Introduction
Spinal cord ischemia-reperfusion injury (SCII) is a
secondary injury resulting from the temporary interruption of blood
supply to the spinal cord and may result in irreversible vascular
damage with subsequent paraplegia or other neurological deficits
(1). This severe complication is
frequently observed in transient ischemic insults of the spinal
cord and following surgical repair of thoracoabdominal aortic
aneurysms (2). While the exact
mechanism of secondary SCII remains to be elucidated, there are
multiple contributing cellular responses, including overproduction
of reactive oxygen species and oxygen free radicals that lead to
excessive lipid peroxidation, protein and DNA damage, neuronal
apoptosis and calcium overload (3–5).
Several drugs are currently used for the treatment of SCII,
including glucocorticoids, neurotrophic factors and nerve
regeneration factors. However, a detailed understanding of the
effects of these drugs is lacking (6). Further investigation into the
mechanism of SCII may assist in providing novel therapeutic
targets.
Edema of the spinal cord following the initial
injury may be an important contributing factor to the development
of SCII, posing a major challenge clinically, as it is often
long-lasting and therapy resistant. The transport of water across
cellular membranes contributes to the development of such edematous
conditions, thus proteins involved in this process may present
therapeutic targets. Aquaporin-4 (AQP4) is the most abundant member
of a family of water-conducting integral membrane proteins that is
highly expressed in glial cells of the spinal cord (7,8).
AQP4 expression parallels the changes in water content in the
injured spinal cord (9) and has
been observed to be directly involved in spinal cord
injury-associated edema (10).
Furthermore, AQP4 has been implicated in spinal cord damage in
multiple sclerosis (11) and
amyotrophic lateral sclerosis (12). Thus, the development of an
effective and safe therapeutic agent targeting AQP4 expression may
be beneficial for the treatment of SCII.
A commonly used traditional remedy for numerous
medical conditions, including central nervous system and
neurodegenerative disorders, is ginseng (13). While the active agent remains to be
elucidated, it is hypothesized to be ginsenoside, an extract from
the stem and leaf of ginseng. Currently, >50 different
ginsenoside monomers have been isolated (14–16),
each with different effects. Ginsenoside Rg has an anti-fatigue
effect and is able to improve memory and learning ability (15,17).
Ginsenoside Rg2 is also able to improve myocardial ischemia and
hypoxia in the treatment and prevention of coronary heart disease
(18,19). Ginsenoside Rg3 can promote tumor
cell apoptosis and inhibit tumor cell growth (20,21).
Ginsenoside Rb2 acts as an antioxidant, removing free radicals and
improving recovery from myocardial ischemic-reperfusion injury
(22). Ginsenoside Rh1 suppresses
the central nervous system and may have an hypnotic effect
(23). However, the most abundant
and representative single monomer, ginsenoside Rb1, has
demonstrated therapeutic potential for a variety of conditions.
Several studies have revealed that the Rb1 monomer is
anti-apoptotic (24,25) and has the ability to alleviate
oxidative stress by scavenging oxygen free radicals (26), blocking calcium channels (27) and attenuating ischemic-reperfusion
injury (28,29). As there is evidence that
ginsenoside Rb1 may reduce the damaging effects of myocardial and
cerebral ischemia, it was hypothesized that it may also have a
protective effect against SCII. Therefore, the effect of
ginsenoside Rb1 treatment on abdominal aortic occlusion-induced
SCII was examined. Furthermore, the expression of AQP4 was assessed
as a potential mechanism for the neuroprotective effects of
ginsenoside Rb1 treatment.
Materials and methods
Animals
A total of 96 male and female 8–10 week-old
Sprague-Dawley rats (weighing 220–260 g) were obtained from the
animal experimental center of Jilin University (Changchun, China)
and maintained in an automatic light/dark cycle with ad
libitum access to food and water. Rats were acclimated for 1
week prior to experimentation. Animal care and procedures were
approved by the Ethics Committee of Jilin University (Changchun,
China).
Drug treatment and dosage
Ginsenoside Rb1
(C54H92O23, molecular weight:
1109.29 Da) was provided by the Department of Organic Chemistry,
Jilin University. The appropriate dosage of ginsenoside Rb1 for
rats was established with preliminary experiments to determine the
minimum effective concentration of ginsenoside Rb1 following SCII
in rats. Concentrations of ginsenoside Rb1 >10 mg/kg/day
exhibited toxicity in rat organs. Therefore, the maximum dosage of
10 mg/kg/day was administered as a once-daily intraperitoneal (IP)
injection in the present experiments.
Spinal cord ischemic-reperfusion injury
model
Animals were randomly assigned to one of the four
following groups with a total of 24 animals in each group: i) Blank
control condition, ii) sham surgery procedure; iii) SCII induced by
abdominal aortic occlusion control group or iv) SCII group treated
with ginsenoside Rb1. All rats were anesthetized with an IP
injection of 10% chloral hydrate. The model of transient SCII by an
abdominal aortic occlusion was implemented according to a
previously reported method (30).
