Introduction
Doxorubicin (Dox), isolated from cultures of
Streptomyces peucetius var. caesius, is a cytotoxic
anthracycline antibiotic and is commonly used for cancer treatment
(1). Although it has been well
demonstrated that Dox exerted robust antitumor activity, the
associated dose-dependent cardiotoxicity restricted its clinical
use (1–3). A novel therapeutic regime, in which
one compound is employed to enhance its antitumor activity while
decreasing the severe side effects, may improve the care of cancer
patients.
The mechanism of cardiac injury induced by Dox,
which may involve free radical stress, calcium overloading,
mitochondrial dysfunction or dysregulation of iron hemostasis, has
been investigated (1,4). The exact molecular mechanisms,
however, in which reactive oxygen species (ROS) are considered to
be central remain unclear (1,5). It
was reported that Dox has been observed to transform into a
semiquinone radical, which then reduces oxygen to produce
superoxide and reacts with polyunsaturated fatty acids to yield
lipid hydroperoxide (6). The
antioxidants, which cleaved the generated ROS, were hypothesized to
exhibit protective effects against Dox-induced cardiomyopathy
(7,8). Notably, a number of antioxidants,
including thrombopoietin, schisandrin B and probucol, as well as
the FDA-approved dexrazoxane, have been shown to prevent and treat
Dox induced-cardiomyopathy (7–10).
Thus, novel antioxidants, which enhance the anticancer activity of
Dox, may be a potential candidate for a novel combination
therapeutic regime.
The ginsengs have been employed in Asian societies
for thousands of years and used as herbal medication for a variety
of disorders (11). The major
pharmacological properties of ginseng were attributed to the
ginsenosides, the active ingredients, which have been documented to
possess diverse activities, including neuroprotective,
cardioprotective, antioxidant and anticancer properties (11,12).
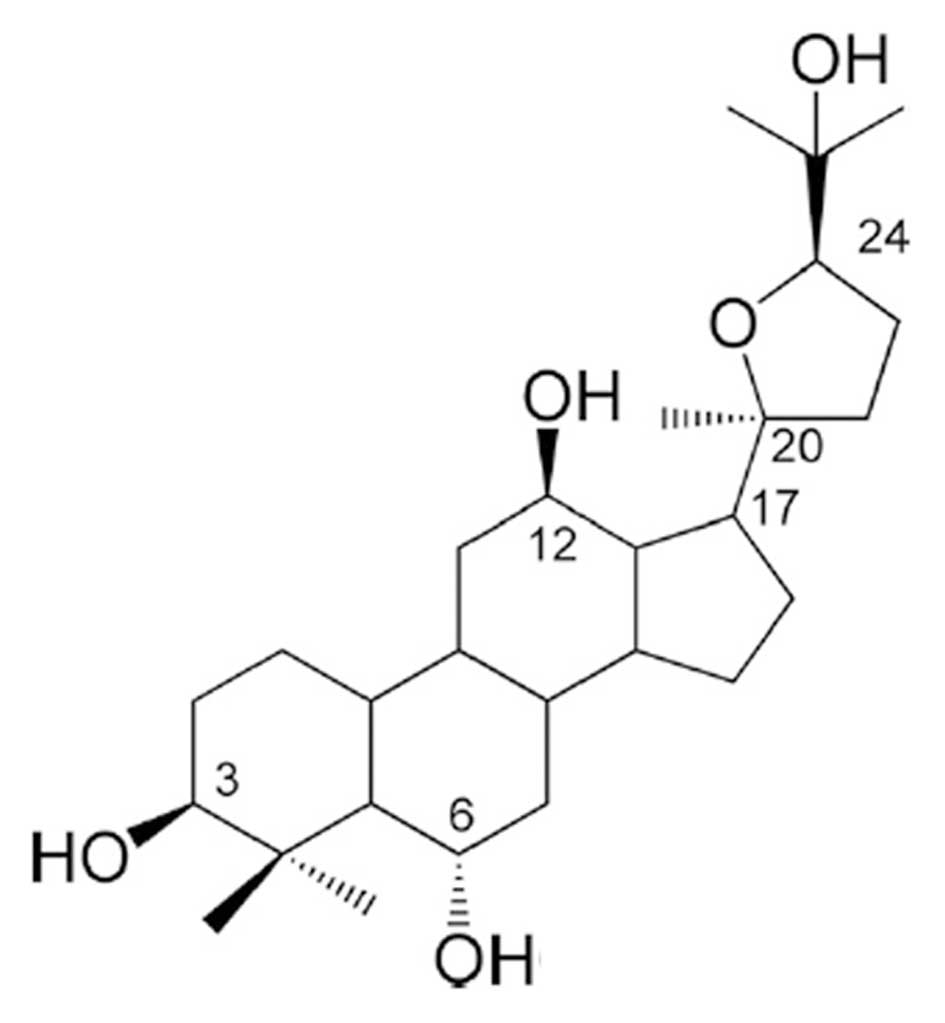
Ocotillol (Fig. 1), a derivative
of pseudoginsenoside F11 from American ginseng, was recently
reported to potentiate the anticancer activity of Dox (13). However, the effect of ocotillol on
Dox-induced cardiac injury, which is the most severe and lethal
toxic effect of Dox, remains to be fully understood. In the current
study, an in vivo model was developed to determine the
effect of ocotillol on Dox-induced acute and chronic
cardiomyopathy.
