Introduction
Endothelial progenitor cells (EPCs) have the ability
to differentiate ex vivo into endothelial-phenotyped cells
and were initially detected in the peripheral circulation in 1997
via the isolation of cells expressing the CD34 antigen (1,2).
EPCs develop into endothelial cells (ECs) during embryogenesis and
human hematopoietic stem cytogenesis (3,4). It
has also been reported that hemangioblasts were the multipotent
precursor cells of EPCs and hematopoietic stem cells (HSCs). EPCs
are known to contribute to the growth of vessels and it was
reported that they may induce prolonged vascular recovery from
ischemia (5,6). In addition, the transplantation of
healthy EPCs to compensate for the role of their dysfunctional
counterparts demonstrated promising improvements in numerous animal
models of ischemic disease (7–9).
EPCs may enter into the circulation by detaching from activated or
damaged vessels. Studies have reported the number of circulating
EPCs was significantly increased under several pathological
conditions that involved vascular injury or instability, including
myocardial infarction and cancer (10,11).
This therefore suggested that circulating EPCs may have provided an
endogenous repair mechanism that counteracted the ongoing risk
factor-induced endothelial injury and therefore protected against
the development of vascular injuries, such as myocardial infarction
(10–13). Therefore, the present study
hypothesized that changes in circulating levels of EPCs may act as
a predictor for the incidence of acute myocardial infarction.
Due to its low toxicity, superparamagnetic iron
oxide (SPIO) nanoparticles coated with bioprobes were developed for
highly specific labeling of targeted tumors in tumor examination
and treatment (14–17). Magnetic resonance imaging (MRI) was
demonstrated to be effective in tracking transplanted stem cells by
labeling cells with superparamagnetic iron oxide (SPIO)
nanoparticles (18). The present
study investigated the intracellular iron content, labeling
efficiency and cell viability of SPIO-labeled EPCs as well as
analyzed the MRI results in order to set up a theoretical
foundation for the application of autograft EPCs in
vivo.
Materials and methods
Cells
The present study was approved by the ethics
committee of Xijing Hospital, Fourth Military Medical University
(Xi’an, China). EPCs were derived and cultured as previously
described (19). In brief,
mononuclear cells (MNC) from minipig (the minipig was purchased
from the Experimental Animal Center, Forth Military Medical
University, Xi’an, China) bone marrow were first isolated using
density gradient centrifugation. MNCs were plated on un-coated
tissue culture flasks at a density of 1×106/ml in
Dulbecco’s modified Eagle’s medium (DMEM; HyClone, Thermo Fisher
Scientific, Waltham, MA, USA) containing 10% fetal bovine serum
(FBS; HyClone). Non-adherent cells were then collected and plated
on culture flasks following four days of culture and the medium was
replaced. Following a further 24 h of culture, non-adherent cells
were collected and seeded into culture flasks at a density of
1×106/ml in DMEM supplemented with 10% FBS, vascular
endothelial growth factor (VEGF; 10 ng/ml; PeproTech EC Ltd,
London, UK) and basic fibroblast growth factor (bFGF; 10 ng/ml;
PeproTech EC Ltd). Cells were then maintained at 37°C and 5%
CO2. Media was observed daily and changed every two to
three days.
Fluorescence-activated cell sorting
(FACS) analysis
Flow cytometric staining and analyses were performed
as previously described (20). In
brief, EPCs were resuspended in 100 ml rinsing buffer and incubated
with the following monoclonal antibodies: Phycoerythrin
(PE)-conjugated mouse anti-human CD31 (1:50), fetal liver kinase
(Flk)-1 (1:100) and factor VIII (1:16,000) (Santa Cruz
Biotechnology, Inc, Dallas, TX, USA) for 30 min at room
temperature. Following washing with phosphate-buffered saline (PBS;
pH 7.35), the expression of membranous antigen on the cells was
detected using a FACSCaliburTM flow cytometer (BD
Biosciences, San Jose, CA, USA) equipped with the Cell Quest
software (BD Biosciences). Flow cytometric data were analyzed using
appropriate controls (cat no.: 1-001-A; R&D Systems, Inc.,
Minneapolis, MN, USA) with isotype-matched immunoglobulin G and
unstained controls.
SPIO labeling in vitro
Concentrations of SPIO (Bayer Healthcare
Pharmaceuticals, Montville, NJ, USA) in cell culture medium were as
follows: Group 1, 17.5 μg/ml; group 2, 35 μg/ml; group 3, 70 μg/ml;
and group 4, 140 μg/ml, the control group consisted of DMEM without
SPIOs. EPCs labeled with SPIO were incubated at 37°C and 5%
CO2 atmosphere, EPCs were then washed with culture DMEM
and subsequently used for in vitro studies. Following
incubation for one day, the growth and morphology of EPCs in
culture were observed daily.
Cell viability
Following incubation for one week, all EPCs were
washed three times with DMEM, trypsinized, counted and then
resuspended. Cells from each group (18 wells/group) were initially
seeded in un-coated 96-well plates and cultured in 37°C and 5%
CO2. Following incubation for a further 24 h, cells from
each group were added to 500 μl MTT (Beyotime Institue of
Biotechnology, Haimen, China) and assessed using a standard MTT
assay for 4 h. Supernatant fluid was then discarded and 150 μl
dimethylsulfoxide (DMSO) was added to each well for 10 min with
agitation. The light absorption of cells was measured using an
ELISA reader (iMark; Bio-Rad Laboratories, Inc., Hercules, CA,
USA). Finally a MTT bar graph was drawn.
Electron microscopy
In order to detect the iron concentration within
EPCs and observe the morphology of EPCs in culture, SPIO-labeled
EPCs were grown in a six-well tissue culture plate until they
reached 80% confluence. Following incubation, the medium was
removed and the plate was gently washed twice with sterile PBS.