Briefly, animals were anesthetized and body temperature was
maintained at 37°C with a heating pad. The rats were placed in a
right lateral position and the abdomen was opened through a midline
incision to expose the abdominal aorta. The aorta and its branches
were meticulously dissected from the caudal to the left renal
artery. A microvessel clamp was placed across the aorta just distal
to the left renal artery to occlude blood supply to the lumbar
spinal cord. Following aortic declamping, the abdominal wall was
closed and the animal was permitted to regain consciousness.
Transient spinal cord ischemia was induced for 60 min, followed by
60 min of reperfusion. In the sham group, the animals underwent the
laparotomy only, with no aortic occlusion. In the SCII control
group, an IP injection of 0.9% sodium chloride was administered,
whereas in the treatment group, a dose of 10 mg/kg/day of
ginsenoside Rb1 was administered after 60 min of reperfusion. For
each group, rats were sacrificed at 1, 3, 5 and 7 days after the
procedure (n=6 per time-point) and the spinal cord was quickly
removed for further analyses.
Behavioral analysis of spinal cord
injury
To assess neurological deficits, locomotor activity
was examined and measured in all rats using the Basso Beattie
Bresnahan (BBB) rating scale (31). This commonly used method for
assessment of recovery from spinal cord injury provides a measure
of hindlimb nerve function by rating activity on a scale between 0
and 21, with a score of 21 indicating full recovery.
Morphological analysis
Spinal cord specimens were embedded in paraffin and
sectioned at 4 μm. Sections from the lumbar (L3) level of each
spinal cord were deparaffinized and underwent hematoxylin and eosin
(H&E) or Nissl staining. They were subsequently mounted on
slides and coverslipped with Permount (Thermo Fisher Scientific,
Waltham, MA, USA). Neuronal injury, cell morphology and Nissl
bodies were observed and evaluated under a light microscope
(magnification, ×400; CX21; Olympus Corp., Tokyo, Japan).
Quantification of apoptosis using the
terminal deoxynucleotidyl transferase dUTP nick end labeling
(TUNEL) technique
Paraffin-embedded spinal cord specimens were
sectioned (4 μm) and deparaffinized. Sections were then treated
with a TUNEL reaction mixture (Roche, Basel, Switzerland) for 60
min at 37°C followed by a 60 min incubation at 37°C in a
Converter-POD solution (Roche) and reacted with
3,3′-diaminobenzidine for visualization. Apoptotic cells were
observed with a light microscope (magnification, ×400) and appeared
as cells with brown stained nuclei, with normal cells appearing
blue. Six sections from each group were examined, with ~500 cells
counted on each section. The apoptotic rate was calculated based on
the number of TUNEL-positive cells out of the total number
counted.
Analysis of AQP4 protein level by western
blotting
Protein levels from spinal cord extracts were
analyzed using western blotting. Total protein was extracted from
the spinal cord tissue and 50 μg of the extract from each isolate
was separated on a 10% SDS-PAGE gel and transferred onto a
nitrocellulose filter (Sartorius, Goettingen, Germany) for
immunodetection. The filters were incubated with rabbit anti-rat
polyclonal AQP4 (1:1,000, ab46182) or β-actin (1:1,000; ab8227)
antibodies (Abcam, Cambridge, UK), followed by horseradish
peroxidase-conjugated goat anti-rabbit polyclonal IgG (1:800;
sc2004; Santa Cruz Biotechnology, Inc., Dallas, TX, USA).
Immunoreactive bands were visualized with enhanced
chemiluminescence (Santa Cruz Biotechnology, Inc.) followed by
optical density analysis.
Analysis of AQP4 protein level using
immunohistochemistry
Antigen retrieval was performed by incubating spinal
cord sections in a citrate-buffered saline solution (pH 6.0) at
98°C for 10 min. Subsequently, the sections were allowed to cool,
then rinsed for 3 min in phosphate-buffered saline (PBS) three
times, incubated with 1% Triton X-100 in PBS for 20 min and then
incubated with goat serum at 37°C for 60 min. Sections were rinsed
three times with PBS, followed by a 12 h incubation with rabbit
anti-rat polyclonal AQP4 antibody (1:500; ab46182) and a 60 min
incubation in goat anti-rabbit IgG heavy and light chain
DyLight®488 polyclonal secondary antibody (1:2,000;
ab96899; Abcam). Staining was visualized by fluorescence microscopy
(BX51TF; Olympus Corp.) and quantified by measuring the integrated
optical density using Image-Pro Plus 6.0 imaging software (Media
Cybernetics Inc., Rockville, MD, USA).