Materials and methods
Materials
Ocotillol was isolated from American Ginsengs by the
Shandong Luye Pharmaceutical Company (Yantai, China) and obtained
as white powder with the molecular formula
C30H52O5 and a molecular weight of
492. The purity of the compound was checked by high-performance
liquid chromatography and was observed to be >98.5%. In
vivo, ocotillol and Dox (Beyotime Institute of Biotechnology,
Hangzhou, China) were dissolved in 1% carboxymethycellulose sodium
(CMCS; Shandong Luye Pharmaceutical Company) and 0.9% sodium
chloride as the proposed doses, respectively.
Animals
Male Swiss mice (weight, 18–22 g) were obtained from
Shandong Luye Pharmaceutical Company. The animals were housed in a
light- and temperature-controlled room (21–22°C; humidity, 60–65%)
and had ad libitum access to a standard diet and water. All
the experiments were performed in accordance with the Guideline for
Care and Use of Experimental Aniamls of Experimental Animal
Research Committee of Yantai University.
Model of Dox-induced acute
cardiomyopathy
Male Swiss mice were randomly divided into two
groups (n=10 per group). The control group was administered with
one dose of Dox dissolved in 0.9% NaCl intraperitoneally (i.p.) at
20 mg/kg on day 1 and 10 doses of CMCS gavage (i.p.) daily. The
pretreated groups received a total of 10 doses of ocotillol at 10
mg/kg daily with the first administration 24 h prior to
administration of one dose of Dox. The animals were checked twice
daily and the number of dead mice were recorded continuously for 12
days post-dose administration. The survival curve was presented and
the difference was compared between the two groups.
Model of Dox-induced chronic
cardiomyopathy
Male Swiss mice were randomly divided into four
groups (n=10 per group). The control group was administered a total
of six doses of 0.9% NaCl i.p. every other day and eight doses of
CMCS daily orally (p.o.). The Dox group was administrated a total
of six doses Dox dissolved in 0.9% NaCl i.p. at 3 mg/kg
(accumulative dose 18 mg/kg) every other day, and a total of eight
doses of CMCS p.o. daily. The pretreated groups received a total of
eight doses ocotillol at 5 mg/kg and 10 mg/kg daily with the first
administration 24 h prior to Dox injection.
Blood sampling and tissue preparation were performed
under anesthesia with ketamine (Peking Union Medical College,
Beijing, China) at 50 mg/kg and xylazine (EnoGene, Nanjing, China)
at 20 mg kg 24 h after the final dose. Blood samples were drawn
into heparinized tubes and divided into two parts. One was for the
assays of white blood cell counts (WBC), red blood cell counts
(RBC) and platelets (PLT). The other part was immediately
centrifuged (2,500 × g for 10 min at 4°C), and the plasma was
stored at −80°C, and the content of creatine kinase (CK) and CK MB
fraction (CK-MB) in plasma were measured with a Hitachi 7060 Fully
Automated Biochemistry Analyzer (Hitachi, Tokyo, Japan).
Following the sacrifice of the mice by carbon
dioxide asphyxiation, the hearts were rapidly removed. Half the
tissue was weighed and homogenized in ice-cold normal saline (1/9,
w/v) and centrifuged (5,000 × g for 10 min at 4°C). The suspension
was stored at −80°C and the content of glutathione (GSH) and
malondialdehyde (MDA) in the heart tissue were determined using
commercial kits provided by Nanjing Jiancheng Bioengineering
Institute (Nanjing, China). The protein concentration was
determined by the bicinchoninic acid kit (Beyotime Institute of
Biotechnology) and was used to normalize the data.
