Introduction
Cyclin-A2 has an important role in the regulation of
the cell cycle due to its two-point control. Cyclin-A2 interacts
with CDK1 to control the G1/S transition and also
interacts with CDK1 and CDK2 to control the G2/M phase
(1,2). Cyclin-A2 has therefore previously
been recognized as a key regulator of the cell cycle.
Downregulation of cyclin A following birth has demonstrated time
concordance with terminal cardiomyocyte cell-cycle exit in mammals
(3). A recent study by Li et
al (4) indicated that
cyclin-A2 expression levels were increased following acute
myocardial infarction (AMI), suggesting that cyclin-A2 may be
involved in myocardial self repair. The study additionally reported
that newborn cardiomyocytes were immature and that certain
ventricular cardiomyocytes in rodents were multinucleated. Since
this self repair following AMI was unable to prevent cardiac
remodeling and the expression levels of cyclin-A2 were low
(4), the present study
hypothesized that overexpression of cyclin-A2 may promote the
re-initiation of the cardiomyocyte cell cycle and cell division.
However, overexpression of cyclin-A2 may result in excessive
multiplication and potentially contribute to the development of
cancer in other organs (1).
Adeno-associated virus (AAV) has previously been
used as a gene therapy vector due to its low immunogenicity and
sustained transgene expression (5). The inflammatory response caused by
AAV is almost identical to that caused by saline or plasmids
(5,6). This feature makes AAV superior to
other vectors, including adenovirus, herpes virus and lentivirus.
For this reason, AAV vectors are able to provide safe, long-term
gene transfer into several organs, including the lung (7), liver (8), brain (9), retina (10) and heart in animal models (11). Recently, AAV vectors exhibiting
cardiac tropism facilitated cardiac transgene expression following
intravenous injection (11). AAV
serotype 9 (AAV9) has been proven to be a useful vector for gene
therapy in cardiovascular disease via its specific transfection in
the myocardium. Intravenous delivery or intrapericardial injection
of AAV9-enhanced green fluorescence protein was demonstrated to
produce higher gene expression levels in the myocardium than that
of AAV5 and AAV6 (12–16). The recombinant AAV9 was therefore
selected as a vector for cyclin-A2, driven by cytomegalovirus
(CMV), for its superior cardiac tropism. The gene was transfected
via tail vein injection and its efficiency was evaluated. The
safety of the procedure was evaluated by detecting expression
levels of cyclin-A2 and proliferation-associated proteins in the
heart, lung, kidney and liver.
Materials and methods
Experimental animals
Male C57BL/6J mice (21–23 g), aged 10–12 weeks, were
purchased from the animal center of Xinjiang Medical University
(Urumqi, China). The mice were maintained at a temperature of
21–25°C, in a light/dark cycle. Common feed was provided ad
libitum. The present study was conducted in accordance with the
recommendations of the Guide for the Care and Use of Laboratory
Animals of the National Institutes of Health (Bethesda, MD, USA).
The animal use protocol was reviewed and approved by the
Institutional Animal Care and Use Committee of Xinjiang Medical
University (Urumqui, China).
AAV9 recombinant (rAAV9)
The AAV9 vector (rAAV9-cyclinA2-CMV) was constructed
by Virovek, Inc. (Hayward, CA, USA). The primers used were as
follows: Forward, 5′-ATATGAATTCCACCAT GCCGGGCACCT CGAGGCA-3′ and
reverse, 5′-GGCCGTCGACTCACACA CTTAGTGTCTCTG-3′. The PCR reaction
conditions were as follows: Initial denaturation at 95°C for 5 min,
35 cycles of denaturation at 95°C for 30 sec, annealing at 56°C for
30 sec, extension at 72°C for 40 sec, and extension at 72°C for 5
min. Following amplification by polymerase chain reaction, a final
concentration of 2.39×1013 genome copies (GC)/ml
recombinant was obtained.
Experimental groups
Sixty C57BL/6J mice were randomly divided into
control and experimental groups (n=30 per group). The experimental
group were injected with 2×1010 GC rAAV9-cyclinA2-CMV
recombinant in 200 μl saline into the tail vein, while the control
group were injected with the equivalent volume of saline.
Observations were made at two and four weeks following
injection.
Tissue samples
Two weeks following injection (Tg-2w), the heart,
liver, lung and kidney were harvested following sacrification of
the animals by diastolic arrest induced by 0.2 mol/l KCl (control
group, n=7; experimental group, n=8). Remnant blood and fat tissue
were removed from the myocardium and divided into two sections. One
of these sections was immediately submerged in liquid nitrogen and
the remaining section was fixed in paraformaldehyde or Bouin’s
fluid (HT10132; Sigma-Aldrich, St. Louis, MO, USA). Samples were
stored at −80°C until used. The liver, kidney and lungs were also
stored. The procedure was repeated with the remaining mice at four
weeks following injection (Tg-4w) (control group, n=5; experimental
group, n=7).
Western blot analysis
Tissue in
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid buffer with 0.5
mg/ml leupeptin, 10 μg/ml aprotinin and 1 mM phenylmethanesulfonyl
fluoride was homogenized in a grinder according to the
bicinchoninic acid assay method (BCA Protein Assay kit; cat no.
23225; Pierce Biotechnology, Inc., Rockford, IL, USA). Firstly,
diluted albumin (BSA) standards were prepared. Briefly, working
reagents were prepared by mixing 50 parts of BCA™ Reagent A with 1
part of BCA™ Reagent B (50:1, Reagent A:B). A total of 0.1 ml of
each standard and samples were added to appropriately labeled test
tubes, and 2 ml working reagent was added to each at 37°C.