Analysis of AQP4 mRNA levels
Expression of AQP4 mRNA was measured in spinal cord
tissue samples using reverse transcription-quantitative polymerase
chain reaction (RT-qPCR). Total RNA was extracted from spinal cord
tissue with TRIzol reagent (Invitrogen Life Technologies, Carlsbad,
CA, USA) according to the manufacturer’s instructions. RNA was
converted into cDNA with M-MLV reverse transcriptase (Promega
Corporation, Madison, WI, USA). Quantitative detection of
transcripts was performed by RT-PCR with SYBR Green using the
following gene-specific primers for AQP4 and rat β-actin: AQP4,
sense 5′-ATT GGG AGT CAC CAC GGT TCA T-3′ and antisense 5′-TGG ATT
CAT GCT GGC TCC GGT AT-3′ (211 bp product) and β-actin, sense
5′-TCA CCC ACA CTG TGC CCA TCT ACG-3′ and antisense 5′-GGA TGC CAC
AGG ATT CCA TAC CCA-3′ (343 bp product). A total of 40
amplification cycles were performed, consisting of 94°C for 30 sec,
58°C (for AQP4) or 57°C (for β-actin) for 30 sec and 72°C for 40
sec. The results were analyzed using the 2−ΔΔCT method
(32) and values were normalized
and expressed relative to the blank group levels.
Statistical analysis
Data are presented as the mean ± standard deviation
or standard error of the mean. Differences between groups were
assessed by analysis of variance using SPSS 14.0 statistics
software (SPSS, Inc., Chicago, IL, USA). P<0.05 was considered
to indicate a statistically significant difference.
Results
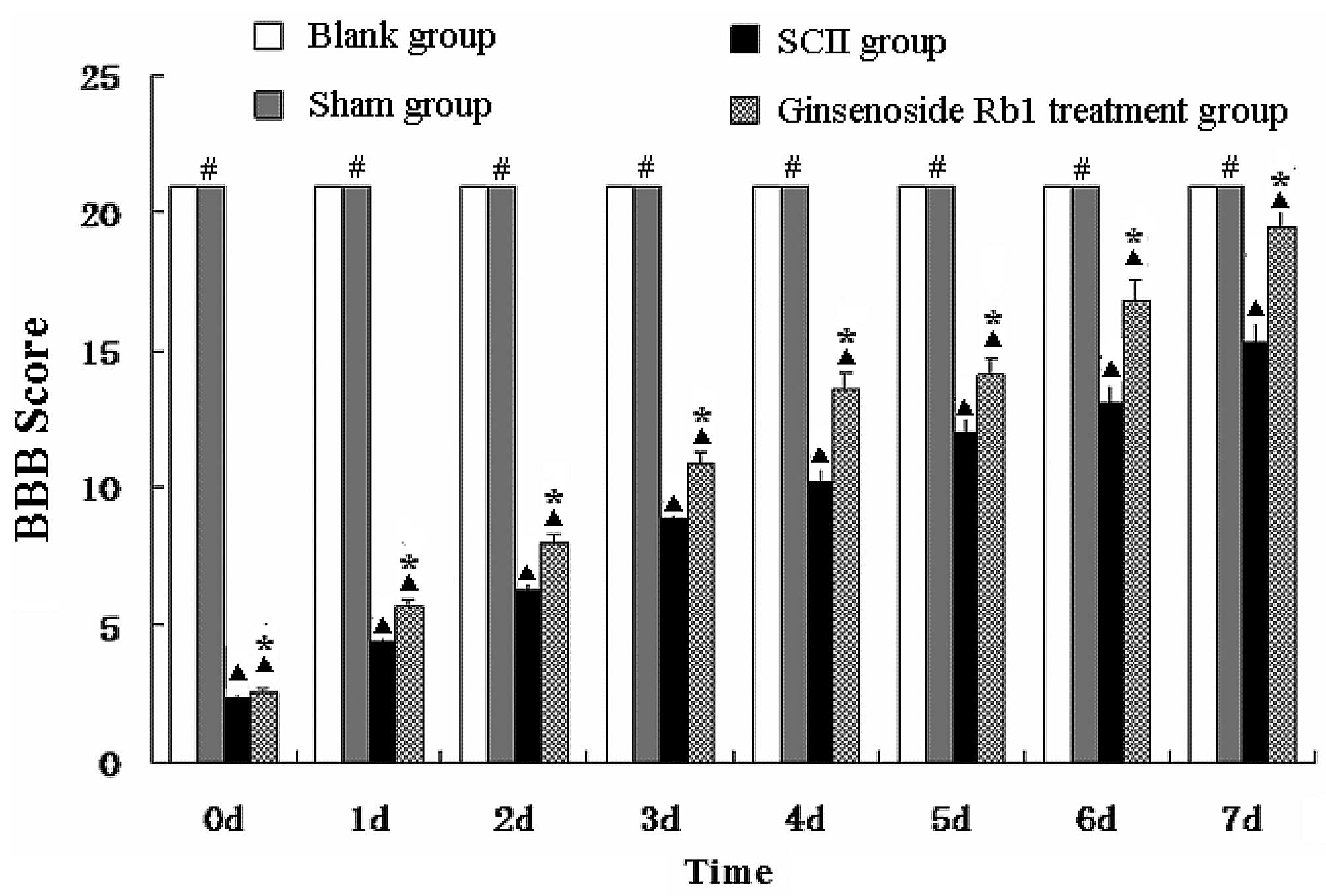
Ginsenoside Rb1 enhances hindlimb nerve
function in SCII rats
The BBB scores for the blank group and the sham
group were 21. The BBB scores of the untreated SCII group and
ginsenoside Rb1-treated SCII group (treatment group) at day 1 were
2.34±0.20 and 2.62±0.36, respectively, and then gradually recovered
reaching 15.42±1.36 and 18.73±1.55, respectively, at day 7. BBB
scores from the untreated SCII group were lower than those of the
sham group at all time points (P<0.05) and the BBB scores of the
treatment group were higher than those of the untreated SCII group
at all time points (P<0.05; Fig.