The other sections of heart tissue were fixed with
4% formaldehyde overnight, dehydrated in ascending grades of
alcohol and embedded in paraffin. Serial sections were sliced at 5
μm and stained with hematoxylin and eosin. The sections were
analyzed and images were captured using a Nikon Eclipse 50i
microscope (Nikon, Chiyoda, Japan) by two pathologists with blind
investigation and the representative images are presented.
Statistical analysis
Survival rates were compared by Kaplan-Meier
log-rank test. Other data in this study are expressed as the mean ±
standard deviation and analyzed by one-way analysis of variance.
The difference between two groups was determined by Student’s
t-test. P<0.05 was considered to indicate a statistically
significant difference.
Results
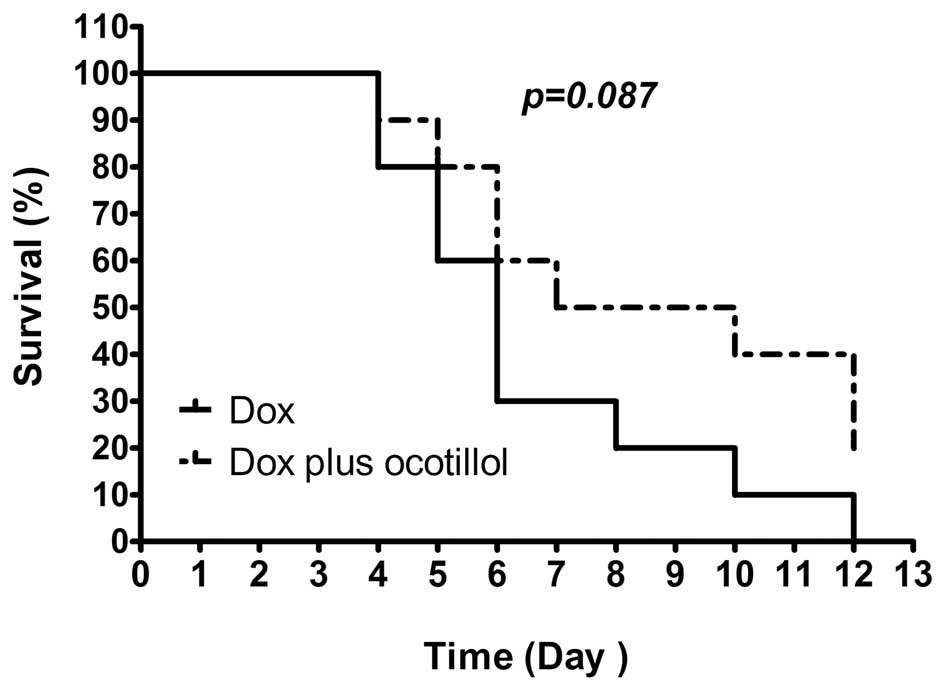
Ocotillol prolongs the survival time in a
model of acute Dox-induced cardiomyopathy
The mice were injected once with Dox at dosage of 20
mg/kg and the morbidity was observed twice daily. As shown in
Fig. 2, all animals in the Dox
group succumbed within 12 days post-dose. However, co-treatment
with ocotillol at a dosage of 10 mg/kg, prolonged the survival rate
(p=0.087, compared with the Dox group), in which 2 of 10 animals
remained alive at the end of the experiment.
Ocotillol exerts a protective effect in a
model of chronic Dox-induced cardiomyopathy
Plasma CK and CK-MB
The levels of CK and CK-MB are biomarkers of heart
tissue damage (14). As a
cardiotoxic agent, Dox significantly increased the level of CK and
CK-MB in the treated animals (Fig.
3; P<0.05, vs. the control group), which indicated the
occurrence of heart tissue injury. Co-treatment with ocotillol at a
dose of 10 mg/kg was observed to significantly decrease the
elevated levels of CK and CK-MB (Fig.
3, P<0.05, vs. Dox group). Ocotillol alone exhibited no
marked effect at the tested dosage.