Samples were then digested using trypsin, transferred into a tube
and centrifuged at 1,500 × g for 15 min. The supernatant was
removed and cells were fixed with 4% paraformaldehyde and
visualized using an electron microscope (IX83; Olympus Corp.,
Tokyo, Japan).
Magnetic resonance imaging of
SPIO-labeled EPCs in vitro
Different concentrations of SPIO-labeled EPC
solution (SPIO, 35 μg/ml) were collected by removing the free SPIO
and washed three times with PBS. The EPCs were suspended in 1%
agarose prior to being transferred into 1.5-ml microcentrifuge
tubes. Concentrations of SPIO-labeled EPC cells were as follows:
Group a, control; group b, 1.0×104/ml; group c,
5.0×104/ml; group d, 1.0×105/ml; and group e,
5.0×105/ml. In vitro MRI of the tubes was then
conducted.
MRI scans were performed on a clinical 3.0T
whole-body MRI system (MAGNETOMTrioTim; Siemens Healthcare,
Erlangen, Germany) following 24 h culture. T2-weighted fast
spin-echo (T2WITSE) MRI measurements were initially obtained in
axial and sagittal planes. Imaging parameters for these images
were: Repetition time/echo time, 2652110 ms; flip angle, 90°; field
of view, 210 mm; slice thickness, 3 mm; matrix size, 205×256.
Statistical analysis
Statistical analysis was performed using SPSS
(version 13.0; SPSS Inc., Chicago, IL, USA). Values are expressed
as the mean ± standard deviation and analyzed using the one-way
analysis of variance. P<0.05 was considered to indicate a
statistically significant difference between values.
Results
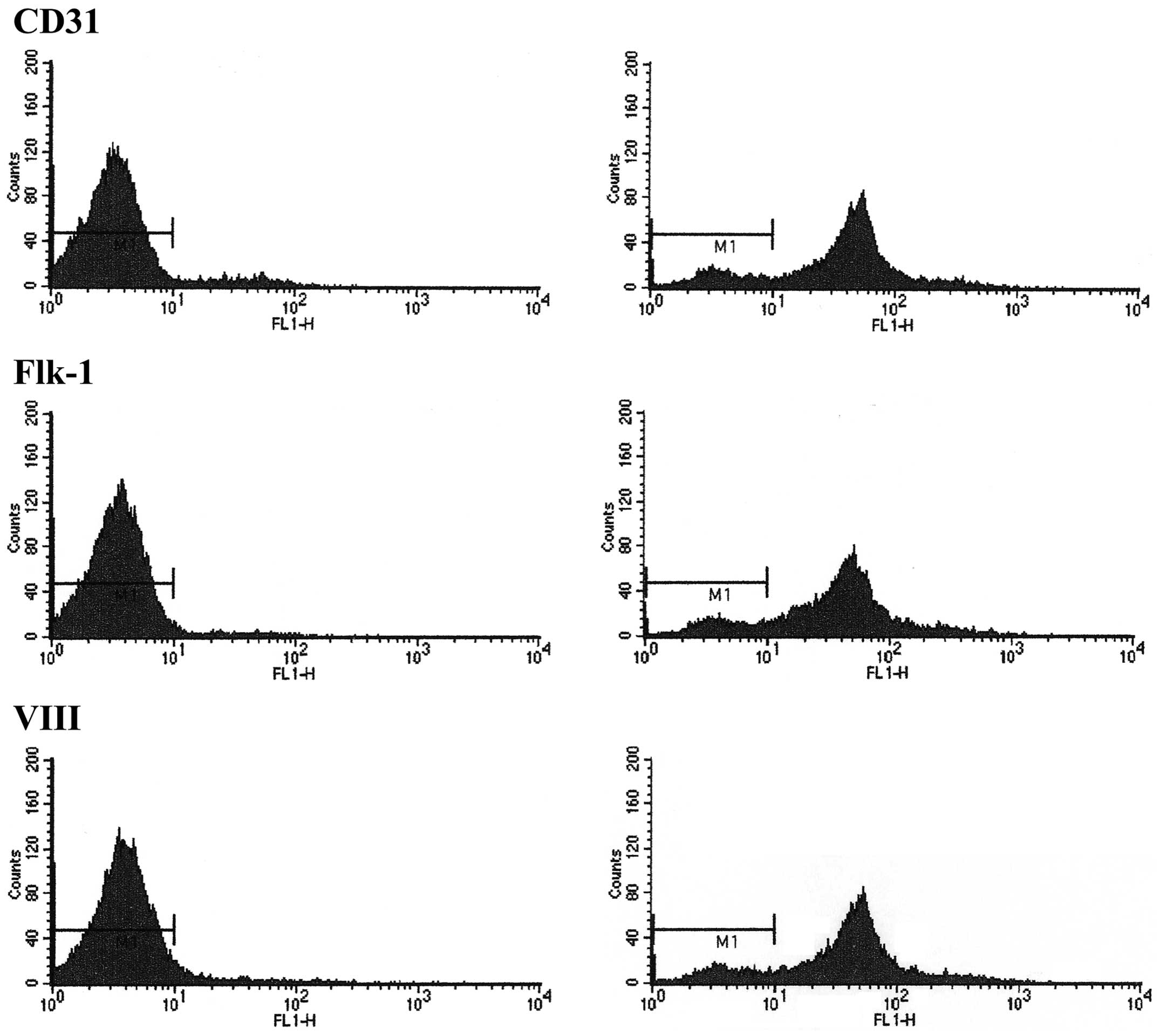
FACS analysis confirms the identity of
EPCs
A large number of molecular markers were reported to
be associated with EPCs (21–23);
in the present study, several of these molecular markers were
detected using flow cytometry in order to identify EPCs. The
results of the FACS analysis revealed EPCs were positive for the
markers CD31, Flk-1 and factor VIII in 83.52, 85.34 and 83.86% of
cells, respectively (Fig. 1). Thus
these cells were identified as EPCs.