Subsequently, the absorbance of the samples was measured at 562 nm
(SKanit for Multiskan GO 3.2; Thermo Fisher Scientific Inc.,
Rockford, IL, USA), in order to produce a standard curve. A
standard curve was prepared by plotting the average blank-corrected
562 nm measurement for each BSA standard versus its concentration
in μg/ml. Secondly, a microplate procedure was performed to
determine the protein concentration. Briefly, 25 μl of each
standard or unknown sample was pipetted into a microplate well
(working range = 20–2,000 μg/ml). A total of 200 μl of the working
reagent was added to each well and the plate was mixed thoroughly
on a plate shaker for 30 sec. The plate was then covered and
incubated at 37°C for 30 min. Subsequently, the plate was cooled to
room temperature. The absorbance was measured at or near 562 nm on
the microplate reader. The average 562 nm absorbance measurement of
the blank standard replicates was subtracted from the 562 nm
measurements of all other individual standard and unknown sample
replicates. For electrophoresis, 50 μg total protein from
homogenized total tissue was purified by 12% SDS-PAGE (Invitrogen
Life Technologies, Carlsbad, CA, USA) and subsequently blotted onto
a 0.45 μm polyvinylidene fluoride membrane. Mouse monoclonal
immunoglobulin G1 (IgG1) anti-human cyclin-A2
(Santa Cruz Biotechnology, Inc., Dallas, TX, USA; 1:500; sc53227;
54 KDa), mouse monoclonal IgG2a anti-human proliferating cell
nuclear antigen (PCNA; Cell Signaling Technology, Inc., Danvers,
MA, USA; 1:1,000; 2586; 36 KDa) and rabbit polyclonal IgG
anti-human phospho-histone H3 (H3P; Abcam, Cambridge, UK; 1:1,000;
ab115152; 17 KDa) were used as the primary antibodies. Rabbit
anti-human GAPDH (Cell Signaling Technology, Inc.; 1:1,000; 36 KDa)
was used as reference. Following reaction with the primary
antibodies at 4°C overnight, the membrane was washed with PBST and
then incubated with secondary anti-rabbit (WP2007; Invitrogen Life
Technologies) and anti-mouse IgG antibodies (WP2006; Invitrogen
Life Technologies) for 2 h at room temperature. Then, the membranes
were washed three times for 5 min and visualized on a gel imager
(Gel Doc XR+; BioRad, CA, USA) to determine the optical density
ratio with GAPDH.
Immunohistochemistry
Tissues were sectioned into 5-μm slices following
Bouin’s fixation. Paraffin-embedded samples were deparaffinized in
xylol for 20 min followed by a descending series of ethanol (100,
95 and 70%) and distilled water. Subsequently, the sections were
exposed to 3% H2O2 for 20 min to block
unspecific antigens, prior to being washed three times in
phosphate-buffered saline (PBS) and incubated with sodium citrate
for antigen retrieval at 92–98°C for 10 min. Sections were washed
three times in PBS following recovery at room temperature and goat
serum was used to block unspecific antigens, following which the
primary antibodies were added to the sections at the appropriate
dilutions (cyclin-A2, 1:500; PCNA, 1:1,000; H3P, 1:1,000).
Immunofluorescence
Immunofluorescence was used to detect the expression
of cyclin-A2 in cardiomyocytes. Samples stored at −80°C were
gradually warmed to room temperature. Subsequently, the sections
were washed three times in PBS for five minutes. Goat serum was
added to block the unspecific antigen. The primary antibodies for
cyclin-A2, PCNA and H3P diluted in 5% bovine serum albumin were
incubated with the tissue overnight at 4°C. Corresponding secondary
antibodies (goat anti-rabbit IgG and donkey anti-Mouse IgG CF™ 594;
Thermo Fisher Scientific Inc.) were incubated at 37°C for one hour
and subsequently the nuclei were stained with DAPI for seven
minutes at room temperature. Following three washes in PBS, images
were captured of the stained sections using a Leica Photomicrograph
(Leica Microsystems GmbH, Wetzlar, Germany).
Statistical analysis
Values are expressed as the mean ± standard
deviation. Statistical significance between two groups was examined
by Student’s t-test and multigroup comparisons were made using
one-way analysis of variance. Data were analyzed using SPSS 16.0
(SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to
indicate a statistically significant difference between values.