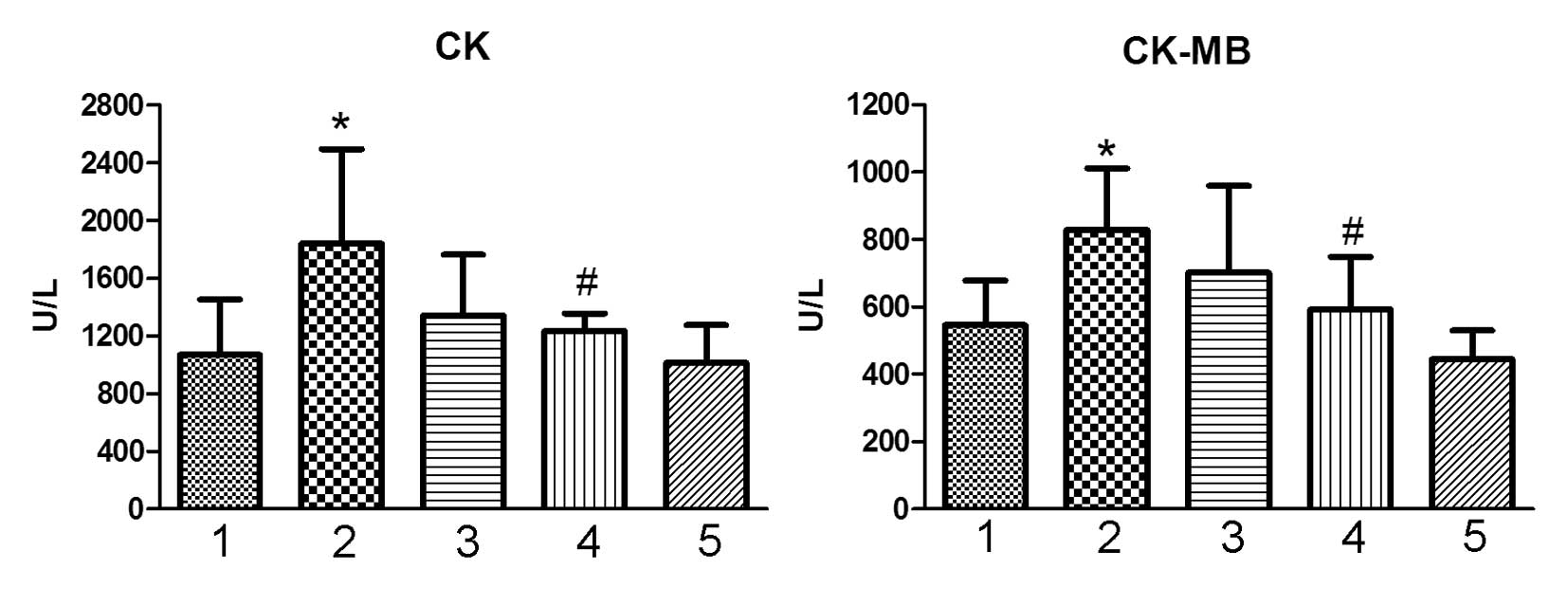 | Figure 3Effect of ocotillol on serum CK and
CK-MB in the model of Dox-induced chronic cardiomyopathy. Animal
serum was prepared and the serum CK and CK-MB were detected. 1,
control group; 2, Dox group (3 mg/kg); 3, Dox (3 mg/kg) plus
ocotillol group (5 mg/kg); 4, Dox (3 mg/kg) plus ocotillol group
(10 mg/kg); and 5, ocotillol group (10 mg/kg). All data are
expressed as the mean ± standard deviation (n=10).
*P<0.05, vs. the control group;
#P<0.05, vs. the Dox group. Dox, doxorubicin; CK,
creatine kinase; CK-MB, creatine kinase MB fraction. |
Tissue GSH and MDA
Tissue GSH is a significant antioxidant biomolecule
against oxidative stress (15).
Following treatment with Dox, the contents of GSH in the heart
tissue was significantly decreased (Fig. 4; P<0.05, vs. the control group).
The content of MDA was significantly increased in the animals
treated with Dox (Fig. 4;
P<0.05, vs. control group). Co-treatment with ocotillol,
however, significantly alleviated the reduction of GSH and
elevation of MDA (Fig. 4;
P<0.05, vs. Dox group). Ocotillol alone exhibited no marked
effect on the content of GSH and MDA in heart tissue at the tested
dosage.
