Introduction
Cardiac stem cells (CSCs) may be used as a potential
source for the study of cardiac repair. Previous studies have
isolated CSCs from enzymatically digested cardiac tissue, by cell
sorting based on cell surface markers (c-kit+, Sca-1+) (1,2). The
antigen c-kit is a cytokine receptor that is expressed on the
surface of stem cells, and can initiate the growth of certain types
of cells (1). Cells that are
generated from cardiac tissue, express c-kit and differentiate into
numerous cell lineages may be regarded as CSCs. A recent study
conducted by the authors of the present study, showed that c-kit
positive CSCs from dog and rat hearts have the potential to
differentiate into sinus node-like cells in vitro (3). However, the proportion of sinus
node-like cells was small and the intrinsic mechanisms behind this
kind of differentiation remain to be elucidated. Therefore, one aim
of the present study was to identify an ideal way to generate large
numbers of sinus node-like cells. The present study selected
angiotensin II (Ang II) to enhance the efficiency of CSC
differentiation. Ang II is the main effector peptide in the
renin-angiotensin system. This peptide has systemic and local
effects and is associated with cell growth and differentiation,
through the activity of four types of receptors (4). Numerous studies have confirmed the
effectiveness of Ang II in the differentiation of stem/progenitor
cells (5,6). However, whether Ang II may promote
the differentiation of CSCs into pacemaker-like cells remains
unclear. The two pacemaker-associated genes
hyperpolarization-activated cyclic nucleotide-gated (HCN)2 and HCN4
encode isoforms 2 and 4 of the hyperpolarization-activated channel
that is highly expressed in the sinus node, and is required for
mature cardiac pacemaker activity (7). Cells that are cardiomyocyte-like and
express HCN2 and HCN4 may be regarded as pacemaker-like cells.
CSCs were intended to be used as seed cells for a
biological pacemaker study. Based on previous data, it may be
hypothesized that Ang II could promote the differentiation of CSCs
into pacemaker-like cells. To test this hypothesis, several
independent approaches were used: Biological characterization of
the mouse CSCs; treatment of CSCs with Ang II in order to promote
differentiation into cardiac pacemaker-like cells; and
investigation into the growth and differentiation of Ang II-induced
cells, by analyzing the expression levels of cardiac conduction
system-specific Tbx2 and Tbx3, and cardiac-specific connexin (Cx)
Cx30.2 and Cx45. Tbx2 and Tbx3 are known to have a role in the
diversification of the specialized conduction system during
vertebrate embryogenesis (8–10).
In addition, the occurrence of the pacemaker current
(If ) was recorded using the patch clamp
technique.
Materials and methods
The present study protocol was approved by the
institutional animal care and use committees of North Sichuan
Medical College (Sichuan, China) and the First Clinic College of
Wuhan University (Hubei, China).
Breeding and sorting of endogenous
cardiac stem cells
Mice were supplied by Wuhan University Center for
Animal Experiment (Wuhan, China) and maintained in the following
conditions: Room temperature and specific pathogen-free conditions.
Heart tissues of three female one-month-old C57BL/6 mice were
isolated and cultured according to a previous study with minor
modifications (11,12). Mice were sacrificed by breaking the
neck in the absence of anaesthesia (to avoid myocardial damage) and
tissues from the cardiac apex were minced into 1–2 mm3
pieces in a 3 ml vial using ophthalamic scissors, washed with
phosphate-buffered saline (PBS; Hyclone Laboratories, Inc., Logan,
UT, USA) and digested three times with 0.1% collagenase II
(Sigma-Aldrich, St. Louis, MO, USA) and three times with 0.25%
trypsin (Hyclone Laboratories, Inc.) alternatively (each digestion,
4 min), at room temperature (20°C). After digestion, the remaining
tissue fragments were cultured in tissue culture medium [Iscove’s
modified Dulbecco’s medium (IMDM; Invitrogen Life Technologies,
Carlsbad, CA, USA) supplemented with 20% fetal calf serum (FCS;
Gibco Life Technologies, Carlsbad, CA, USA), 100 U/ml penicillin G,
100 μg/ml streptomycin (Hyclone Laboratories, Inc.), 0.1 mmol/l 2
mmol/l L-glutamine and 2-mercaptoethanol] at 37°C and 5%
CO2. Following 4–5 days of culture, small, bright cells
began to migrate above the fibroblast layer that had formed from
the adherent tissue fragments. The small and bright cells were
collected and incubated with the sorting antibody c-kit (bsF-0672R;
Biosynthesis Biotech Co., LTD, Beijing, China). The c-kit-positive
(c-kit+) cells were subsequently sorted by flow cytometry, using a
BD LSR II flow cytometer and BD FACSDiva™ software (BD Biosciences,
Franklin Lakes, NJ, USA). Subsequentyl certain sorted cells were
stained with fluorescein isothiocyanate (FITC)-conjugated
antibodies against CD45 and CD34 (Santa Cruz Biotechnology, Santa
Cruz, CA, USA) in order to detect the positive rate of CD45 and
CD34, respectively, through fluorescence activated cells sorting
(FACS). A phase contrast microscope (OLYMPUS BX51; Olympus
Corporation, Tokyo, Japan) was used in the cell culture
process.
Treatment by Ang II
For spontaneous differentiation, a proportion of
sorted cells (n=4×104) were cultivated with cell culture
medium (35% IMDM/65% Dulbecco’s modified Eagle’s medium-Ham F-12
mix (Hyclone Laboratories, Inc.) containing 10% FCS, 0.1 mmol/l
2-mercaptoethanol, 0.1 U/ml thrombin, 100 U/ml penicillin G, 100
μg/ml streptomycin, 0.1 mmol/l 2 mmol/l L-glutamine). Two days
after cell sorting, the sorted cells were transferred into 6-cm
diameter petri dishes and cultured. Various concentrations of Ang
II (0.1, 1.0, 10 μmol/l; Sigma-Aldrich) were added to the culture
medium at day 3, for 48 h. On day 5, the Ang II-treated and control
untreated sorted cells (n=4×104) were plated onto
gelatin-coated 6-cm tissue culture dishes (Nunc, Thermo Fisher
Scientific, Waltham, MA, USA) and cultivated in the previously
mentioned growth medium, supplemented with 10 ng/ml epidermal
growth factor and 20 ng/ml basic fibroblast growth factor. The
sorted cells-derived cells were subsequently analyzed by reverse
transcription-polymerase chain reaction (RT-PCR),
immunofluorescence and FACS analysis. The single-cell suspensions
were then stained with the following antibodies: FITC-conjugated
anti-c-kit, anti-CD45, anti-CD34 and isotype control immunoglobulin
G. In brief, certain single-cell suspensions stained with
FITC-conjugated antibodies against c-kit were treated three times
using a flow cytometry to determine the positive rate of c-kit in
the total cells. In addition, under sterile condition, different
single-cell suspensions were sorted using the flow cytometry in
order to obtain purified c-kit+ cells. Sorted cell were then
serially reseeded into 12-well and six-well plates and 6-cm culture
dishes for further expansion. Furthermore, certain single-cell
suspensions obtained from sorted c-kit+ cell, were stained with the
FITC-conjugated antibodies against CD45 and CD34 in order to detect
the positive rate of CD45 and CD34. The differentiated cells were
investigated using the patch clamp method at different stages of
differentiation in vitro.
A previous study showed that higher concentrations
of Ang II increased cardiac differentiation (13). In order to identify the effective
concentration of Ang II, three concentrations were tested. The
cells treated with 0.1 and 10 μM Ang II did not grow well;
therefore, 1.0 μM Ang II was chosen for further study. The growth
curves of the cells were constructed according to mean values
measured by cell counting on days 3, 5, 8, 12, 15, 18 and 22. The
cell growth curves were drawn with the culture time as the abscissa
and the cell number as the ordinate.