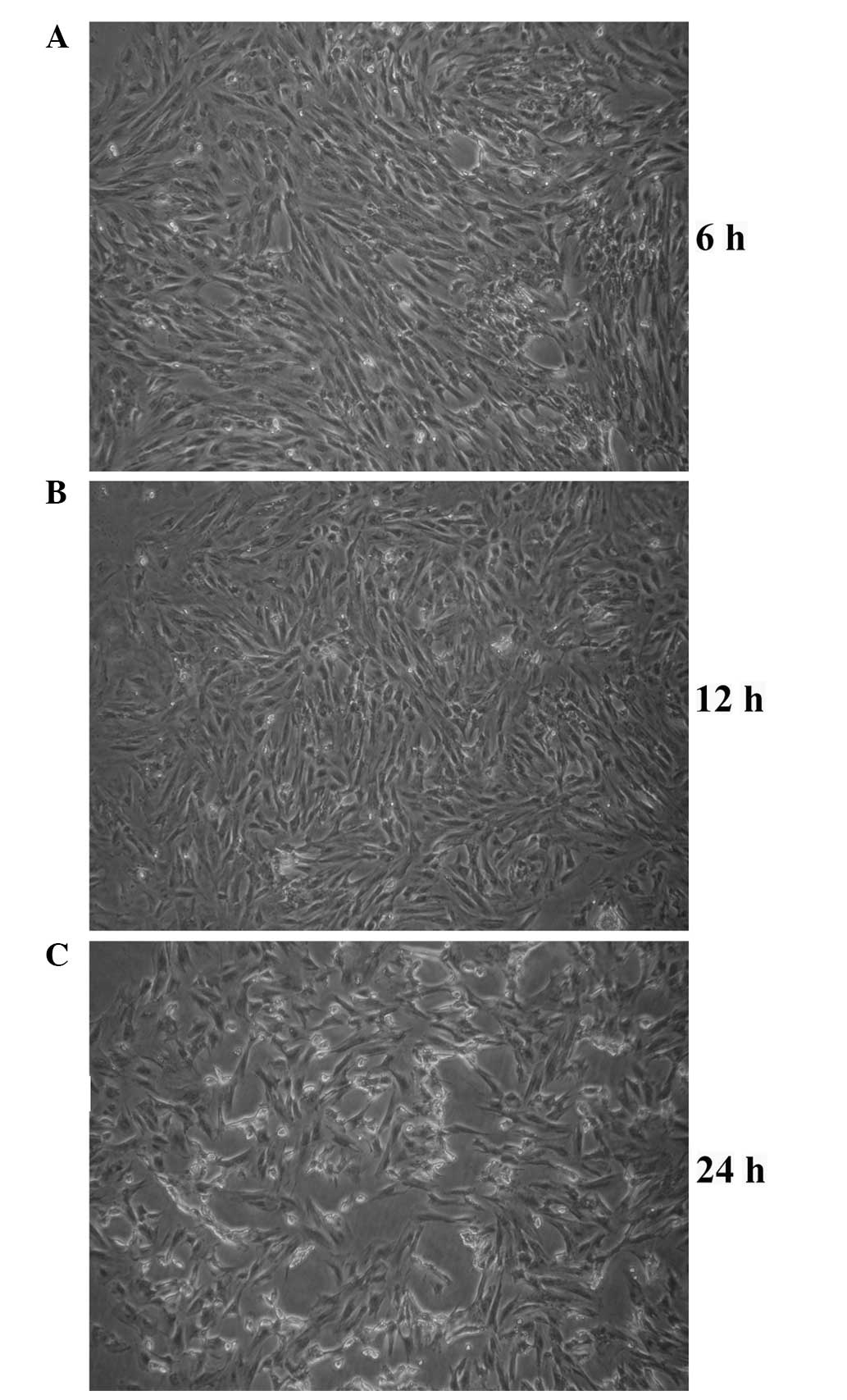
Optical microscopy confirms the labeling
efficiency of SPIO
Endocytosis of SPIO particles was observed in EPCs
using optical microscopy. Following 6 h of incubation of EPCs with
35 μg/ml SPIO, characteristic granular SPIO particles were detected
within the cytoplasm (Fig. 2).