Results
Expression and location of cyclin-A2 in
the myocardium
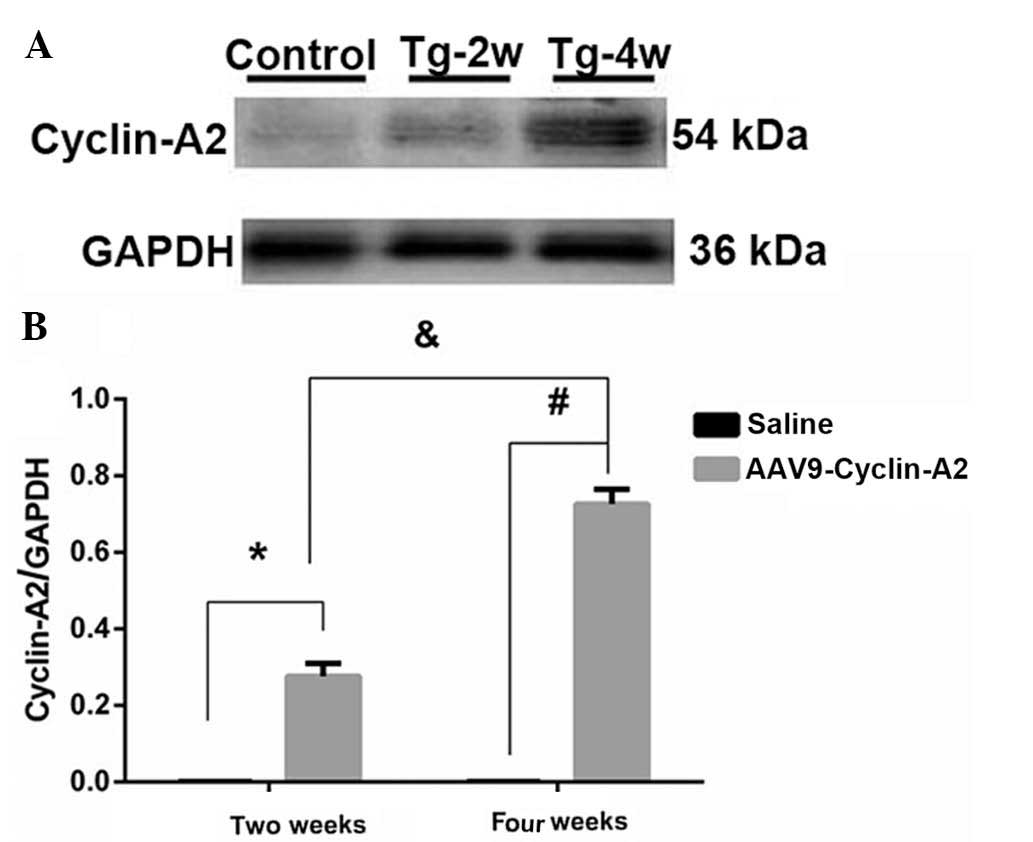
Western blot analysis indicated that expression of
cyclin-A2 commenced two weeks (27.1±3.33%) following injection and
expression levels had increased four weeks following injection
(74.4±3.36%) in comparison to those at two weeks. Significantly