Immunocytochemistry
For immunocytochemistry, the sorted cell outgrowths
were placed on gelatin-coated cover slips (18×18 mm, n=3 in 60 mm
tissue culture plate) and were either fixed with 4%
paraformaldehyde (Sigma-Aldrich) in PBS at room temperature (RT)
for 20 min, depending on the antibody used. Subsequent to rinsing
three times in PBS, bovine serum albumin (BSA, 5% in PBS) was used
to inhibit unspecific labeling (30 min) at RT. Following
permeabilization with 0.2% Triton X-100 (Sigma-Aldrich) for 5 min,
the cells were incubated with the primary antibodies at specific
dilutions overnight at 4°C. The samples were washed three times
with PBS and incubated with fluorescence-labeled secondary
antibodies (diluted in 1% BSA in PBS), at 37°C for 45 min. The
cells were then incubated with 4′,6-diamidino-2-phenylindole (5
μg/ml in PBS; Sigma-Aldrich) at 37°C for 10 min, in order to stain
the nuclei. Subsequent to washing three times in PBS, the specimens
were embedded in mounting medium (glycerol; Promega Corp., Madison,
WI, USA).
The following primary antibodies were used for
immunocytochemistry staining, all were purchased from Santa Cruz
Biotechnology: Rat monoclonal anti-c-kit (1:00; cat. no. sc-19619;
Santa Cruz Biotechnology), rabbit polyclonal anti-cardiac troponin
(cTnI; 1:100; cat. no. sc-15368; Santa Cruz Biotechnology), mouse
monoclonal anti-smooth muscle actin (SMA; 1:100; cat. no. sc-53015;
Santa Cruz Biotechnology), rabbit polyclonal anti-CD31 (1:00; cat.
no. sc-8306; Santa Cruz Biotechnology) and rabbit polyclonal
anti-HCN4 (1:100; cat. no. sc-28750; Santa Cruz Biotechnology).
Isotype-matched antibodies were used as control. Fluorescein
isothiocyanate-conjugated secondary antibodies (cat. nos. sc-2012
and sc-2010; 1:100, Santa Cruz Biotechnology) were used to detect
c-kit, SMA and HCN4, respectively. Rhodamine-conjugated goat
anti-rabbit immunoglobulin G (cat. no. sc-2091; 1:100; Santa Cruz
Biotechnology) was used to detect rabbit anti-mouse cTnI and
CD31.
RT-PCR
Total RNA was extracted from induced and uninduced
CSCs using TRIzol® reagent (Promega Corp.).
Transcriptional expression levels of Nkx2.5, GATA4, HCN2, HCN4,
Cx30.2, Cx45, Tbx2 and Tbx3 genes were determined using
semi-quantitative RT-PCR, according to the manufacturer’s
instructions. Transcript levels were standardized to the
corresponding mouse GAPDH levels. Tbx2 and Tbx3 are known to have a
role in diversification of the specialized conduction system,
during vertebrate embryogenesis. Connexins were also analyzed,
including Cx30.2, which is a marker of the conduction system and is
usually detected in the sinus and the atrioventricular nodes of the
adult mouse heart (14). The
primers for RT-PCR (Promega Corp.) are listed in Table I.
 | Table IPrimer sequences for reverse
transcription-polymerase chain reaction. |
Table I
Primer sequences for reverse
transcription-polymerase chain reaction.
| Gene | Primer sequence
(forward/reverse) | Product size
(bp) | NCBI accession
no. |
|---|
| Nkx2.5 |
CGACGGAAGCCACGCGTGCTC
CGCTGTCGCTTGCACTTG | 181 | NM_008700.2 |
| GATA4 |
AAACGGAAGCCCAAGAACCTGAAT
GAGCTGGCCTGCGATGTCTGAGTG | 311 | NM_008092.3 |
| HCN2 |
GAAGATGTACTTCATCCAGCA
CTGGCCAAGCTCTGCCTG | 391 | NM_008226 |
| HCN4 |
GGAGTATCCCATGATGCGGAG
GGCAGGAGAGGGCTCAATCCA | 513 | NM_001081192 |
| Connexin 30.2 |
GCGCCGCCGGTGCTGTTCGT
GCCGCCTCGCCCTCGCTGTC | 525 | AJ414561.1 |
| Connexin 45 |
ATCATCCTGGTTGCAACTCC
CTCTTCATGGTCCTCTTCCG | 169 | AY390397.1 |
| Tbx2 |
TTCCACAAACTGAAGCTGAC
GCTGTGTAATCTTGTCATTCTG | 204 | NM_009324.2 |
| Tbx3 |
CAGCCGCGGTTCCACATCGTCA
GGGCCGTGCTCCTCCTTGCTCTC | 410 | NM_011535.2 |
| GAPDH |
ACCACAGTCCATGCCATCAC
TCCACCACCCTGTTGCTGTA | 452 | NM_008084.2 |
The thermal conditions of the PCR were set, using a
C1000 PCR thermocycler (Bio-Rad Laboratories, Inc. Hercules, CA,
USA), as follows: 95°C for 5 mins, followed by 30 cycles of 30 sec
at 95°C, with 1 min annealing intervals; followed by 1 min
extension at 72°C. Additional 10 min incubation at 72°C was
included following completion of the final cycle. A total of 5 μl
PCR product was electrophoresed on a 1% agarose gel (Promega Corp.)
and imaged using a gel imaging instrument (UVIdoc HD5; UVitec Ltd,
Cambridge, UK).
Electrophysiological recording
The membrane currents of the cultured CSCs were
studied using whole-cell recording configuration of the patch clamp
technique. Equipement used for electrophysiological recording
included a standard and advanced two-microelectrode voltage-clamp
amplifier (CA-1B; DAGAN, Minneapolis, MN, USA), pCLAMP software
(Axon Instruments, Foster City, CA, USA) and a patch pipette (P-97;
Sutter Instrument Co., Novato, CA, USA). For recording the inward
sodium current the patch pipette solution consisted of (in mM)
Na2ATP 5, MgCl2 5, EGTA 11, CaCl2
1, HEPES 10, CsCl 120, Glucose 11 (pH 7.4), and the bath solution
to measure ionic current consisted of (mM): KCl 5.4,
CaCl2 1.8, NaCl 135, NaH2PO4 0.33,
HEPES 10, MgCl2 1, Glucose 10 (pH 7.2). For detecting
the inward calcium current the patch pipette solution consisted of
(in mM): CaCl2 1, CsCl 120, Na2ATP 5, EGTA
11, MgCl2 5, Glucose 11, HEPES 10 (pH 7.3), and the bath
solution to measure ionic current consisted of (in mM): CsCl 10,
chloride choline 120, BaCl2 10, MgCl2 1,
Glucose 10, HEPES 10 (pH 7.4) (15,16).
For recording the If current, the pipette
solution contained (in mM): KCl 20, K-gluconate 125,
MgCl2 1, NaCl 5, HEPES 10, MgATP 5 (pH 7.2), and the
bath solution consisted of (in mM): KCl 5.4, NaCl 140,
MgCl2 1, glucose 5.5, CaCl2 1.8, HEPES 5 (pH
7.4) (2,17).
Statistical analysis
Statistical analyses were performed using SPSS
version 11.0 (SPSS Inc., Chicago, IL, USA) and GraphPad Prism
version 6 (GraphPad Software, Inc., La Jolla, CA, USA). The values
represent the mean ± standard error. P<0.05 was considered to
indicate a statistically significant difference.