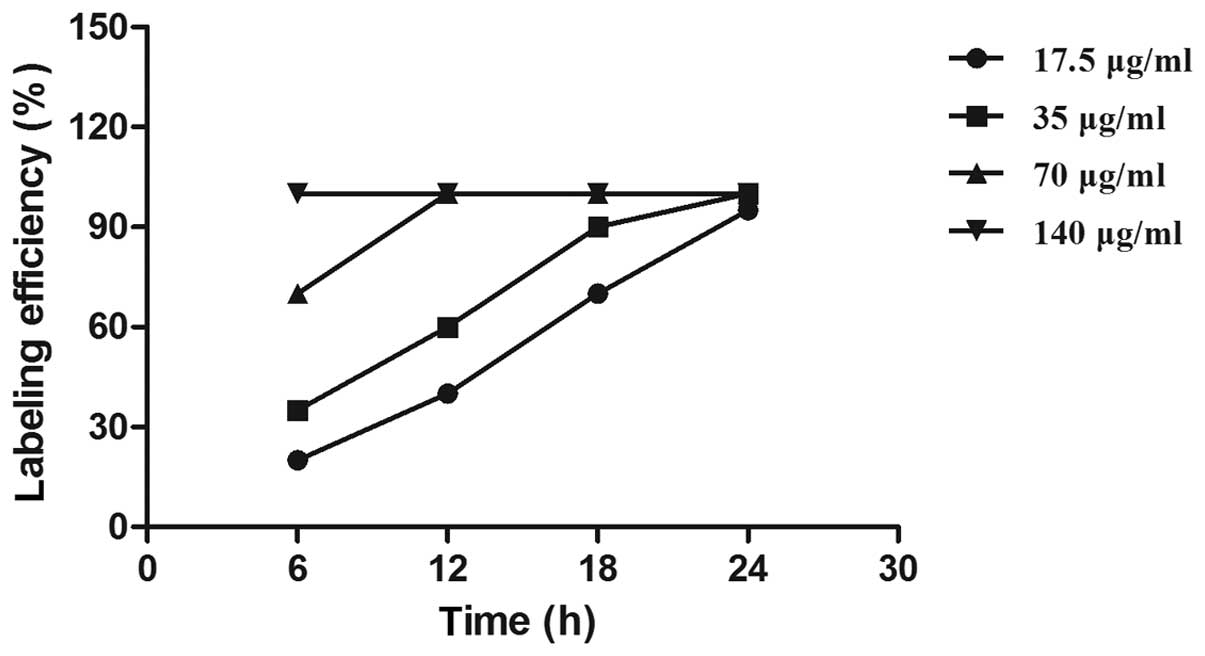
Following 24 h, the SPIO labeling efficiency increased to 100% in
all groups and the endocytotic rate of SPIO particles positively
correlated with the concentration of SPIO. However, no significant
difference was found among the labeling efficiencies of the groups
(Fig. 3). Furthermore,
characteristic intracytoplasmic granular SPIO particles were
observed following 28 days in culture (data not shown).
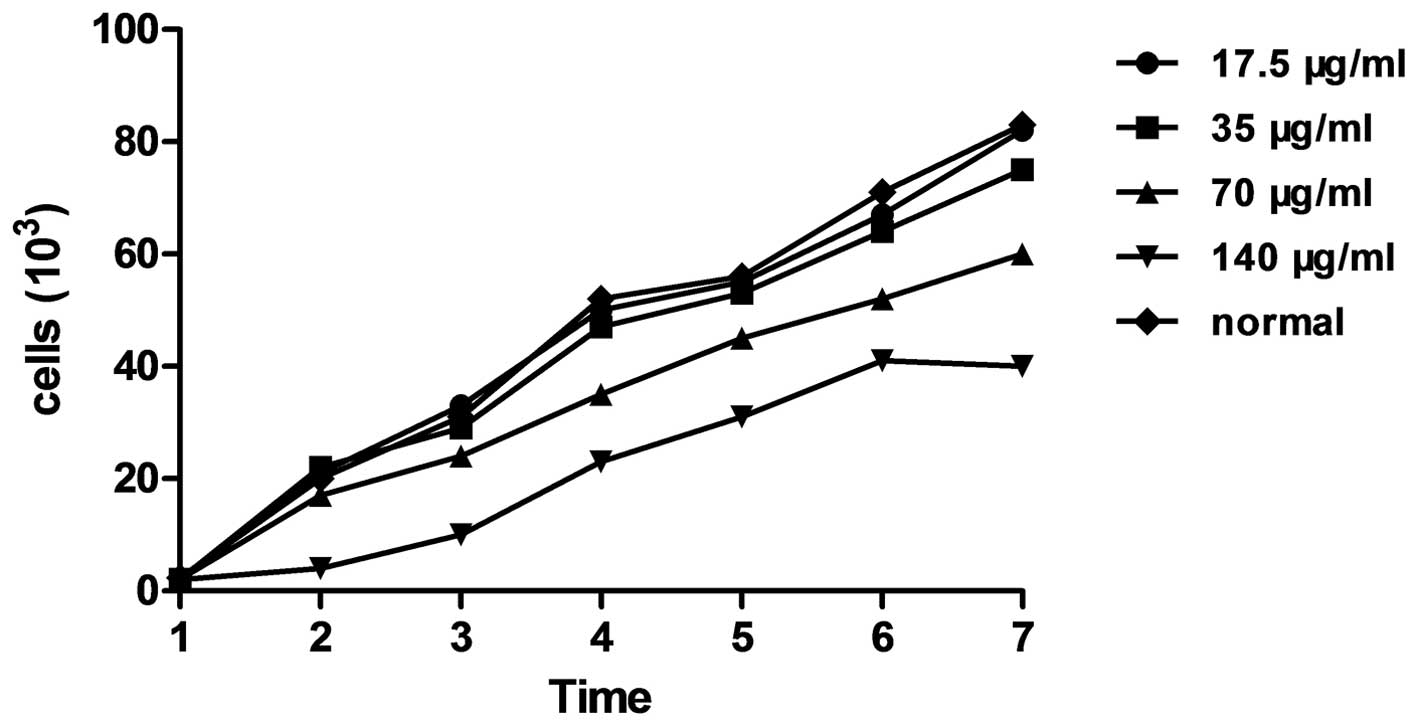
Cell growth and viability of EPCs labeled
with different concentrations of SPIO
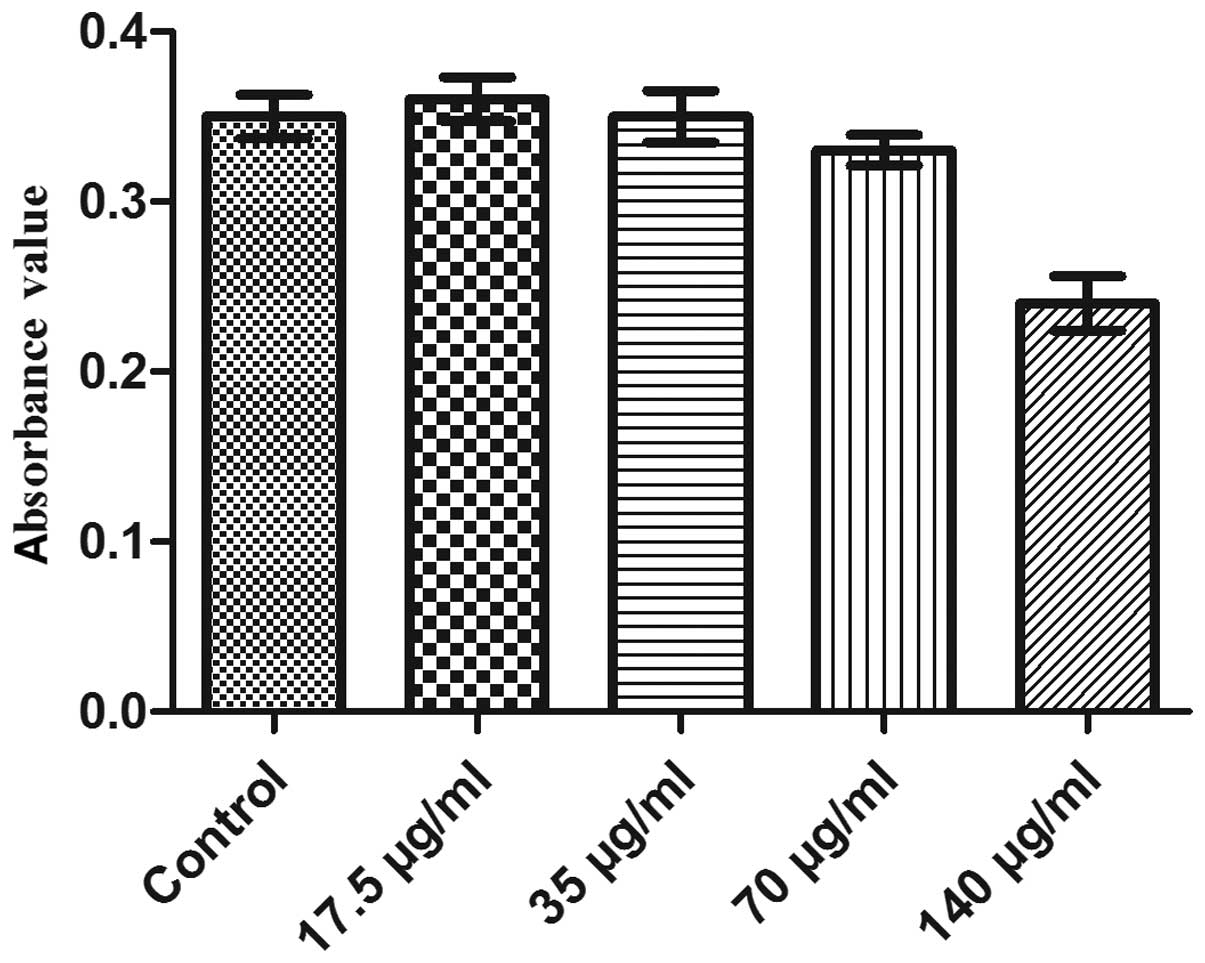
Fig. 4 shows the
growth curve of EPC labeling with different concentration of SPIO
(17.5, 35, 70 and 140 μg/ml, respectively) following one week in
culture. In groups 1, 2 and 3, the trends of cell growth were not
significantly different to those of the control group (P>0.05);
in addition, MTT analysis of cell viability revealed no significant
difference between the absorbance (490 nm) of groups 1, 2 and 3
compared with that of the control (P>0.05) (Fig. 5). However, the cell growth and
viability were markedly suppressed in the fourth group (140 μg/ml)
as well as cell growth for group 3 (70 μl/mg).