1).
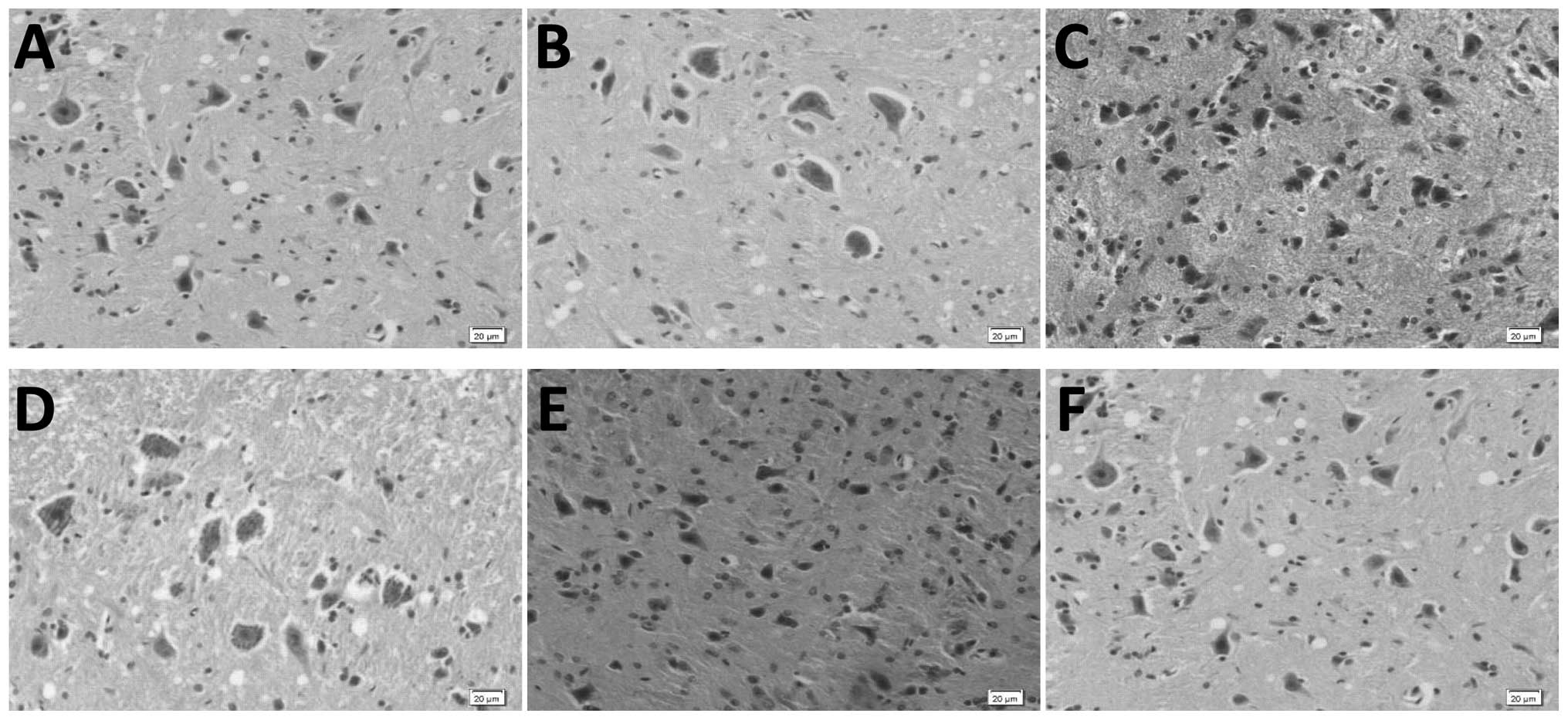
Ginsenoside Rb1 treatment alleviates
neuronal damage in SCII rats as observed by H&E staining
Microscopic analysis of H&E stained spinal cord
sections revealed normal morphology and clear cell structure in the
blank and sham group specimens. Certain neurons in the untreated
SCII group were damaged and enlarged, with pyknotic cell nuclei and
tissue interstitial hyperemia. These features were not observed in
specimens from ginsenoside Rb1-treated rats (Fig. 2).