lower expression levels were observed in the control group
(P<0.0001; 0.146±0.013 and 0.142±0.107%, respectively; Fig. 1A and B; Table I). No significant difference was
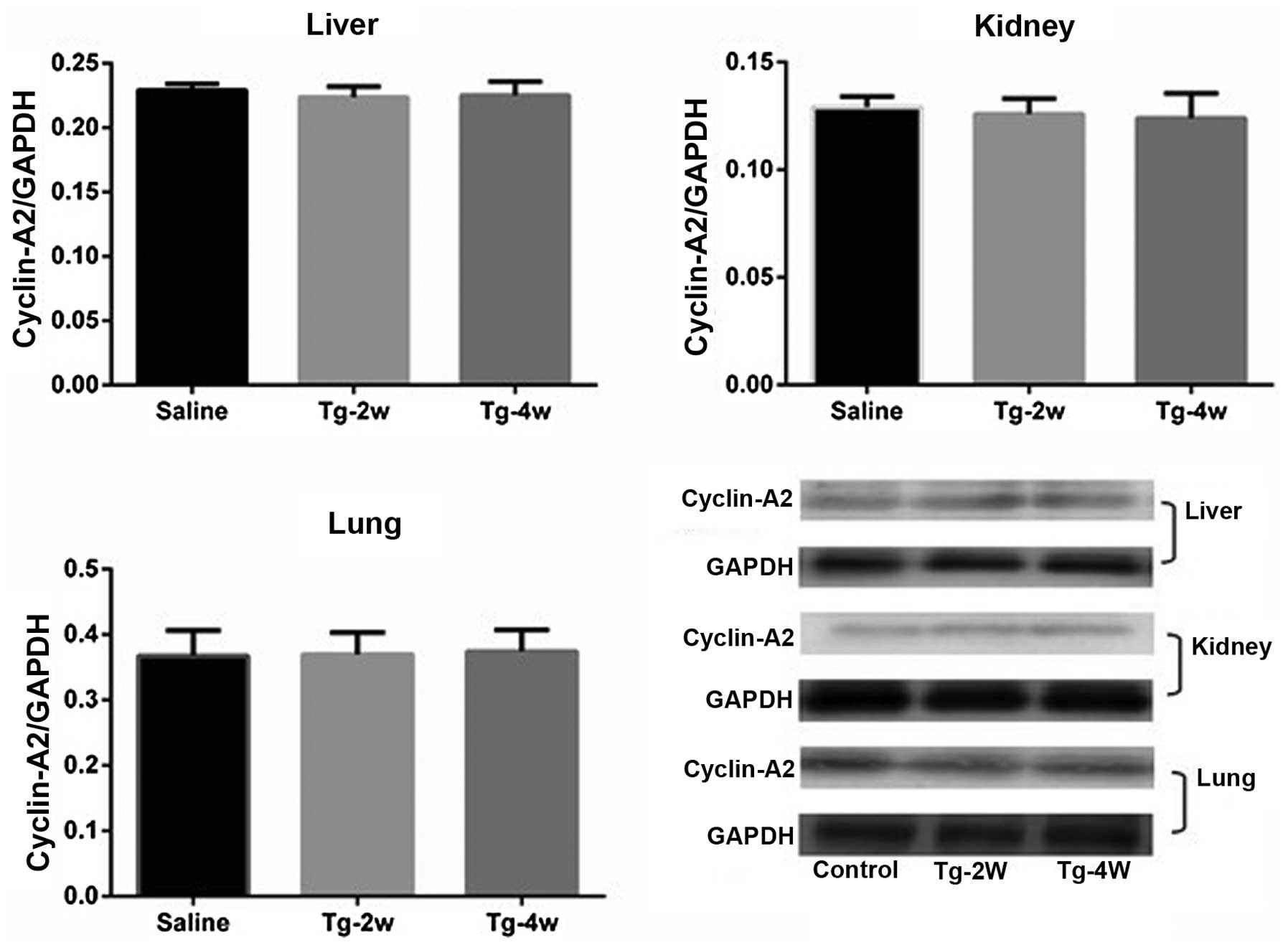
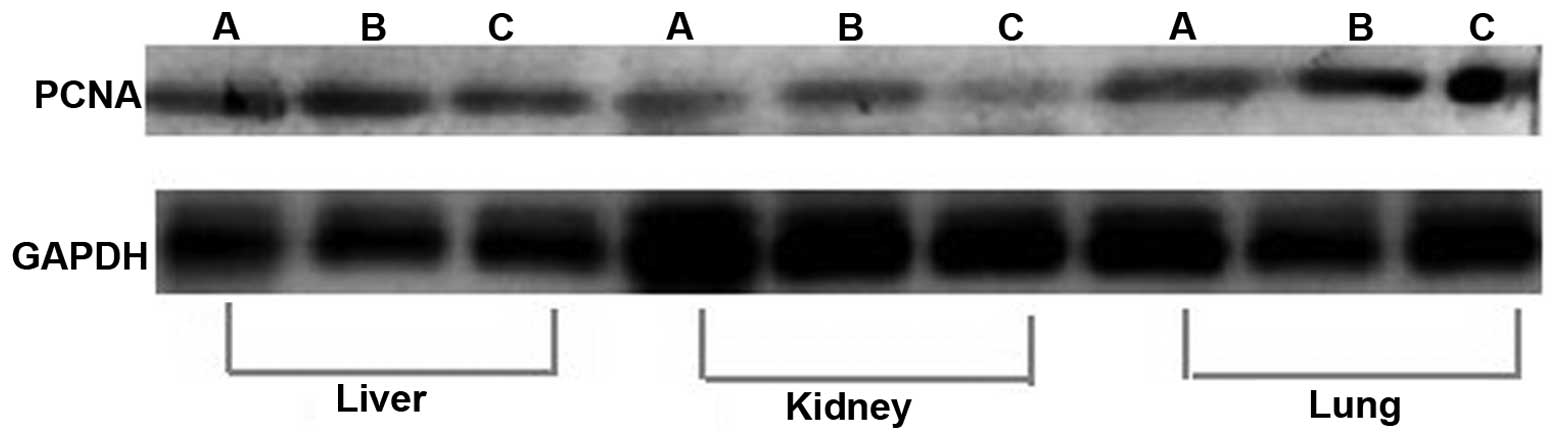
observed in expression levels between the two groups in the liver,