Results
Isolation, culture and expansion of
CSCs
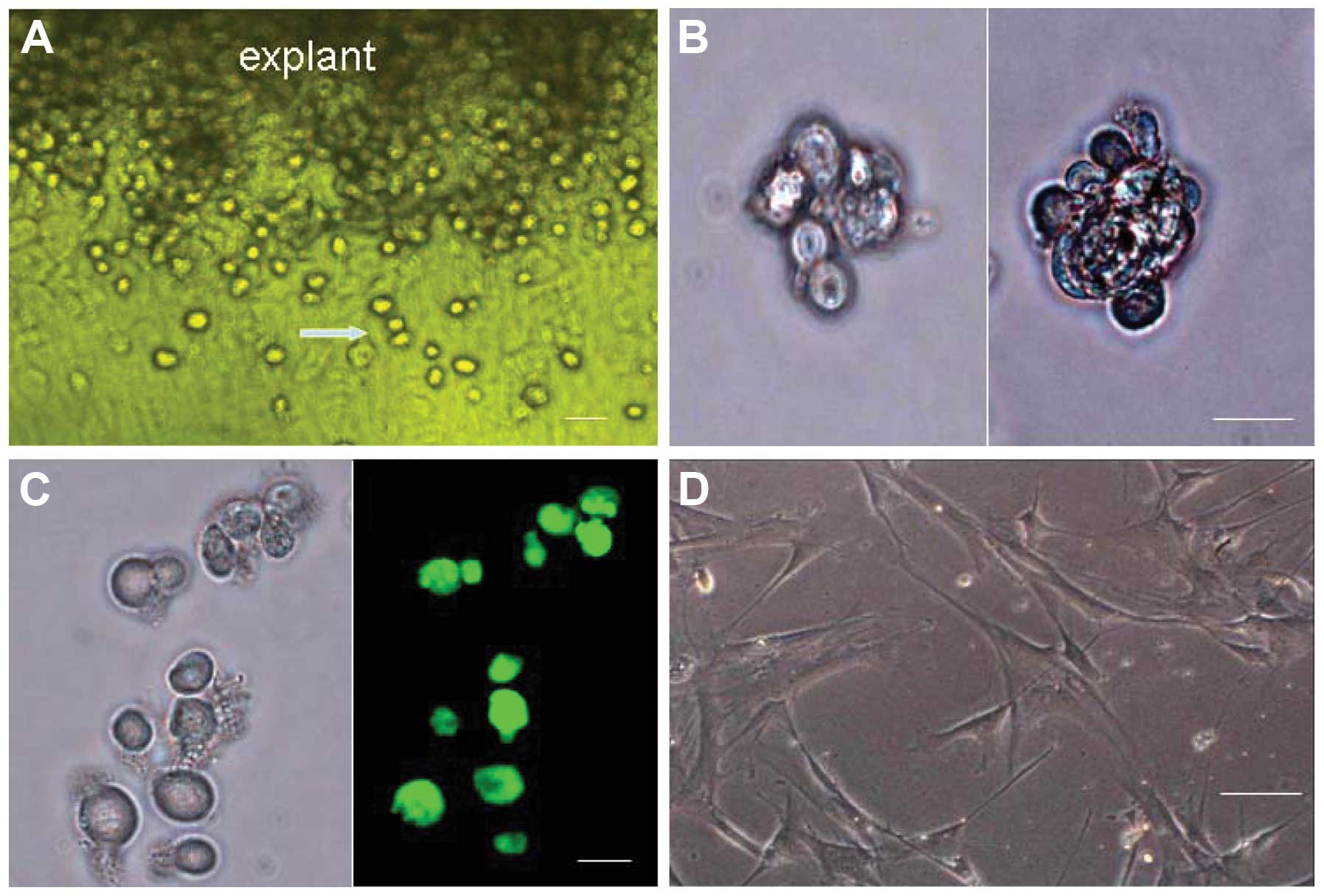
Two to four days after explantation of the minced
mouse cardiac tissue, the round, phase-bright cells began to
migrate above the layer of fibroblasts, from the edge of the
adherent explants (Fig. 1A).
Successful cell outgrowth was obtained in 27 out of 30 cases. A
phase contrast microscope was used to visualize the cells following
cell sorting by FACS. The purified c-kit+ cells were shown to be
small, round and bright (Fig.
1A–C). The majority of these cells were capable of
proliferating, and some began to aggregate monoclonally and form
cardiac spheres in suspension (Fig.
1B). The single and clumping cells slowly increased in size and
gradually adhered to the plate. The adherent cells were able to
produce new round, bright cells. Two weeks later, the majority of
c-kit+ cells had differentiated into cells with a fusiform or
irregular shape (Fig. 1D).
However, some c-kit+ cells were still able to divide into round,
bright, c-kit+ cells.
FACS analysis and cell sorting
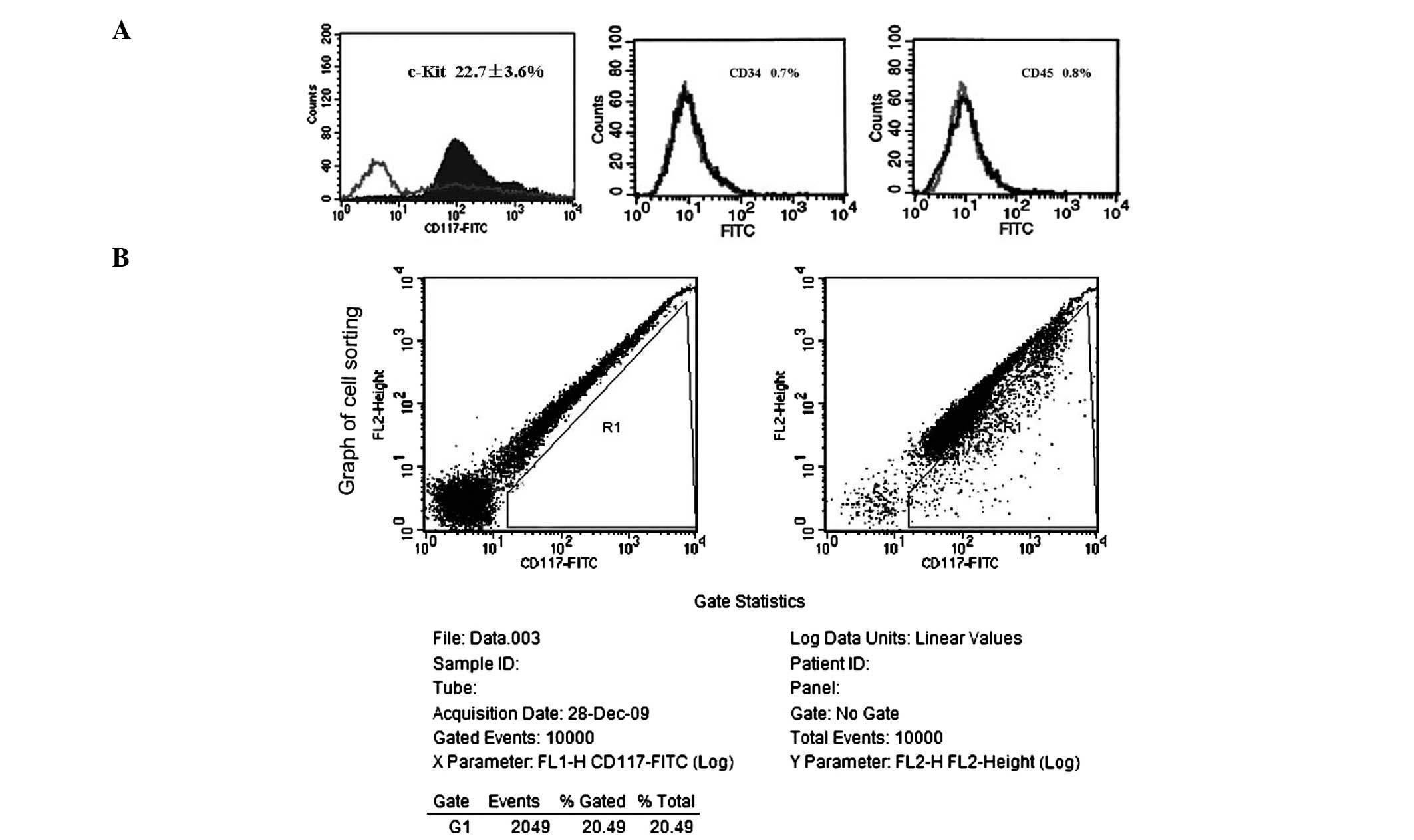
As determined by flow cytometry, 22.7±3.6% of the
detected cells were c-kit+ (Fig.