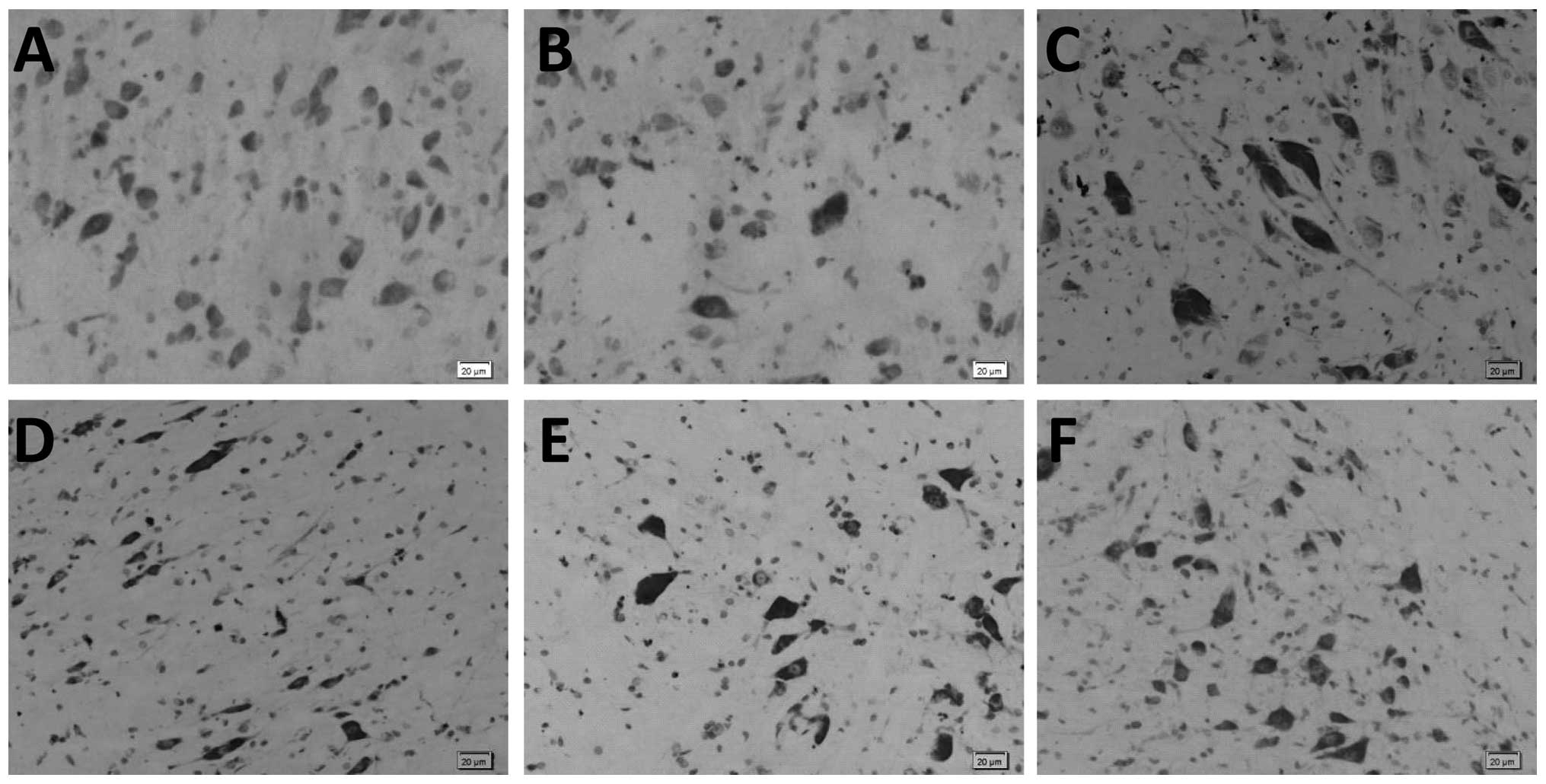
Ginsenoside Rb1 treatment alleviates
neuron damage in SCII rats as observed by Nissl staining
Microscopic analysis of Nissl stained spinal cord
sections demonstrated that the neurons in the blank and sham
control groups were morphologically normal. In sections from the
untreated SCII group, there was a decrease in the number of neurons
and Nissl body structure was unclear, loose and disorganized. In
addition, there was evidence of cytoplasmic condensation and
marginalization, as well as signs of neuron enlargement and unclear
nuclear structure. These appearances are indicative of cell and
tissue damage and were not observed in sections from ginsenoside
Rb1-treated rats (Fig. 3).
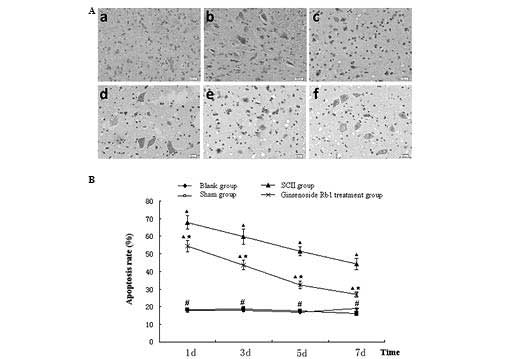
Ginsenoside Rb1 reduces apoptosis in SCII
rats
Following TUNEL staining of spinal cord sections,
the number of apoptotic cells was counted and the rate of apoptosis
was calculated based on the total number of cells (Fig. 4A). The apoptotic rates of the blank
(17.68±1.15%) and sham (18.21±1.26%) groups were lower than those
from the untreated (67.91±3.67%) and ginsenoside Rb1-treated
(54.36±3.26%) SCII groups at day 1(P<0.05). The apoptotic rate
of the treatment group was significantly lower than that of the
untreated SCII group (P<0.05). Ginsenoside Rb1 treatment
decreased the apoptotic rate of SCII between 44.38±3.17 and
26.96±1.44% at day 7 (P<0.05), however, remained higher than
that of the blank control group (18.82±1.16%; P<0.05; Fig. 4B).