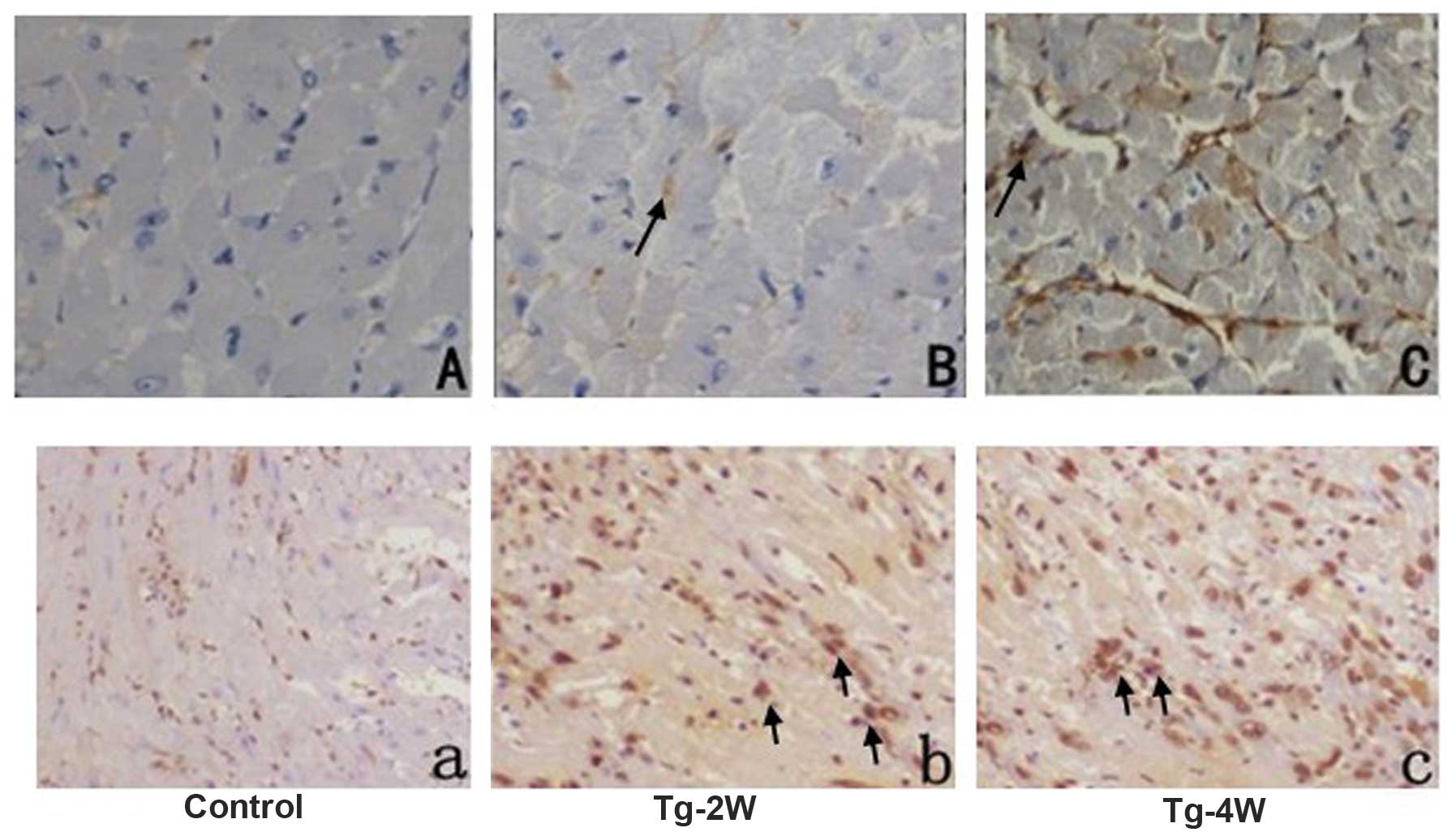
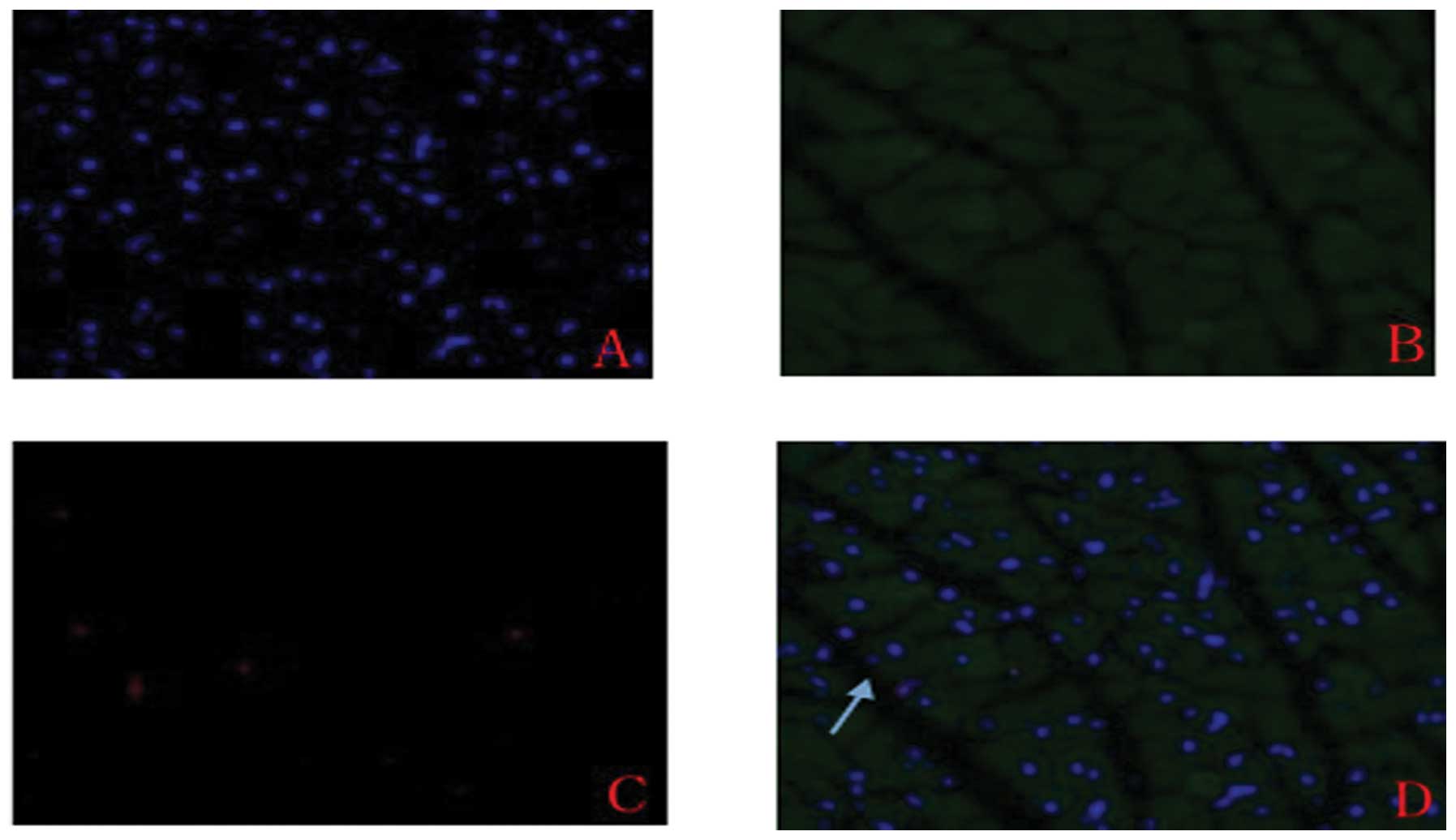
kidney or lung (Fig. 2, Table II). The localization of cyclin-A2
expression was detected by immunohistochemical and
immunofluorescent analysis, which indicated cytoplasmic, but not
nucleic, expression (Figs. 3 and
4).
