthy and W.P. Poh for their assistance.
This study was supported by the National University of Singapore,
the National Research Foundation (Singapore) and the
Singapore-Massachusetts Institute of Technology Alliance for
Research and Technology.
References
|
1
|
Poland GA, Jacobson RM and Targonski PV:
Avian and pandemic influenza: an overview. Vaccine. 25:3057–3061.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
de Wit E and Fouchier RA: Emerging
influenza. J Clin Virol. 41:1–6. 2008.PubMed/NCBI
|
|
3
|
Memoli MJ, Morens DM and Taubenberger JK:
Pandemic and seasonal influenza: therapeutic challenges. Drug
Discov Today. 13:590–595. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rothberg MB, Haessler SD and Brown RB:
Complications of viral influenza. Am J Med. 121:258–264. 2008.
View Article : Google Scholar
|
|
5
|
Watanabe T, Watanabe S and Kawaoka Y:
Cellular networks involved in the influenza virus life cycle. Cell
Host Microbe. 7:427–439. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Karlas A, Machuy N, Shin Y, et al:
Genome-wide RNAi screen identifies human host factors crucial for
influenza virus replication. Nature. 463:818–822. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Stertz S and Shaw ML: Uncovering the
global host cell requirements for influenza virus replication via
RNAi screening. Microbes Infect. 13:516–525. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
König R, Stertz S, Zhou Y, et al: Human
host factors required for influenza virus replication. Nature.
463:813–817. 2010.PubMed/NCBI
|
|
9
|
Fitzgerald KA: The interferon inducible
gene: Viperin. J Interferon Cytokine Res. 31:131–135. 2011.
View Article : Google Scholar
|
|
10
|
Gerber SA and Pober JS: IFN-alpha induces
transcription of hypoxia-inducible factor-1alpha to inhibit
proliferation of human endothelial cells. J Immunol. 181:1052–1062.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang X, Hinson ER and Cresswell P: The
interferon-inducible protein viperin inhibits influenza virus
release by perturbing lipid rafts. Cell Host Microbe. 2:96–105.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Simons K and Ehehalt R: Cholesterol, lipid
rafts, and disease. J Clin Invest. 110:597–603. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Reilly JF, Martinez SD, Mickey G and Maher
PA: A novel role for farnesyl pyrophosphate synthase in fibroblast
growth factor-mediated signal transduction. Biochem J. 366:501–510.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nayak DP, Balogun RA, Yamada H, Zhou ZH
and Barman S: Influenza virus morphogenesis and budding. Virus Res.
143:147–161. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ono A and Freed EO: Role of lipid rafts in
virus replication. Adv Virus Res. 64:311–358. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
van Beek E, Pieterman E, Cohen L, Löwik C
and Papapoulos S: Farnesyl pyrophosphate synthase is the molecular
target of nitrogen-containing bisphosphonates. Biochem Biophys Res
Commun. 264:108–111. 1999.PubMed/NCBI
|
|
17
|
Kavanagh KL, Guo K, Dunford JE, et al: The
molecular mechanism of nitrogen-containing bisphosphonates as
antiosteoporosis drugs. Proc Natl Acad Sci USA. 103:7829–7834.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Guo RT, Cao R, Liang PH, et al:
Bisphosphonates target multiple sites in both cis- and
trans-prenyltransferases. Proc Natl Acad Sci USA. 104:10022–10027.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tan KS, Olfat F, Phoon MC, et al: In vivo
and in vitro studies on the antiviral activities of viperin against
influenza H1N1 virus infection. J Gen Virol. 93:1269–1277. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Matute-Bello G, Winn RK, Jonas M, Chi EY,
Martin TR and Liles WC: Fas (CD95) induces alveolar epithelial cell
apoptosis in vivo: implications for acute pulmonary inflammation.
Am J Pathol. 158:153–161. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yardley V, Khan AA, Martin MB, et al: In
vivo activities of farnesyl pyrophosphate synthase inhibitors
against Leishmania donovani and Toxoplasma gondii.
Antimicrob Agents Chemother. 46:929–931. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rossini M, Adami S, Viapiana O, et al:
Long-term effects of amino-bisphosphonates on circulating γδ T
cells. Calcif Tissue Int. 91:395–399. 2012.
|
|
23
|
Tu W, Zheng J, Liu Y, et al: The
aminobisphosphonate pamidronate controls influenza pathogenesis by
expanding a gammadelta T cell population in humanized mice. J Exp
Med. 208:1511–1522. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Szretter KJ, Brien JD, Thackray LB, Virgin
HW, Cresswell P and Diamond MS: The interferon-inducible gene
viperin restricts West Nile virus pathogenesis. J Virol.
85:11557–11566. 2011. View Article : Google Scholar : PubMed/NCBI
|