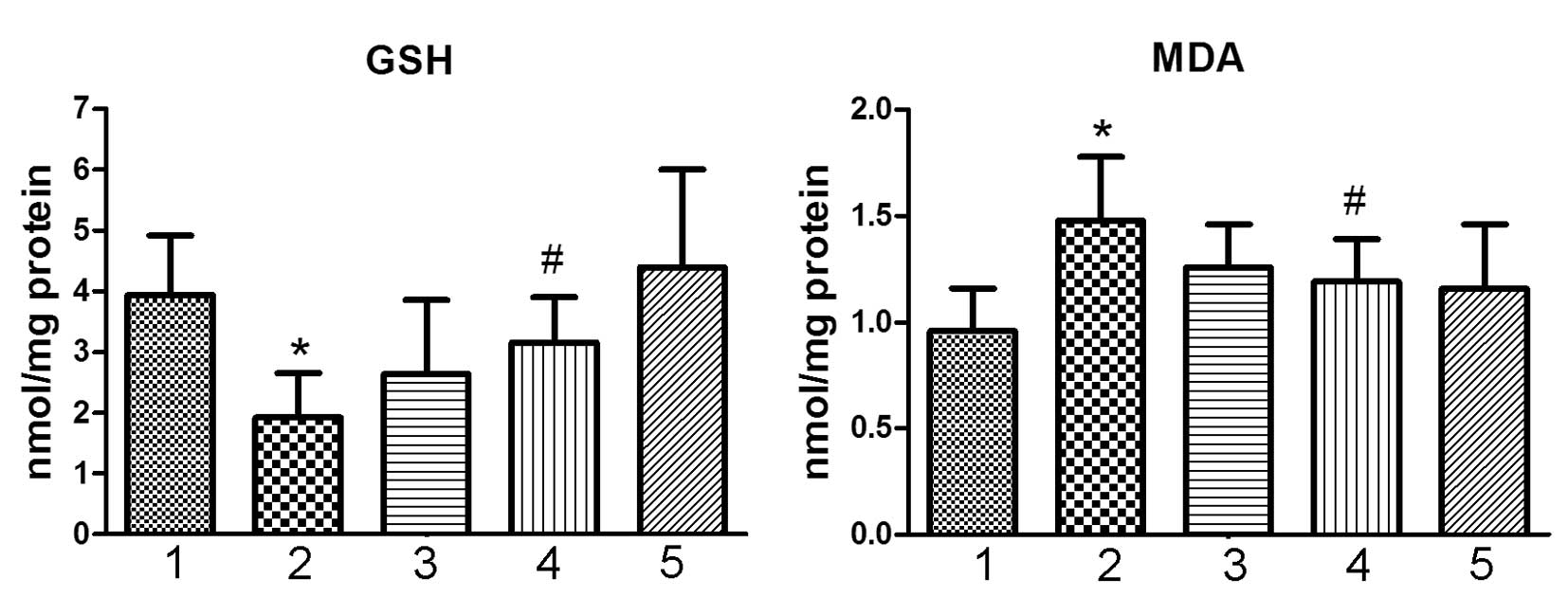 | Figure 4Effect of ocotillol on the content of
GSH and the content of MDA in heart tissue. The tissues were
cooled, homogenized, and then determined for GSH and MDA. 1,
control group; 2, Dox group (3 mg/kg); 3, Dox (3 mg/kg) plus
ocotillol group (5 mg/kg); 4, Dox (3 mg/kg) plus ocotillol group
(10 mg/kg); and 5, ocotillol group (10 mg/kg). All data are
expressed as the mean ± standard deviation (n=10). All data are
expressed as means ± SD (n=10). *P<0.05, vs. control
group; #P<0.05, vs. Dox group. Dox, doxorubicin; GSH,
glutathione; MDA, malondialdehyde. |
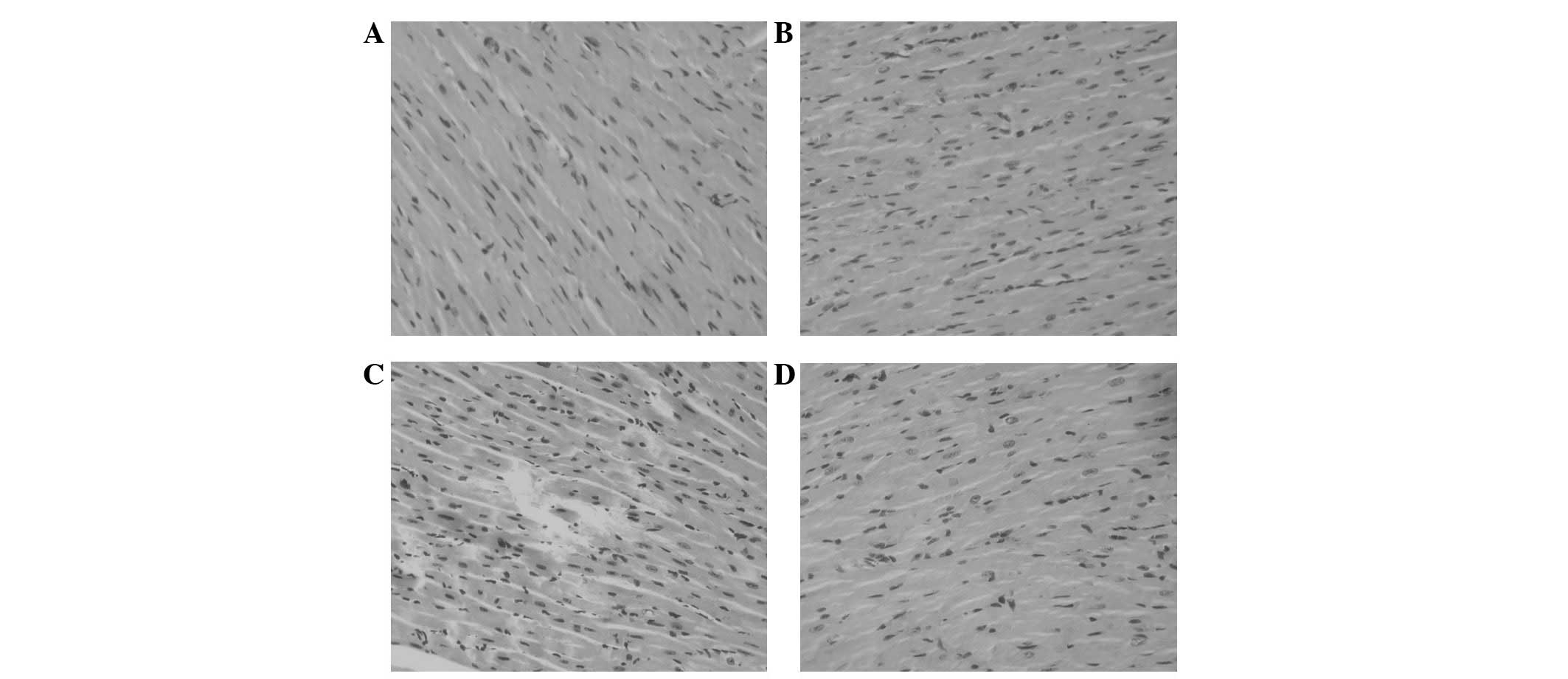
Histological examination
Histological examinations for the left ventricles
were performed as previously described (15). The animals in the control and
ocotillol groups were observed with normal cardiomyocyte
morphology. However, in animals treated with Dox, disorganization
of myofibrillar arrays and cytoplasmic vacuolization were observed
(Fig. 5). When pre-treated with
different dosage of ocotillol, less histopathological changes were
observed. Ocotillol alone had no marked effect at the tested
dosage.
Ocotillol attenuates decreased WBC
count
Following two weeks of administration, Dox markedly
reduced the WBC count (Table I;
P<0.05, vs. control group). However, co-treatment with ocotillol
at a dosage of 10 mg/kg restored the lowered WBC to a greater
degree (Table I; P<0.05,
compared with Dox group). Dox in the presence or absence of
ocotillol had no marked effects on RBC and PLT counts (Table I).
 | Table IEffects of ocotillol and Dox on WBC,
RBC and PLT counts (mean ± standard deviation). |
Table I
Effects of ocotillol and Dox on WBC,
RBC and PLT counts (mean ± standard deviation).