 | Table IExpression levels of cyclin-A2, PCNA
and H3P in the myocardium. |
Table I
Expression levels of cyclin-A2, PCNA
and H3P in the myocardium.
| Tg-2w | Tg-4w |
|---|
|
|
|
|---|
| Saline (n=5) | AAV9-cyclin-A2
(n=7) | Saline (n=5) | AAV9-cyclin-A2
(n=7) |
|---|
| Cyclin-A2/GAPDH | 0.146±0.013 | 27.1±3.33 | 0.142±0.107 | 74.4±3.36 |
| PCNA/GAPDH | 13.1±0.54 | 65.8±3.44 | 13.2±0.55 | 71.2±1.58 |
| H3P/GAPDH | 11.6±0.63 | 11.6±0.78 | 11.7±0.82 | 11.6±0.78 |
 | Table IIExpression of cyclin-A2 in liver,
kidney and lung. |
Table II
Expression of cyclin-A2 in liver,
kidney and lung.
| Tg-2w | Tg-4w |
|---|
|
|
|
|---|
| Saline (n=5) | AAV9-cyclin-A2
(n=7) | Saline (n=5) | AAV9-cyclin-A2
(n=7) |
|---|
| Liver | 0.229±0.005 | 0.217±0.028 | 0.231±0.031 | 0.220±0.025 |
| Kidney | 0.129±0.005 | 0.125±0.008 | 0.127±0.003 | 0.124±0.01 |
| Lung | 11.6±0.63 | 11.6±0.78 | 11.7±0.82 | 11.6±0.78 |
Influence of transfection on
cardiomyocyte regeneration
Expression levels of PCNA were significantly higher
in the cyclin-A2-treated group compared with those of the control
group (two weeks: 13.1±0.54 vs. 65.8±3.44, P<0.001; four weeks:
13.2±0.55 vs. 71.2±1.58; P<0.0001; Fig. 5A–C, Table I). In the cyclin-A2-treated group,
no significant difference in cyclin-A2 expression was observed
between two and four weeks following transfection, which revealed
stable expression of cyclin-A2. This may indicate that persistent
expression of cyclin-A2 promoted cell cycle progression.
Immunohistochemical analysis indicated higher expression levels of
PCNA in the Tg-2w and Tg-4w groups compared with those of the
control group. However, no significant difference was identified in
the expression levels of mitosis-specific protein, H3P, between the
cyclin-A2-treated and control groups (two weeks: 11.6±0.63 vs.
11.6±0.78%, P>0.05; four weeks: 11.7±0.82 vs. 11.6±0.78%,
P>0.05; Fig. 5, Table I).
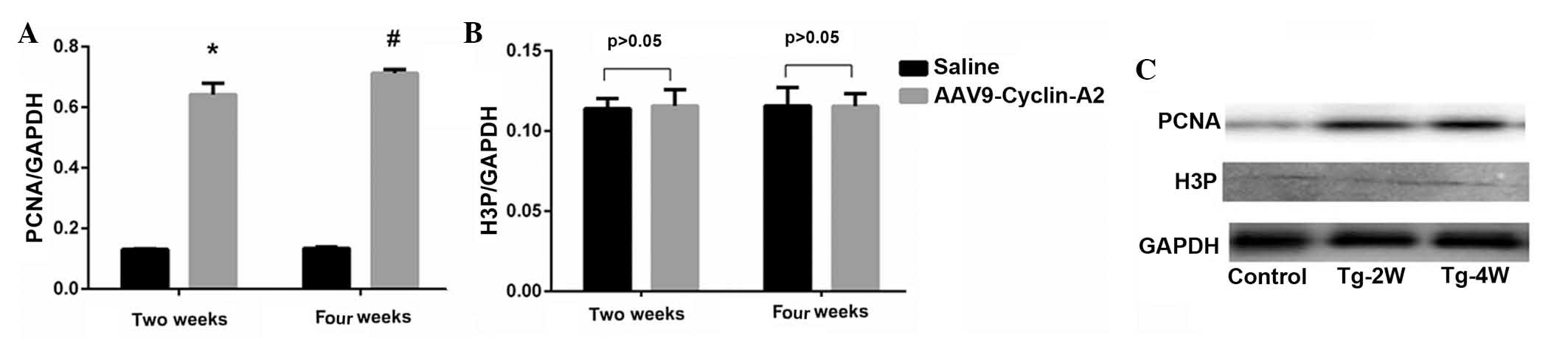 | Figure 5Expression levels of PCNA and H3P
detected by western blot analysis. (A) PCNA expression levels
displayed a significant difference between the control and
experimental groups, two and four weeks following injection.
*P<0.0001, #P<0.0001, AAV9-cyclin-A2
vs. saline group. (B) There was no significant difference in H3P
expression levels between the two groups, P>0.05. (control
group, n=5; experimental group, n=7). Values are expressed as the
mean ± standard deviation. (C) Representative western blot of
control group and experimental group at Tg-2w and Tg-4w. Size of
proteins: PCNA, 36KD; H3P, 17 KDa; GAPDH, 36 KDa. Tg-2w, two weeks
following injection; Tg-4w, four weeks following injection. PCNA,
proliferating cell nuclear antigen; H3P, phospho-histone H3; AAV9,
adeno-associated virus serotype 9. |
Evaluation of safety
Expression of PCNA in the liver, kidney and lung was
used to evaluate the safety of cyclin-A2 gene transfer. Western
blot analysis indicated no statistically significant difference in
PCNA expression levels in the liver, kidney or lung in the
cyclin-A2-treated group compared to those of the control group,
which confirmed the safety of gene transfer (Fig. 6).