2A). After cell sorting of c-kit+ cells by FACS, the positive
expression rates of CD34 and CD45 were determined in the purified
c-kit+ cells. The c-kit+ cells had incredibly low expression of
CD34 (0.7%; Fig. 2A) and CD45
(0.8%; Fig. 2A). A sorting graph
indicated that the positive rate of c-kit in all of the sorted
cells was 20.49% (Fig. 2B).
Effects of Ang II on the growth of c-kit+
cells
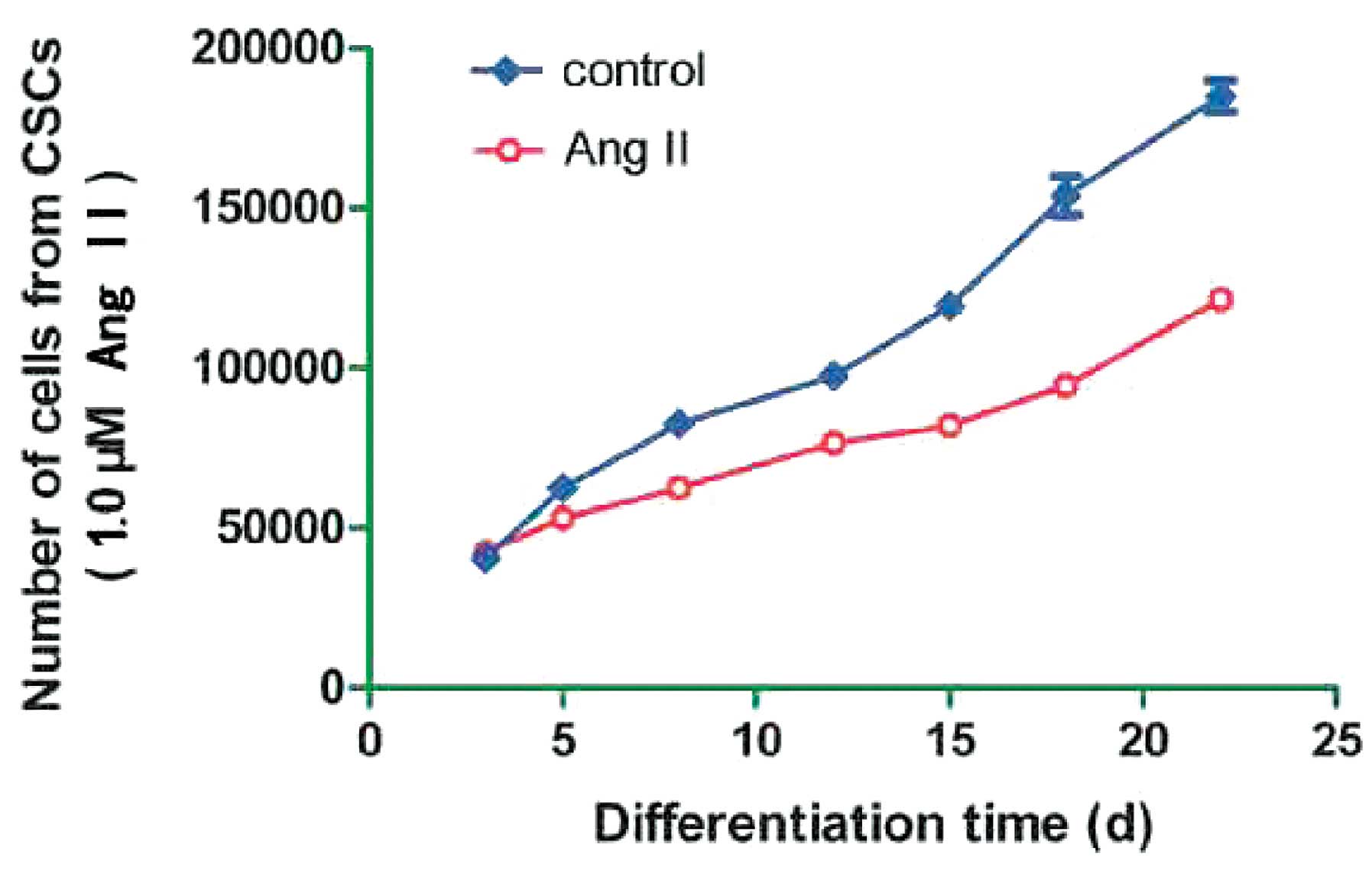
The aim of the present study was to determine the
effects of Ang II, at high concentrations, on the growth of c-kit+
cells. The cells treated with 0.1 and 10 μM Ang II did not have a
good growth pattern; therefore, the growth ability of the sorted
cells treated with 1.0 μM Ang II, was determined at different time
points. Growth curves of the cells showed that treatment with 1.0
μM Ang II hardly inhibited cell growth, particularly at the early
stage of differentiation (Fig.
3).
Characterization of the differentiation
of c-kit+ cells
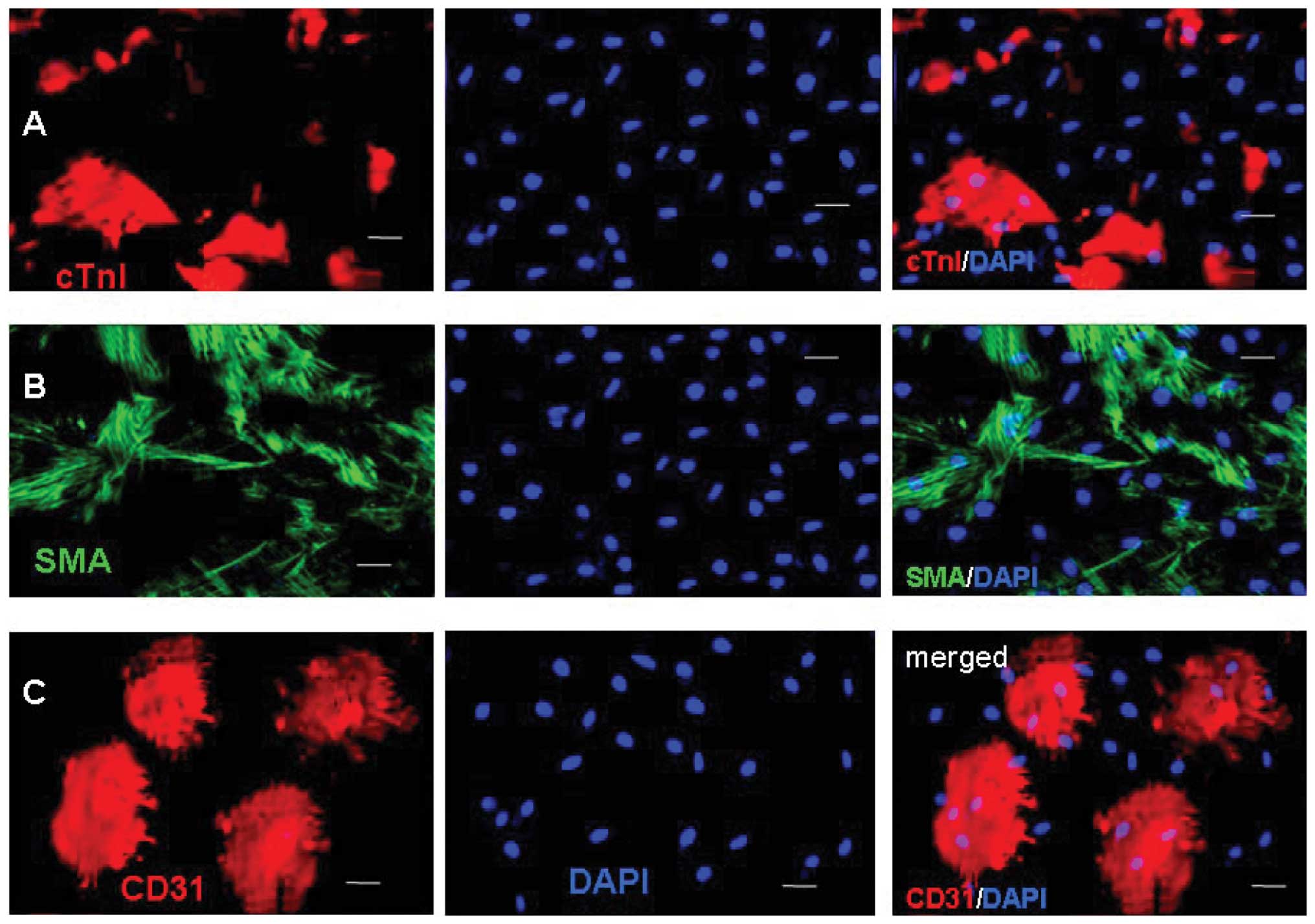
To characterize the differentiation of the c-kit+
cells, the expression levels of cTnI, SMA, CD31 and HCN4 were
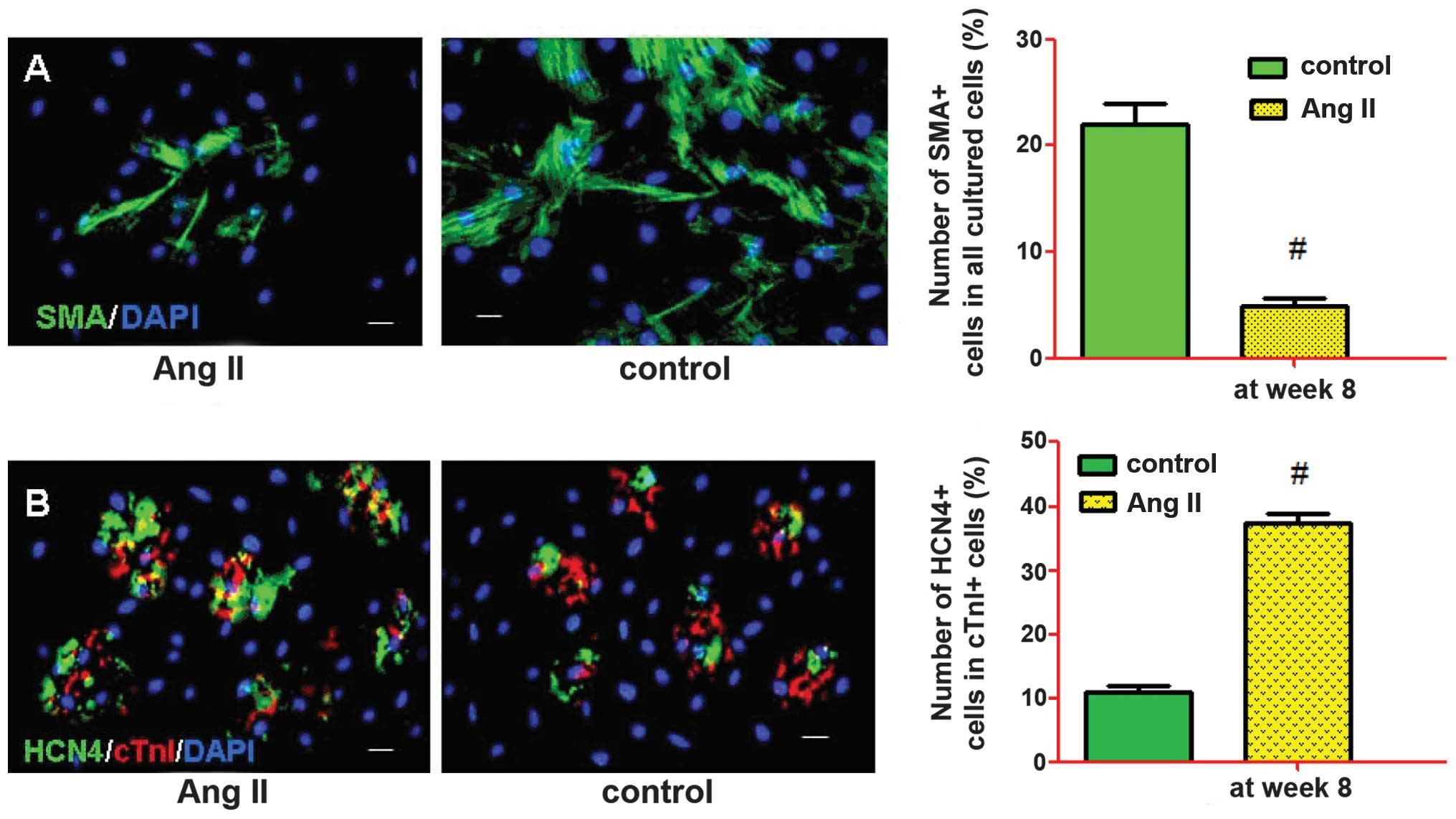
examined by immunostaining after two weeks. The representative
images were captured and the frequencies of cTnI, SMA and CD31 were
determined (Fig. 4). Fluorescence
microscopic analysis revealed that Ang II-treated and control cells
could differentiate into cardiac muscle-like cells (cTnI), smooth