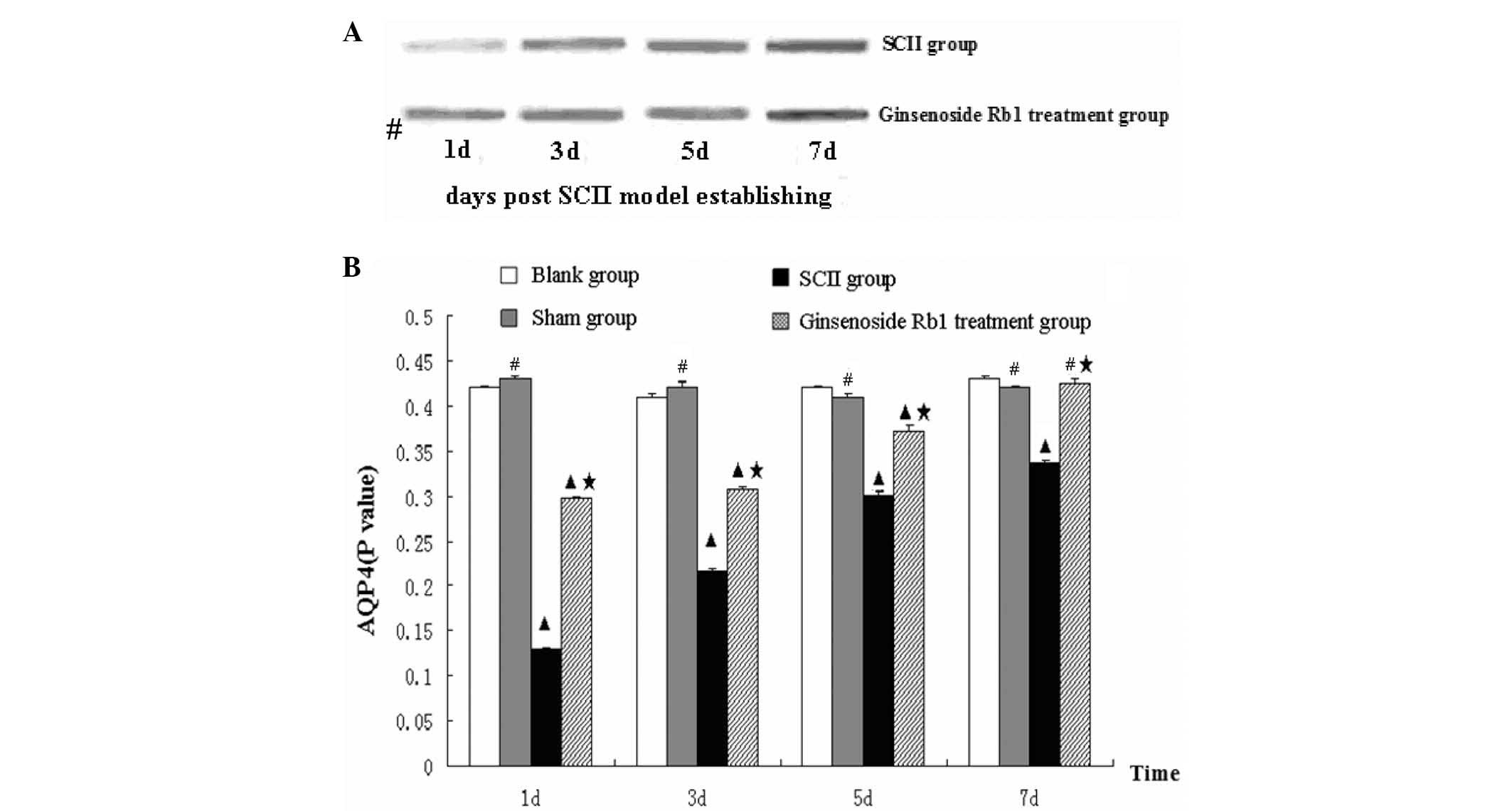
Ginsenoside Rb1 attenuates the decrease
in AQP4 protein expression in SCII rats as measured by western
blotting
Evaluation of AQP4 protein expression by western
blotting revealed that protein levels in the blank and sham control
groups were consistent and no significant differences were
detected, suggesting that the sham injury had no effect on spinal
cord AQP4 protein levels. Expression of AQP4 protein in the
untreated SCII group was significantly reduced to one third of the
level of the blank group and gradually increased at days 3, 5 and 7
after the procedure. Although the level at day 7 increased to 79.9%
of the level of the blank group, it remained lower than the levels
identified in the blank and sham groups at days 3, 5 and 7
(P<0.05). Treatment with ginsenoside Rb1 for 1 day significantly
increased AQP4 protein expression compared with the untreated SCII
group (P<0.05). While protein expression in the treated SCII
group was lower at day 1 compared with the blank and sham control
groups, it gradually increased after 3 and 5 days and recovered to
the blank control group level by day 7 (Fig. 5).