Discussion
The present study aimed to evaluate the effect of
cyclin-A2 transfection into the myocardium via rAAV9. The
associated safety issues were also assessed. Studies previously
indicated that delivery of cyclin-A2 via adenovirus or transgenesis
restarted the myocardial cell cycle and enhanced cardiac
regeneration following myocardial infarction (17,18).
Another study demonstrated that cyclin-A2 expression levels peaked
at two weeks following transfection and subsequently gradually
decreased so that by the fourth week, expression levels had almost
disappeared. This confirmed that gene transfer of cyclin-A2 via
adenovirus did not result in long term expression (17). A further study revealed long term
expression of cyclin-A2 in myocardial regeneration following
myocardial infarction (18).
However, these studies did not investigate the safety of cyclin-A2
gene transfer or whether the adenovirus vector may provide
unsustained cyclin-A2 expression. A recent study discovered
re-expression of cyclin-A2 following AMI, suggesting that cyclin-A2
may participate in myocardial self repair (4). Meanwhile, of cyclin-A2 also peaked at
two weeks following AMI and levels were significantly decreased by
four weeks, which was almost consistent with the observed effects
of cyclin-A2 gene transfer (17).
Therefore, to investigate the number of ways in which the exogenous
cyclin-A2 gene performed, an rAAV9-cyclinA2-CMV complex was
constructed and injected into the myocardium of normal C57BL/6J
mice via the tail vein, in order to observe the expression levels
and effects on the myocardial cell cycle following gene
transfer.
Cyclin-A2 expression was observed two weeks
following gene transfection and persisted for at least four weeks,
whereas no expression was observed in the control group. The
expression likely began at two weeks following transfection, as the
single-stranded DNA of the AAV must be converted into
double-stranded DNA prior to transcription (14). This process occurs rapidly in
actively dividing cells, due to the presence of DNA polymerases
(15). However, in post-mitotic
cells and in particular, cardiomyocytes, the process is
significantly delayed. The results of the present study are
consistent with Svensson et al’s (19) study on mice, which indicated that
expression of cyclin-A2 may be sustained for a minimum of one month
following rAAV transfection. rAAV is superior to the adenovirus in
terms of expression duration, which was confirmed by the detection
of lasting cyclin-A2 expression via rAAV9 transfer. It has been
confirmed that an intermediate dose of AAV9 (2.5×1010
GC) provides high-level gene transfer to the heart, whereas
transfer is less via alternative AAV serotypes (16). Furthermore, at an intermediate
dose, AAV9 expression is limited almost exclusively to the heart,
with only a small number of positive cells detectable in the liver
(12). The results of the present
study demonstrated that expression levels of cyclin-A2 in the
liver, lung and kidney showed no significant difference at two or
four weeks following transfection compared with those of the
control group. This therefore confirmed that AAV9 was the most
suitable cardiotropic AAV serotype for gene transfer to the
myocardium.
Following gene transfer, proliferation-associated
proteins were also detected to evaluate the safety and efficiency
of gene transfer. Higher expression of PCNA, a typical indicator of
DNA synthesis, was observed in the cyclin-A2-treated group compared
with that in the control group following gene transfer. This
demonstrated that transfection with cyclin-A2 restarted the
myocardial cell cycle and promoted DNA synthesis. Furthermore, no
significant difference was observed in expression levels of PCNA
between two and four weeks following transfection, indicating that
exogenous cyclin-A2 was regulated by cell cycle-associated
proteins. However, H3P, a mitosis-specific protein, exhibited no
significant difference between the cyclin-A2-transfected and
control groups; this was attributed to the cytoplasmic localization
of cyclin-A2 following gene transfer. It has been confirmed that
cyclin-A2 is localized predominantly in the nucleus during the S
phase; at the end of G2 phase, it is re-localized to the
centrosomes in the cytoplasm, where it binds to the poles of
mitotic spindles (2). To
facilitate its association with CDK2, cyclin-A2 is shuttled between
the nucleus and cytoplasm and the nucleic localization of cyclin-A2
is required for mitosis (20,21).
However, cyclin-A2 expression via rAAV9 driven by CMV resulted in
cytoplasmic localization, which may explain why no increase in
cardiomyocyte mitosis was detected.
Following gene transfer, cyclin-A2 expression levels
in the liver, lung and kidney demonstrated no significant
difference to those of the control group. This conclusion was
confirmed by evaluating the expression levels of
proliferation-associated proteins, PCNA and H3P, in the liver, lung
and kidney. No significant difference was detected in PCNA or H3P
expression levels between the liver, lung and kidney of the
cyclin-A2-transfected group and those of the control group. This
may reflect cardiac tropism from an alternative perspective but
confirmed the safety of gene transfer.
In the present study, the safety and efficiency of
gene transfer by rAAV9 was only confirmed in normal mice; further
study should evaluate the effect of cyclin-A2 on the infarcted
myocardium. Previous studies indicated that expression of genes
transfected by adenovirus only lasted for four weeks; therefore,
four weeks was selected as the experimental end-point. Further
study should be conducted with an extended observation period,
which may establish the extent of long-term gene expression
following rAAV9 delivery. Further research is also required
regarding myocardial regeneration.
In conclusion, the present study confirmed that the
delivery of cyclin-A2 via an rAAV9 vector restarted the myocardial
cell cycle and resulted in steady and specific cyclin-A2 expression
in the myocardium. This may provide a novel therapeutic route for
myocardial regeneration following cardiac injury.