muscle-like cells (SMA) and endothelium-like cells (CD31), with
various levels of effectiveness. The percentage of differentiated
cells expressing cTnI, SMA and CD31 at week 8 were 31.6±4.2,
6.3±4.3 and 20.4±8.1% in the Ang II-treated cells, and 22.5±5.8,
21.5±5.1 and 21.9±4.5% in the control cells, respectively. There
were a significantly increased number of Ang II-treated cells
expressing cTnI (P<0.05) and a significantly decreased number of
Ang II-treated cells expressing SMA (P<0.01), as compared with
the control cells.
Treatment of the c-kit+ cells with 1.0 μM Ang II
resulted in a significant increase in the number of
cardiomyocyte-like cells, and a suppression of smooth muscle-like
cells, at week 8 (Fig. 5A). The
number of anti-cTn1 and HCN4-labeled cells increased at the
advanced differentiation stage (week 8) (Fig. 5B). Treatment with Ang II also
resulted in the formation of irregular and fragile cells; however,
the size of the CSCs did not significantly differ from the
non-treated control CSCs. Notably, the increase in the percentage
number of anti-cTnI and anti-HCN4-labeled cells was not observed by
immunocytochemical analysis, when the CSCs were treated with
different concentrations of Ang II (0.1, 10 μM) or were treated at
earlier time points (2 and 4 weeks). Therefore, the CSCs treated
with 1.0 μM Ang II were selected for further study. These results
indicate a concentration and time-dependent influence of Ang II on
the differentiation of CSCs.