Morphological changes of EPCs labeled
with SPIO
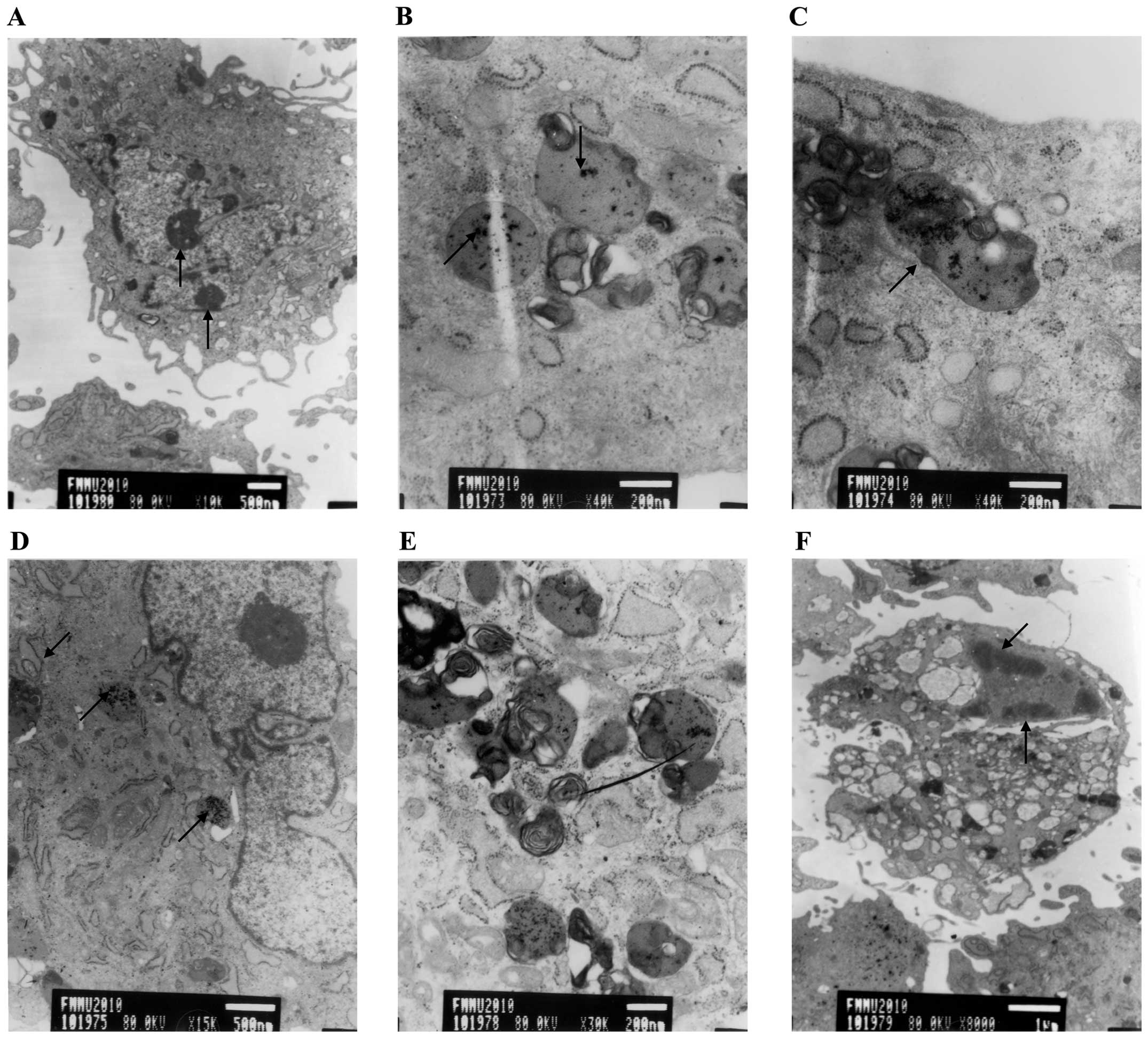
Following one week in culture, electron microscopy
revealed micorvilli on the surface of control cells as well as
numerous intracellular organelles and prominent nuclear euchromatin
with sharp nucleoli (Fig. 6A).
EPCs labeled with SPIO at concentrations of 17.5 μg/ml and 35 μg/ml
revealed identical characteristics, including figure, shape and
nucleolus structure, compared to those of the control group
(Fig. 6B and C). All
concentrations of SPIO-labeled EPCs contained endolysosomal iron
particles, whereas these were not observed in EPCs of the control
group (Fig. 6B–D); in addition,
EPCs labeled with 70 μg/ml SIPO exhibited an increased number of
endolysosomes (Fig. 6E). However,
EPCs labeled with 140 μg/ml SIPO underwent apoptotic cell death;
morphologically, EPCs in this group demonstrated a reduced quantity
of microvilli, a highly concentrated cytoplasm and the nucleus was
located at the edge of the nuclear membrane (Fig. 6F). These results indicated that 35
μg/ml SPIO was a safe concentration for EPC-labeling, without
affecting the biological characteristics of cells.
In vitro MRI
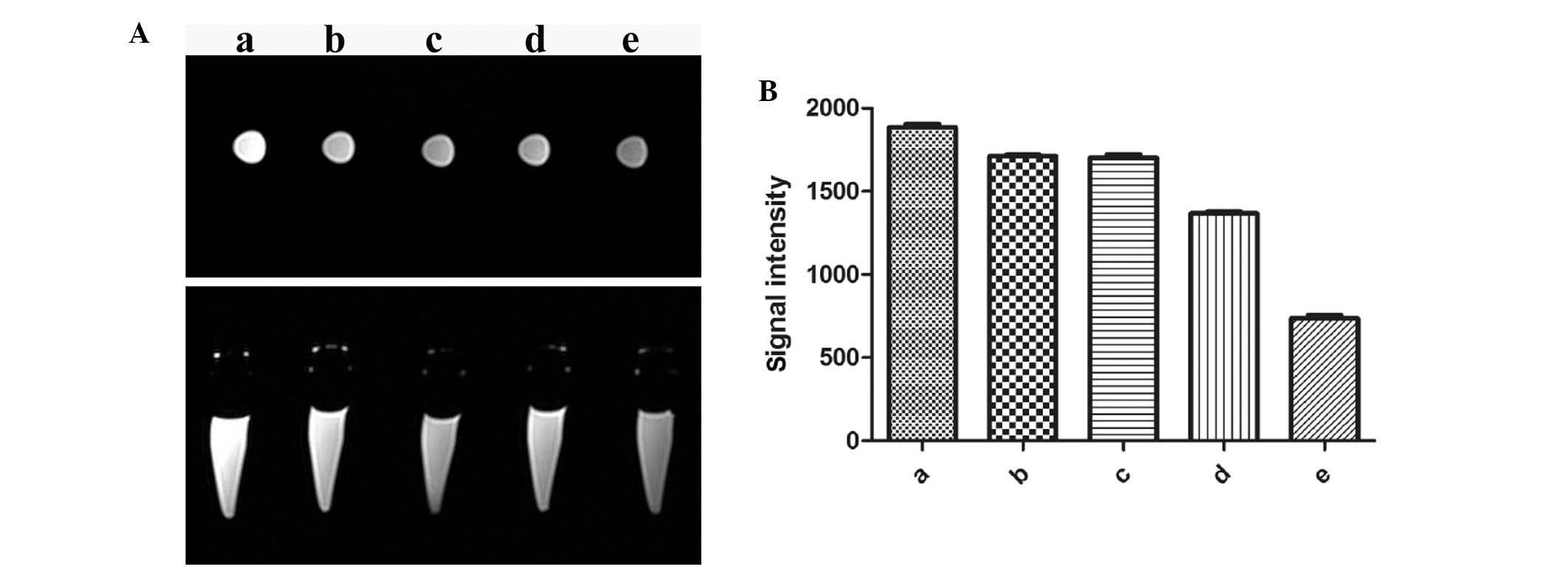
EPCs were labeled with 35 μg/ml SPIO and MRI was
performed on different concentrations of SPIO-labeled cells
(control, 1.0×104, 5.0×104,
1.0×105 and 5.0×105/ml) in vitro
(Fig. 7A). As shown in Fig. 7B, the T2WI signal intensity of
SPIO-labeled EPCs decreased with increasing concentration of EPCs;
in addition, 5×104/ml SPIO-labeled EPCs was the lowest
concentration of cells observed within the imaging parameters.