| Group (n=10) | WBC
(106/ml) | RBC
(109/ml) | PLT
(106/ml) |
|---|
| Control | 11.45±1.74 | 12.86±1.40 | 555.00±145.56 |
| Dox 3 mg/kg | 3.24±0.33a | 10.48±1.49 | 406.67±80.40 |
| Dox 3 mg/kg+Ocotillol
5 mg/kg | 5.13±1.10b | 11.03±1.20 | 476.52±188.43 |
| Dox 3 mg/kg+Ocotillol
10 mg/kg | 7.13±1.67b | 10.18±2.04 | 576.33±298.49 |
| Ocotillol 10
mg/kg | 11.2±2.89 | 12.76±1.08 | 658.00±135.01 |
Discussion
In previous studies, repeated administration of Dox
lead to frequent and devastating cardiomyopathy, and complications
commonly lead to a reduced quality of life for the patient and/or
morbidity (1,3). A non-toxic ‘sensitizer’, which
enhanced the potential of Dox without increasing its toxic effect,
is likely to improve the treatment of cancer patients. Ocotillol
was recently reported to enhance the potential of Dox (15) and in the current study, evidence is
provided that ocotillol may also exert cardioprotective effects
against Dox-induced cardiomyopathy.
Since ocotillol had been shown to enhance the
antitumor activity of Dox, it was important to determine whether
ocotillol may also increase its toxicity, particularly for its
dose-dependent and irreversible cardiotoxicity. In the model of
Dox-induced acute cardiomyopathy, co-treatment with ocotillol did
not decrease the survival time, but exhibited protective activity
against Dox-induced morbidity. Dox-ocotillol combination therapy,
therefore, may not only increase the antitumor effects, but may
also decrease the toxic effects, which in turn may provide clinical
benefits.
The chronic cardiac injury model was performed to
determine the effect of ocotillol on the Dox-induced
cardiomyopathy. CK and CK-MB are well established diagnostic
markers for myocardial function (14). During cardiac myocyte injury, these
enzymes were leaked into the serum, which was easily detected in
the blood samples. In the current study, cumulative doses of Dox
(18 mg/kg) caused a significant increase in CK and CK-MB, which
indicated that Dox exhibited severe cardiotoxicity. The increased
plasma enzymes were suppressed by pre-treatment with ocotillol,
which indicated that ocotillol was capable of attenuating
Dox-induced cardiac injury. Notably, the histological examination
of the heart tissue also showed that pre-treatment with ocotillol
could significantly alleviate the Dox-induced histopathologic
lesion.
It has been well documented that the cardiotoxicity
of Dox was mediated by ROS (5). In
view of the importance of oxidative stress to cardiac injury, a
number of studies have suggested that the cardioprotective effects
of ginseng ingredients, including ginsenoside Rg1 and Rh2, were
associated with the reduction in oxidative stress by enhancing
endogenous antioxidant reserve (15,16).
Based on our previous results, ocotillol is capable of exerting
cardioprotective effects on myocardial injury induced by
isoproterenol in rats by enhancing the antioxidative potency of the
heart (17). Pre-treatment with
ocotillol may increase the content of GSH in heart tissue and as a
consequence, the MDA may be cleared by these anti-oxidant
biomolecules.
Bone marrow suppression is a major toxic property of
cytotoxic drugs, including Dox and paclitaxel, which has been
primarily observed with leukopenia and neutropenia (18,19).
In the current study, the decreased extents of the leukopenia in
the mice treated with Dox were significantly attenuated by
co-treatment with ocotillol, which indicated that ocotillol was
capable of alleviating the bone marrow toxicity of Dox.
The effect of ocotillol on the toxicity of Dox was
completely different to its effect on the potency of Dox, the exact
mechanism of which remains unknown. One possible interpretation is
that this occurred in a cell/tissue-dependence manner (20), in which these selective
characteristics are likely to further benefit its co-administration
clinically. This finding, which was also observed in a number of
published compounds, including Rh2 and schisandrin (8,15),
supported the further investigation for ocotillol as a protector
against Dox-induced cardiotoxicity.
In conclusion, the present study showed the
protective effect of ocotillol against Dox-induced cardiomyopathy,
which may be associated with the role of ocotillol in the
maintenance of the endogenous anti-oxidant status in heart tissue.
Combined with the previous findings, ocotillol was capable of
enhancing the antitumor activity of Dox, the data implied that use
of ocotillol with Dox may be an improved therapeutic strategy.
Acknowledgements
This study was supported by grants from Taishan
Scholar Project, A Project of Shandong Province Higher Educational
Science and Technology Program (grant no. J12LM53), and the
National Natural Science Foundation of China (grant no.
81202038).