Abbreviations:
|
PCNA
|
proliferating cell nuclear antigen
|
|
H3P
|
phospho-histone H3
|
|
rAAV9
|
recombinant adeno-associated virus
serotype 9
|
|
CDK
|
cyclin-dependent kinase
|
|
eGFP
|
enhanced green fluorescent protein
|
|
CMV
|
cytomegalovirus
|
References
|
1
|
Yam CH, Fung TK and Poon RY: Cyclin A in
cell cycle control and cancer. Cell Mol Life Sci. 59:1317–1326.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pagano M1, Pepperkok R, Verde F, Ansorge W
and Draetta G: Cyclin A is required at two points in the human cell
cycle. EMBO J. 11:961–971. 1992.PubMed/NCBI
|
|
3
|
Yoshizumi M, Lee WS, Hsieh CM, Tsai JC, Li
J, Perrella MA, Patterson C, Endege WO, Schlegel R and Lee ME:
Disappearance of cyclin A correlates with permanent withdrawal of
cardiomyocytes from the cell cycle in human and rat hearts. J Clin
Invest. 95:2275–2280. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li Y, Hu S, Ma G, Yao Y, Yan G, Chen J, Li
Y and Zhang Z: Acute myocardial infarction induced functional
cardiomyocytes to re-enter cell cycle. Am J Transl Res. 5:327–335.
2013.
|
|
5
|
Mingozzi F and High KA: Therapeutic in
vivo gene transfer for genetic disease using AAV: progress and
challenges. Nat Rev Genet. 12:341–355. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wright MJ, Wightman LM, Lilley C, de Alwis
M, Hart SL, Miller A, Coffin RS, Thrasher A, Latchman DS and Marber
MS: In vivo myocardial gene transfer: optimization, evaluation and
direct comparison of gene transfer vectors. Basic Res Cardiol.
96:227–236. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Flotte TR: Recent developments in
recombinant AAV-mediated gene therapy for lung diseases (Review).
Curr Gene Ther. 5:361–366. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sands MS: AAV-mediated liver-directed gene
therapy. Methods Mol Biol. 807:141–157. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mandel RJ: CERE-110, an adeno-associated
virus-based gene delivery vector expressing human nerve growth
factor for the treatment of Alzheimer’s disease. Curr Opin Mol
Ther. 12:240–247. 2010.PubMed/NCBI
|
|
10
|
Rolling F: Recombinant AAV-mediated gene
transfer to the retina: gene therapy perspectives (Review). Gene
Ther. 11(Suppl 1): S26–S32. 2011. View Article : Google Scholar
|
|
11
|
Pacak CA and Byrne BJ: AAV vectors for
cardiac gene transfer: experimental tools and clinical
opportunities (Review). Mol Ther. 19:1582–1590. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bish LT, Morine K, Sleeper MM, Sanmiguel
J, Wu D, Gao G, Wilson JM and Sweeney HL: Adeno-associated virus
(AAV) serotype 9 provides global cardiac gene transfer superior to
AAV1, AAV6, AAV7, and AAV8 in the mouse and rat. Hum Gene Ther.
19:1359–1368. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bostick B, Ghosh A, Yue Y, Long C and Duan
D: Systemic AAV-9 transduction in mice is influenced by animal age
but not by the route of administration. Gene Ther. 14:1605–1609.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zincarelli C, Soltys S, Rengo G and
Rabinowitz JE: Analysis of AAV serotypes 1–9 mediated gene
expression and tropism in mice after systemic injection. Mol Ther.
16:1073–1080. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fang H, Lai NC, Gao MH, Miyanohara A, Roth
DM, Tang T and Hammond HK: Comparison of adeno-associated virus
serotypes and delivery methods for cardiac gene transfer. Hum Gene
Ther Methods. 23:234–241. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Inagaki K, Fuess S, Storm TA, Gibson GA,
Mctiernan CF, Kay MA and Nakai H: Robust systemic transduction with
AAV9 vectors in mice: Efficient global cardiac gene transfer
superior to that of AAV8. Mol Ther. 14:45–53. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Woo YJ, Panlilio CM, Cheng RK, Liao GP,
Atluri P, Hsu VM, Cohen JE and Chaudhry HW: Therapeutic delivery of
cyclin A2 induces myocardial regeneration and enhances cardiac
function in ischemic heart failure. Circulation. 114(1 Suppl):
I206–I213. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cheng RK, Asai T, Tang H, Dashoush NH,
Kara RJ, Costa KD, Naka Y, Wu EX, Wolgemuth DJ and Chaudhry HW:
Cyclin A2 induces cardiac regeneration after myocardial infarction
and prevents heart failure. Circ Res. 100:1741–1748. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Svensson EC, Marshall DJ, Woodard K, Lin
H, Jiang F, Chu L and Leiden JM: Efficient and stable transduction
of cardiomyocytes after intramyocardial injection or intracoronary
perfusion with recombinat adeno-associated virus vectors.
Circulation. 99:201–205. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang X, Song Y, Ren J and Qu X:
Knocking-down Cyclin A(2) by siRNA suppresses apoptosis and
switches differentiation pathways in K562 cells upon administration
with doxorubicin. PLoS One. 4:e66652009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jackman M, Kubota Y, den Elzen N, Hagting
A and Pines J: Cyclin A- and cyclin E-Cdk complexes shuttle between
the nucleus and the cytoplasm. Mol Biol Cell. 13:1030–1045. 2002.
View Article : Google Scholar : PubMed/NCBI
|