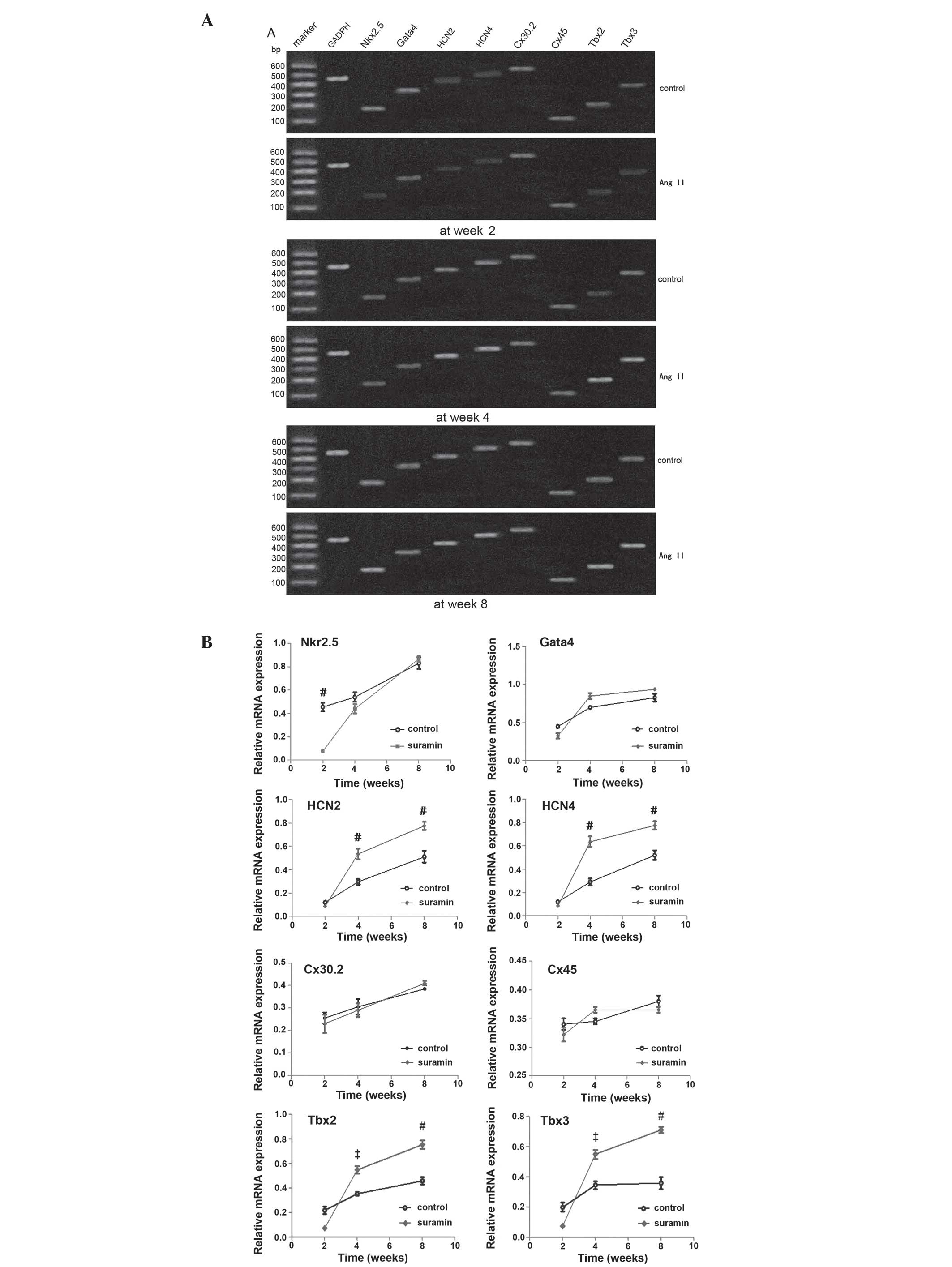
mRNA expression levels
To further investigate the development of Ang
II-induced sinus node-like cells, the mRNA expression levels of
specific genes in the cells differentiated from the CSCs, treated
with 1.0 μM Ang II and control, were assessed at weeks 2, 4 and 8.
The detected genes were associated with either cardiomyocyte or
sinus node cells: Nkx2.5, GATA4, HCN2, HCN4, Cx30.2, Cx45, Tbx2 and
Tbx3. The upregulation of GATA4, Tbx2 and 3 expression levels at
the advanced stages of differentiation were correlated with the
increased HCN2 and HCN4 expression levels, in the Ang II-induced
cells. There was no difference in the expression levels of Cx30.2
and Cx45 between the Ang II-treated and control cells. Furthermore,
the expression levels of Nkx2.5 were downregulated in the Ang
II-induced cells, as compared with the control cells (Fig. 6).
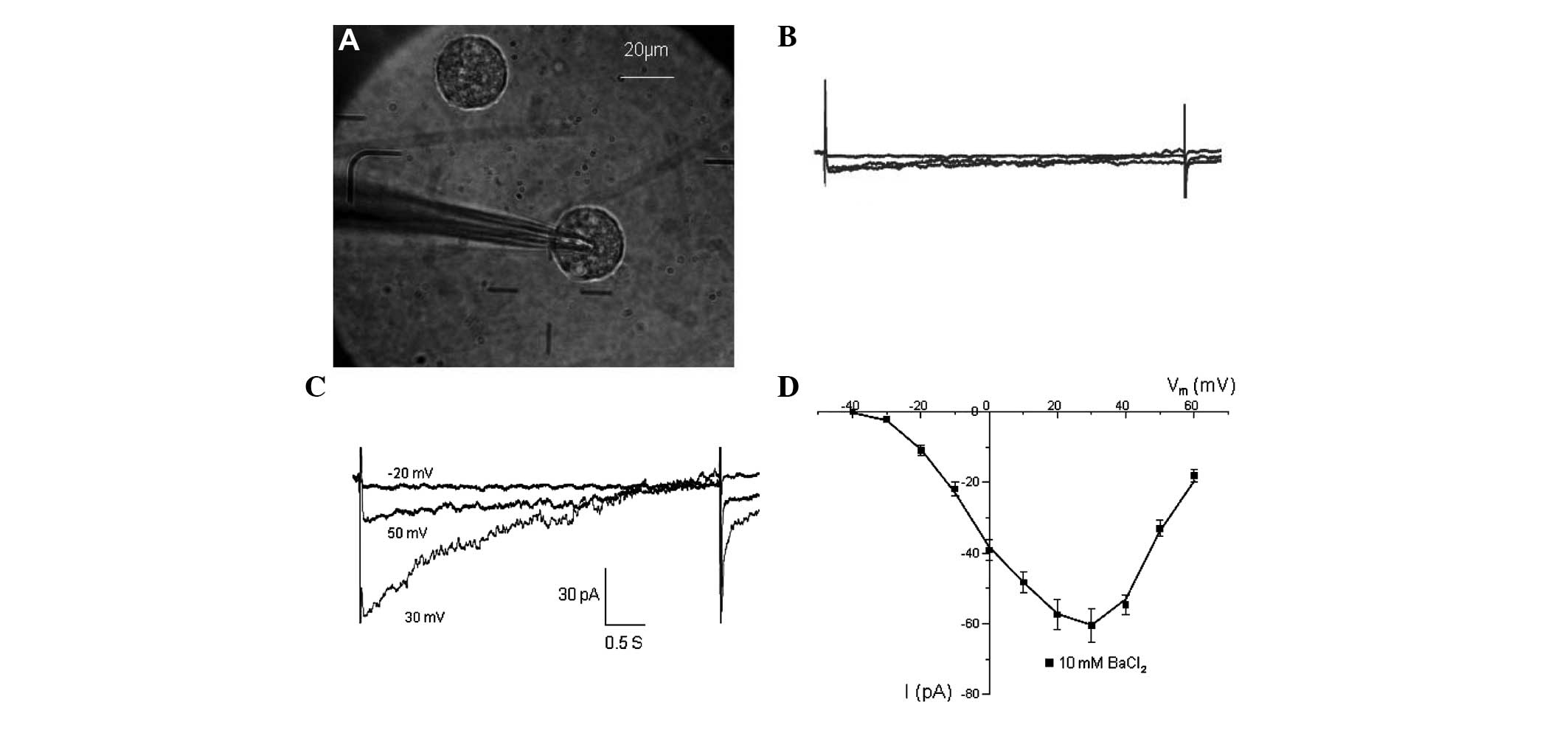
Inward currents of CSCs
Detection of the inward sodium current was initially
attempted following cell sorting. However, the inward sodium
current could not be recorded in all of the CSCs being
investigated. The presence of functional Ca2+ channels
was then assessed. At 2 mM external Ca2+ there was
little inward current being recorded (Fig. 7B). However, after switching to 10
mM Ba2+, inward currents were recorded (Fig. 7D). The currents were activated at
~−40 mV and peaked at 20–30 mV (Fig.