Furthermore, the T2WI signal intensity significantly decreased at
labeled cell concentrations of 5.0×105/ml and
1.0×105/ml compared with that of the control group
(P<0.05).
Discussion
EPCs are widely considered to be an effective
therapeutic agent for the treatment of certain vascular diseases
(24). Numerous studies have
confirmed that EPCs may be used as an alternative cell-based
approach for the enhancement of angio- and vasculogenic responses
(25). Therefore, it was
hypothesized that autograft EPCs may yield promising improvements
in various animal models of ischemic disease. The MRI technique has
numerous advantages, including a wide variety of imaging sequences,
high resolution and improved soft-tissue contrast without radiation
damage, which suggested its potential use for monitoring
transplanted cells (26). However,
the traditional MRI was not able to differentiate the transplanted
stem cells from the histiocytes; therefore, in order improve the
contrast of the cells using MRI, the transplanted cells required
modification. SPIO has been widely used as a negative marker to
label cells (27,28). In order to accommodate the
requirements of preoperative and intraoperative examinations using
simple SPIO without additional indicators, the superior magnetic
characteristics of SPIO required investigation to ensure its safety
in vivo. In the present study, the reaction time, ratio and
appropriate concentrations of SPIO and SPIO-labeled EPCs were
calculated and analyzed. Previous studies confirmed that the
absorption of iron particles had a positive correlation with cell
number, SPIO concentration and the time of incubation (21). The results of the present study
demonstrated that following 6 h of incubation with SPIO iron
particles were phagocytized in all groups and labeling efficiency
reached 100% in the group labeled with 140 μg/ml SPIO, while lower
concentrations of SPIO reached 100% efficiency following 12, 18–24
and 30–36 h, respectively. This therefore indicated that the number
of iron particles absorbed by EPCS increased with incubation
time.
Arbab et al (29) and Himes et al (30) previously confirmed that the SPIO at
concentrations <50 μg/ml produced no side effects on cell
activity. The results of the present study indicated that cell
growth was unaffected by concentrations of SPIO <70 μg/ml;
however, in EPCs labeled with 70 μg/ml SPIO, lysosome enhancement
was observed and concentrations of SPIO >70 μg/ml suppressed the
biological activity of the cells. These results therefore indicated
that it was safe to label EPCs with SPIO at concentrations of 20–70
μg/ml. In addition, the reaction time of cells was elongated at
concentrations of SPIO <20 μg/ml; therefore, 35 μg/ml SPIO was
selected to label target EPCs for subsequent experiments.
Due to the paramagnetism of SPIO, the T2WI signal
significantly decreased during MRI, which was consistent with the
effects of the negative contrast agent. As the cell number
increased, the contrast effect was enhanced, which was thought to
be due to the increased number of cells available to absorb the
iron particles. In vitro MRI demonstrated that
5×104/ml was the lowest observable concentration of
SPIO-labeled EPCs.
In conclusion, the results of the present study
indicated that MRI was able to reflect changes in concentrations of
intracellular iron and therefore has the potential for use in
studying changes in SPIO-labeled EPCs in vivo for the
treatment of myocardial infarction. However, further studies are
required in order to determine the effect of SPIO-labeling of EPCs
in vivo.
Acknowledgements
The present study was supported by grants from the
National Science Foundation (nos. 81201135 and 30370821).
References
|
1
|
Asahara T, Murohara T, Sullivan A, et al:
Isolation of putative progenitor endothelial cells for
angiogenesis. Science. 275:964–967. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ribatti D: The involvement of endothelial
progenitor cells in tumor angiogenesis. J Cell Mol Med. 8:294–300.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Murasawa S and Asahara T: Endothelial
progenitor cells for vasculogenesis. Physiology (Bethesda).
20:36–42. 2005. View Article : Google Scholar
|
|
4
|
Schmidt-Lucke C, Rössing L, Fichtlscherer
S, et al: Reduced number of circulating endothelial progenitor
cells predicts future cardiovascular events: proof of concept for
the clinical importance of endogenous vascular repair. Circulation.
111:2981–2987. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Losordo DW and Dimmeler S: Therapeutic
angiogenesis and vasculogenesis for ischemic disease: part II:
cell-based therapies. Circulation. 109:2692–2697. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tongers J, Roncalli JG and Losordo DW:
Role of endothelial progenitor cells during ischemia-induced
vasculogenesis and collateral formation. Microvasc Res. 79:200–206.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kawamoto A, Katayama M, Handa N, et al:
Intramuscular transplantation of G-CSF-mobilized CD34(+) cells in
patients with critical limb ischemia: a phase IIIa, multicenter,
single-blinded, dose-escalation clinical trial. Stem Cells.