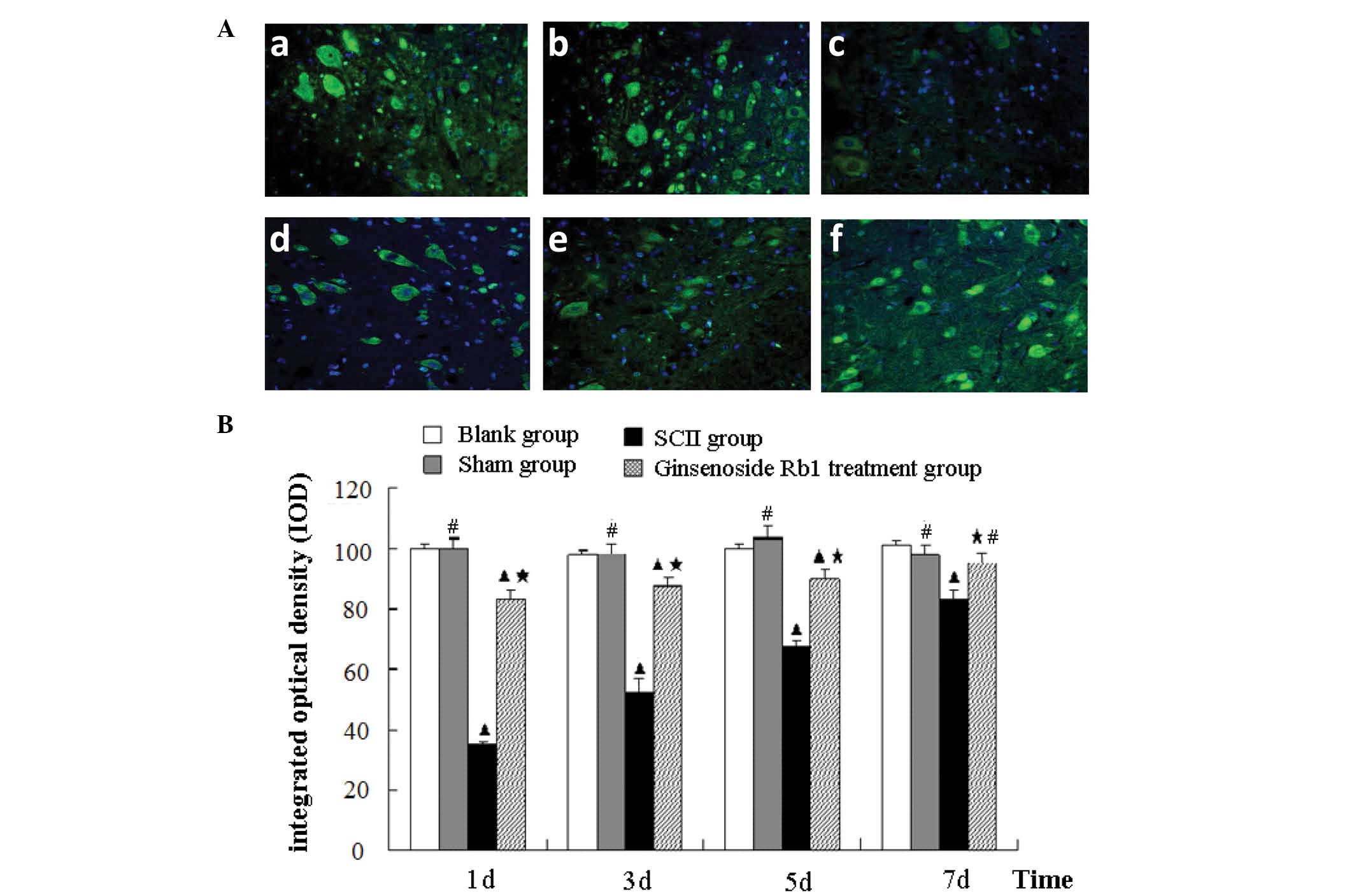
Ginsenoside Rb1 attenuates the decrease
in AQP4 protein expression in SCII rats as measured by
immunohistochemistry
Immunohistochemical analysis of AQP4 expression in
spinal cord sections identified no significant difference between
the blank and sham control groups. AQP4 immunofluorescence in the
untreated SCII group was significantly decreased to 36.9% of the
blank group level at day 1 (P<0.05). AQP4 immunofluorescence
gradually increased to 84.6% of the blank group level at day 7,
however, remained lower than the blank and sham control groups
(P<0.05). Treatment with ginsenoside Rb1 increased the quantity
of AQP4 immunofluorescence compared with the untreated SCII group
at all time points measured (P<0.05), but remained significantly
lower than the blank group on day 1, 3 and 5 (P<0.05). However,
after 7 days, levels of AQP4 immunofluorescence were similar to
those of the blank control group (Fig.