7C), similar to the Ba2+ currents conducted by
L-type Ca2+ channels in other cell types (15,16).
The strongest current was −60.9±3.2 pA at 30 mV.
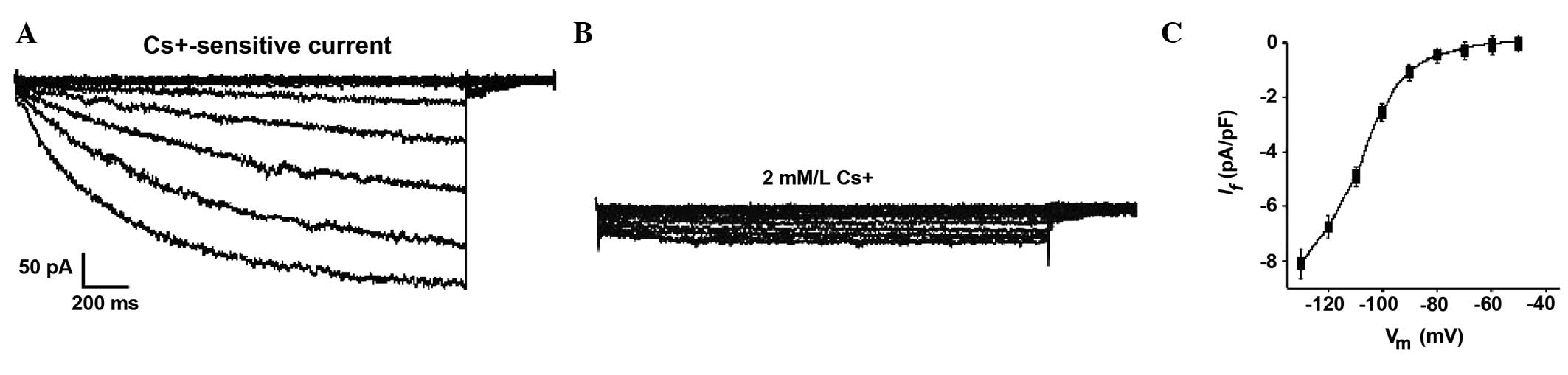
After four weeks, the hyperpolarization-activated
current (If) was detected in the differentiating
c-kit+ CSCs. Subsequent voltage-clamp experiments revealed that
some cells treated with 1.0 μM Ang II (~1/15 cells) had an inward
current that was activated following hyperpolarizing steps from
~−45 mV (Fig. 8A). This
hyperpolarization-activated current became larger and more rapidly
activated at increasingly negative potentials. Furthermore, it was
strongly decreased by 2 mM/l Cs+ (Fig. 8B). These results suggest it may be
characterized as If (18). The occurrence of the
pacemaker current (If) provides further
evidence to confirm the presence of sinus node-like cells derived
from c-kit+ CSCs. The If could not be
recorded at the early stages (before 4 weeks) nor in control
cells.
Discussion
The investigation of CSCs has markedly altered
research regarding the treatment of heart disease (19,20).
Although CSC-derived cardiocytes provide a potential source of
cells capable of functionally integrating into host heart tissues
to reconstitute dead myocardium (21,22),
a number of issues need to be resolved before this approach can be
considered clinically. The present study aimed to explore the
potential for differentiation of c-kit+ CSCs into pacemaker cells.
If under specific induction, CSCs could differentiate into
pacemaker-like cells, the cells may be used as potential seed cells
for a study of biological pacemakers. The results of the present
study demonstrated that treatment of mouse c-kit+ CSCs with Ang II,
and growth factors, promoted the selective differentiation of the
CSCs into sinus node-like cardiac cells, during the definitive
stages of CSC formation, in a time- and concentration-dependent
manner
The c-kit+ cells did not express the hematopoietic
(CD45) or endothelial (CD34) progenitor markers; therefore
confirming that the purified c-kit+ cells were not hematopoietic or
endothelial stem/progenitor cells. In addition, the c-kit+ cells
originated from cardiac tissue and were shown to be capable of
differentiating into several cell lineages (23,24).
Therefore, the sorted c-kit+ cells may be regarded as c-kit+ CSCs.
The transcription factors Nkx2.5 and GATA4, which are essential for
normal heart morphogenesis and regulate the survival, growth, and
proliferation of cardiomyocytes (25,26),
are considered to be the early markers of cardiomyocyte
differentiation. The cells expressing Nkx2.5, GATA4 and cTnI may be
regarded as myocardium-like cells. It is well known that pacemaker
cells are a class of cardiac myocytes with special differentiation.
Therefore, the cells, which expressed cardiomyocyte-related genes
(Nkx2.5, GATA4 and cTnI) and sinus node-related genes (HCN2, HCN4,
Tbx2 and Tbx3), may be regarded as pacemaker-like cells.
In order to study how the CSCs differentiated into
pacemaker-like cardiac cells, the expression levels of Nkx2.5,
GATA4, HCN2, HCN4, Cx30.2, Cx45, Tbx2 and Tbx3 were determined. The
HCN channel family of genes have an important role in physiological
automaticity. Overexpression of the HCN genes may be a promising
measure to be implemented in the development of a biopacemaker
(27,28). The mammalian genome encodes four
HCN genes: HCN1-4. HCN2 and HCN4 channels are expressed in the
sinus node, and determine the hyperpolarization-activated cation
current If and regulate heart rate
(14,29). In the present study, the CSCs
isolated from mouse hearts were shown to be self-renewing and
clonogenic, and could directly differentiate into
cardiomyocyte-like cells in vitro; however, these cells
failed to contract spontaneously. High concentration Ang II could
promote the differentiation of the CSCs into myocardium-like cells
and suppress the differentiation into smooth muscle-like cells.
Furthermore, the number of sinus node-like cells was enhanced in
response to treatment with Ang II at an advanced stage of
differentiation (week 8). Concordantly, the upregulation of HCN2
and HCN4 at the transcript and/or protein level confirmed the
function of Ang II treatment on the induction of the
differentiation of CSCs into sinus node-like cells.
The preferential induction of CSCs into sinus
node-like cells by Ang II in the present study was also supported
by the demonstration of enhanced Tbx2 and Tbx3 transcription levels
in the Ang II-treated cells at a late stage of differentiation
(weeks 4 and 8). Tbx2 and Tbx3 are key regulators in the formation
of the sinus node in vivo, and are expressed in the primary
myocardium and suppress the transformation of primary myocardium
into working myocardium (7,30).
Furthermore, since the activation of Tbx2 and Tbx3 expression in
the developing heart is directly linked to the bone morphogenetic
protein (BMP)/Smad-mediated signaling pathway (31). The BMP-/Smad-signaling mechanisms
may be asoociated with the upregulation of the Tbx2 and Tbx3
transcripts in the Ang II-treated cells. However, this hypothesis
requires further clarification.
The present study observed the temporary
downregulation of Nkx2.5 expression levels following Ang II
treatment at an early stage (at week 2); this result is concordant
with a previous finding that the absence of the cardiac
transcription factor Nkx2.5 in early development is a prerequisite
for the development of sinus nodal cells (32). The downregulation of Nkx2.5 appears
to be necessary for the specific differentiation that results in
the activation of the sinus node-specific genes HCN2, HCN4, Tbx2
and Tbx3. However, the transcript levels of Cx45 and Cx30.2, which
were markers of nodal cells in the adult murine heart (14), were no different between the Ang
II-treated and control cells at all stages observed. These results
suggest that Ang II had little effect on the expression of Cx30.2
and Cx45, but had pleiotropic effects on other pacemaker-associated
genes, such as HCN2, HCN4, Tbx2 and Tbx3.