27:2857–2864. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cho SW, Moon SH, Lee SH, et al:
Improvement of postnatal neovascularization by human embryonic stem
cell derived endothelial-like cell transplantation in a mouse model
of hindlimb ischemia. Circulation. 116:2409–2419. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Losordo DW, Schatz RA, White CJ, et al:
Intramyocardial transplantation of autologous CD34+ stem cells for
intractable angina: a phase IIIa double-blind, randomized
controlled trial. Circulation. 115:3165–3172. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Thal MA, Krishnamurthy P, Mackie AR, et
al: Enhanced angiogenic and cardiomyocyte differentiation capacity
of epigenetically reprogrammed mouse and human endothelial
progenitor cells augments their efficacy for ischemic myocardial
repair. Circ Res. 111:180–190. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Giannoni E, Taddei ML, Parri M, et al:
EphA2-mediated mesenchymal-amoeboid transition induced by
endothelial progenitor cells enhances metastatic spread due to
cancer-associated fibroblasts. J Mol Med (Berl). 91:103–115. 2013.
View Article : Google Scholar
|
|
12
|
Yin M, Liao Z, Yuan X, et al:
Polymorphisms of the vascular endothelial growth factor gene and
severe radiation pneumonitis in non-small cell lung cancer patients
treated with definitive radiotherapy. Cancer Sci. 103:945–950.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu Y, Xia T, Zhang W, et al: Variations
of circulating endothelial progenitor cells and transforming growth
factor-beta-1 (TGF-β1) during thoracic radiotherapy are predictive
for radiation pneumonitis. Radiat Oncol. 8:1892013. View Article : Google Scholar
|
|
14
|
Yang SY, Sun JS, Liu CH, et al: Ex vivo
magnetofection with magnetic nanoparticles: a novel platform for
nonviral tissue engineering. Artif Organs. 32:195–204. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wu CC, Lin LY, Lin LC, et al:
Bio-functionalized magnetic nanoparticles for in vitro labeling and
in vivo locating specific biomolecules. Appl Phys Lett.
92:1425042008. View Article : Google Scholar
|
|
16
|
Oghabian MA, Gharehaghaji N, Amirmohseni
S, et al: Detection sensitivity of lymph nodes of various sizes
using USPIO nanoparticles in magnetic resonance imaging.
Nanomedicine. 6:496–499. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Müller S: Magnetic fluid hyperthermia
therapy for malignant brain tumors - an ethical discussion.
Nanomedicine. 5:387–393. 2009. View Article : Google Scholar
|
|
18
|
Gazeau F and Wilhelm C: Magentic labeling,
imaging and manipulation of endothelial progenitor cells using iron
oxide nanoparticles. Future Med Chem. 2:397–408. 2010. View Article : Google Scholar
|
|
19
|
Cheng K, Wei MQ, Jia GL, et al: Effects of
metoprolol and small intestine RNA on marrow-derived endothelial
progenitor cells applied for autograft transplantation in heart
disease. Eur Rev Med Pharmacol Sci. 18:1666–1673. 2014.PubMed/NCBI
|
|
20
|
Cho SW, Moon SH, Lee SH, et al:
Improvement of postnatal neovascularization by human embryonic stem
cell derived endothelial like cell transplantation in a mouse model
of hind limb ischemia. Circulation. 116:2409–2419. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gill M, Dias S, Hattori K, et al: Vascular
trauma induces rapid but transient mobilization of
VEGFR2(+)AC133(+) endothelial precursor cells. Circ Res.
88:167–174. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gehling UM, Ergün S, Schumacher U, et al:
In vitro differentiation of endothelial cells from AC133-positive
progenitor cells. Blood. 95:3106–3112. 2000.PubMed/NCBI
|
|
23
|
Peichev M, Naiyer AJ, Pereira D, et al:
Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells
identifies a population of functional endothelial precursors.
Blood. 95:952–958. 2002.
|
|
24
|
Moon SH, Kim SM, Park SJ, et al:
Development of a xeno-free autologous culture system for
endothelial progenitor cells derived from human umbilical cord
blood. PLoS One. 8:e752242013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rosell A, Morancho A, Navarro-Sobrino M,
et al: Factors secreted by endothelial progenitor cells enhance
neurorepair responses after cerebral ischemia in mice. PLoS One.
8:e732442013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sun JH, Zhang YL, Nie CH, et al: In vitro
labeling of endothelial progenitor cells isolated from peripheral
blood with superparamagnetic iron oxide nanoparticles. Mol Med Rep.
6:282–286. 2012.PubMed/NCBI
|
|
27
|
Zhang B, Li Q, Yin P, et al:
Ultrasound-triggered BSA/SPION hybrid nanoclusters for
liver-specific magnetic resonance imaging. ACS Appl Mater
Interfaces. 4:6479–6486. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yoo MK, Park IK, Lim HT, et al:
Folate-PEG-superparamagnetic iron oxide nanoparticles for lung
cancer imaging. Acta Biomater. 8:3005–3013. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Arbab AS, Bashaw LA, Miller BR, et al:
Intracytoplasmic tagging of cells with ferumoxides and transfection
agent for cellular magnetic resonance imaging after cell
transplantation: methods and techniques. Transplantation.
76:1123–1130. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Himes N, Min JY, Lee R, et al: In vivo MRI
of embryonic stem cells in a mouse model of myocardial infarction.
Magn Reson Med. 52:1214–1219. 2004. View Article : Google Scholar : PubMed/NCBI
|