Reference
|
1
|
Minotti G, Menna P, Salvatorelli E, Cairo
G and Gianni L: Anthracyclines: molecular advances and
pharmacologic developments in antitumor activity and
cardiotoxicity. Pharmacol Rev. 56:185–229. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Appel JM, Nielsen D, Zerahn B, Jensen BV
and Skagen K: Anthracycline-induced chronic cardiotoxicity and
heart failure. Acta Oncol. 46:576–580. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Singal PK and Iliskovic N:
Doxorubicin-induced cardiomyopathy. N Engl J Med. 339:900–905.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Carvalho C, Santos RX, Cardoso S, et al:
Doxorubicin: the good, the bad and the ugly effect. Curr Med Chem.
16:3267–3285. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Olson RD, Mushlin PS, Brenner DE, et al:
Doxorubicin cardiotoxicity may be caused by its metabolite,
doxorubicinol. Proc Natl Acad Sci USA. 85:3585–3589. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li L, Pan Q, Han W, Liu Z and Hu X:
Schisandrin B prevents doxorubicin-induced cardiotoxicity via
enhancing glutathione redox cycling. Clin Cancer Res. 13:6753–6760.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Siveski-Iliskovic N, Kaul N and Singal PK:
Probucol promotes endogenous antioxidants and provides protection
against adriamycin-induced cardiomyopathy in rats. Circulation.
89:2829–2835. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li L, Lu Q, Shen Y and Hu X: Schisandrin B
enhances doxorubicin-induced apoptosis of cancer cells but not
normal cells. Biochem Pharmacol. 71:584–595. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li K, Sung RY, Huang WZ, et al:
Thrombopoietin protects against in vitro and in vivo cardiotoxicity
induced by doxorubicin. Circulation. 113:2211–2220. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hellmann K: Overview and historical
development of dexrazoxane. Semin Oncol. 25:48–54. 1998.PubMed/NCBI
|
|
11
|
Attele AS, Wu JA and Yuan CS: Ginseng
pharmacology: multiple constituents and multiple actions. Biochem
Pharmacol. 58:1685–1693. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Karmazyn M, Moey M and Gan XT: Therapeutic
potential of ginseng in the management of cardiovascular disorders.
Drugs. 71:1989–2008. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang H, Yu P, Bai J, et al: Ocotillol
enhanced the antitumor activity of Doxorubicin via p53-dependent
apoptosis. Evidence-Based Complementary and Alternat Med.
2013:4685372013.PubMed/NCBI
|
|
14
|
Rajadurai M and Stanely Mainzen Prince P:
Preventive effect of naringin on cardiac markers,
electrocardiographic patterns and lysosomal hydrolases in normal
and isoproterenol-induced myocardial infarction in Wistar rats.
Toxicology. 230:178–188. 2007. View Article : Google Scholar
|
|
15
|
Wang H, Yu P, Gou H, et al:
Cardioprotective effects of 20(S)-ginsenoside Rh2 against
Doxorubicin-induced cardiotoxicity in vitro and in vivo. Evid Based
Complement Alternat Med. 2012:5062142012.PubMed/NCBI
|
|
16
|
Zhu D, Wu L, Li CR, et al: Ginsenoside Rg1
protects rat cardiomyocyte from hypoxia/reoxygenation oxidative
injury via antioxidant and intracellular calcium homeostasis. J
Cell Biochem. 108:117–124. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yu C, Fu F, Yu X, Han B and Zhu M:
Cardioprotective effect of ocotillol, a derivate of
pseudoginsenoside F11, on myocardial injury induced by
isoproterenol in rats. Arzneimittelforschung. 57:568–572.
2007.PubMed/NCBI
|
|
18
|
Papadopoulou LC and Tsiftsoglou AS:
Effects of hemin on apoptosis, suppression of cytochrome c
oxidase gene expression, and bone-marrow toxicity induced by
doxorubicin (adriamycin). Biochem Pharmacol. 52:713–722.
1996.PubMed/NCBI
|
|
19
|
Palma MD, Lombardi G, Donach ME, et al:
Tolerability of PLD/oxaliplatin regimen in recurrent ovarian cancer
patients with previous fragility to carboplatin/paclitaxel
treatment. Am J Clin Oncol. 34:305–308. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang S, Konorev EA, Kotamraju S, Joseph J,
Kalivendi S and Kalyanaraman B: Doxorubicin induces apoptosis in
normal and tumor cells via distinctly different mechanisms.
intermediacy of H2O2- and p53-dependent
pathways. J Biol Chem. 279:25535–25543. 2004. View Article : Google Scholar : PubMed/NCBI
|