The electrophysiological observations of the present
study confirmed that inward currents could be recorded in some
cells derived from the c-kit+ CSCs. The If
pacemaker current was recorded using the patch clamp technique. The
existence of inward current channels reinforces the feasibility of
using CSCs as seed cells in a biopacemaker study. Successful
recording of the If pacemaker provides
significant evidence that the c-kit+ CSCs were capable of
differentiating into sinus node-like cells.
In conclusion, the present study showed that Ang II
could promote the differentiation of CSCs into pacemaker-like
cells. The cardiac differentiation was a result of the pleiotropic
effects on genes by Ang II. These results suggest that CSCs may be
suitable seed cells for use in a future biopacemaker study.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant no. 81270220).
Abbreviations:
|
CSCs
|
cardiac stem cells
|
|
EGF
|
epidermal growth factor
|
|
bFGF
|
basic fibroblast growth factor
|
|
cTnI
|
cardiac troponin I
|
|
SMA
|
smooth muscle actin
|
|
HCN2
|
hyperpolarization-activated cyclic
nucleotide-gated 2
|
|
HCN4
|
hyperpolarization-activated cyclic
nucleotide-gated 4
|
|
RT-PCR
|
reverse transcriptase-polymerase chain
reaction
|
|
FACS
|
fluorescence-activated cell sorter
|
|
Ang II
|
Angiotensin II
|
References
|
1
|
Fukuda K and Yuasa S: Stem cells as a
source of regenerative cardiomyocytes. Circ Res. 98:1002–1113.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tang YL, Shen L, Qian K, et al: A novel
two-step procedure to expand cardiac Sca-1+ cells
clonally. Biochem Biophys Res Commun. 359:877–883. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang J, Huang CX, Wu P, et al: Cardiac
stem cells differentiate into sinus node-like cells. Tohoku J Exp
Med. 222:113–120. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nakamura K, Koibuchi N, Nishimatsu H, et
al: Candesartan ameliorate cardiac dysfunction observed in
angiotensin-converting enzyme 2-deficent mice. Hypertens Res.
31:1953–1961. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zambidis ET, Park TS, Yu W, et al:
Expression of angiotensin-converting enzyme (CD143) identifies and
regulate sprimitive hemangioblastsderived from human pluripotent
stem cells. Blood. 112:3601–3614. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ishizuka T, Goshima H, Ozawa A, et al:
Effect of angiotensin II on proliferation and differentiation of
mouse induced pluripotent stem cells into mesodermal progenitor
cells. Biochem Biophys Res Commun. 420:148–155. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Stieber J, Herrmann S, Feil S, et al: The
hyperpolarization-activated channel HCN4 is required for the
generation of pacemaker action potentials in the embryonic heart.
Proc Natl Acad Sci USA. 100:15235–15240. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hoogaars WM, Tessari A, Moorman AF, et al:
The transcriptional repressor Tbx3 delineates the developing
central conduction system of the heart. Cardiovasc Res. 62:489–499.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hoogaars WM, Engel A, Brons JF, et al:
Tbx3 controls the sinoatrial node gene program and imposes
pacemaker function on the atria. Genes Dev. 21:1098–1112. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Christoffels VM, Hoogaars WM, Tessari A,
et al: Tbox transcription factor Tbx2 represses differentiation and
formation of the cardiac chambers. Dev Dyn. 229:763–770. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Messina E, De Angelis L, Frati G, et al:
Isolation and expansion of adult cardiac stem cells from human and
murine heart. Circ Res. 95:911–921. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Oh H, Bradfute SB, Gallardo TD, et al:
Cardiac progenitor cells from adult myocardium: homing,
differentiation, and fusion after infarction. Proc Natl Acad Sci
USA. 100:12313–12318. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim YM, Jeon ES, Kim MR, et al:
Angiotensin II-induced differentiation of adipose tissue-derived
mesenchymal stem cells to smooth muscle-like cells. Int J Biochem
Cell Biol. 40:2482–2491. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kreuzberg MM, Sohl G, Kim JS, et al:
Functional properties of mouse connexin30.2 expressed in the
conduction system of the heart. Circ Res. 96:1169–1177. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Heubach JF, Graf EM, Leutheuser J, et al:
Electrophysiological properties of human mesenchymal stem cells. J
Physiol. 554:659–672. 2004. View Article : Google Scholar
|
|
16
|
Heubach JF, Köhler A, Wettwer E, et al:
T-type and tetrodotoxin-sensitive Ca2+ currents coexist
in guinea pig ventricularmyocytes and are both blocked by mibe-
fradil. Circ Res. 86:628–635. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
van Ginneken AC and Giles W: Voltage clamp
measurements of the hyperpolarization-activated inward current If
in single cells from rabbit sino-atrial node. J Physiol. 434:57–83.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Verkerk AO, Wilders R, van Borren MM, et
al: Pacemaker current (I(f)) in the human sinoatrial node. Eur
Heart J. 28:2472–2478. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rota M, Padin-Iruegas ME, Misao Y, et al:
Local activation or implantation of cardiac progenitor cells
rescues scarred infarcted myocardium improving cardiac function.
Circ Res. 103:107–116. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Passier R, van Laake LW and Mummery CL:
Stem-cell-based therapy and lessons from the Heart. Nature.
453:322–329. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bearzi C, Rota M, Hosoda T, et al: Human
cardiac stem cells. Proc Natl Acad Sci USA. 104:14068–14073. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Padin-Iruegas ME, Misao Y, Davis ME, et
al: Cardiac progenitor cells and biotinylated insulin-like growth
factor-1 nanofibers improve endogenous and exogenous myocardial
regeneration after infarction. Circulation. 120:876–887. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Beltrami AP, Barlucchi L, Torella D, et
al: Adult cardiac stem cells are multipotent and support myocardial
regeneration. Cell. 114:763–776. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Linke A, Müller P, Nurzynska D, et al:
Stem cells in the dog heart are self-renewing, clonogenic, and
multipotent and regenerate infarcted myocardium, improving cardiac
function. Proc Natl Acad Sci USA. 102:8966–8971. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
DiFrancesco D: The role of the funny
current in pacemaker activity. Circ Res. 106:434–446. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ludwig A, Herrmann S, Hoesl E, et al:
Mouse models for studying pacemaker channel function and sinus node
arrhythmia. Prog Biophys Mol Biol. 98:179–185. 2008. View Article : Google Scholar
|
|
27
|
El Chemaly A, Magaud C, Patri S, et al:
The heart rate-lowering agent ivabradine inhibits the pacemaker
current I(f) in human atrial myocytes. J Cardiovasc Electrophysiol.
18:1190–1196. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Habib M, Caspi O and Gepstein L: Human
embryonic stem cells for cardiomyogenesis. J Mol Cell Cardiol.
45:462–474. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Stieber J, Hofmann F and Ludwig A:
Pacemaker channels and sinus node arrhythmia. Trends Cardiovasc
Med. 14:23–28. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Habets PE, Moorman AF, Clout DE, et al:
Cooperative action of Tbx2 and Nkx2.5 inhibits ANF expression
intheatrioventricular canal: implications for cardiac chamber
formation. Genes Dev. 16:1234–1246. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yang L, Cai CL, Lin L, et al: Isl1Cre
reveals a common BMP pathway in heart and limb development.
Development. 133:1575–1585. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mommersteeg MT, Hoogaars WM, Prall OW, et
al: Molecular pathway for the localized formation of the sinoatrial
node. Circ Res. 100:354–362. 2007. View Article : Google Scholar : PubMed/NCBI
|