6).
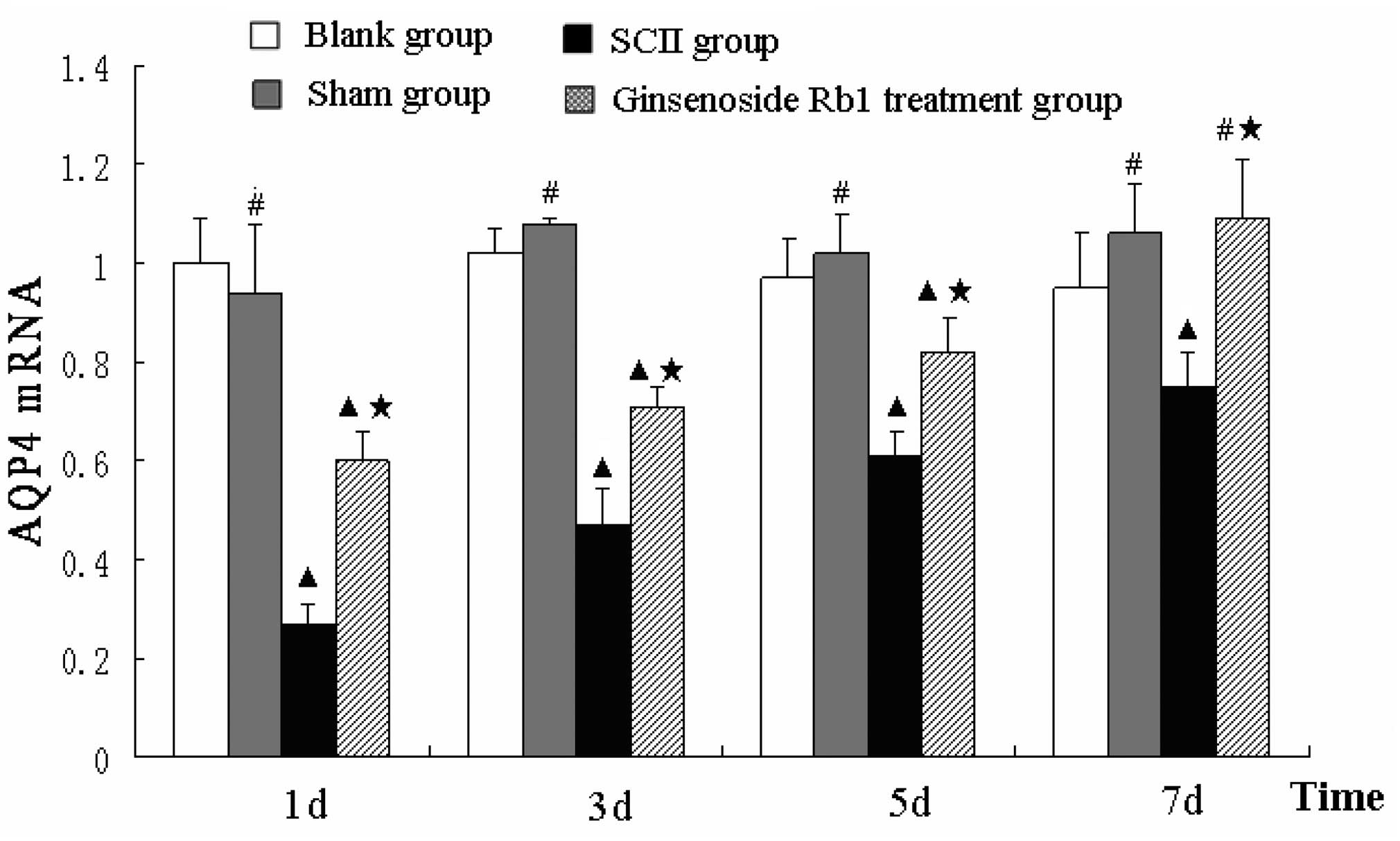
Ginsenoside Rb1 attenuates the decrease
in AQP4 transcription in SCII rats
Alterations in the expression levels of AQP4
transcripts following SCII were similar to the changes observed at
the protein level. The mRNA expression of AQP4 was not
significantly different between the sham and blank control groups.
AQP4 mRNA levels were lower in the untreated SCII group compared
with the blank group and while levels increased over time, they
remained lower than the blank group at day 7 (P<0.05). Treatment
with ginsenoside Rb1 attenuated the decrease in AQP4 mRNA levels in
SCII rats at day 1, 3 and 5 and promoted the recovery to control
group levels by day 7 (Fig.
7).
Discussion
AQP4 is a transmembrane protein widely distributed
throughout the spinal cord that acts as a bidirectional transporter
facilitating the movement of water molecules through the cell
membrane. A disruption in AQP4 expression may lead to a
dysregulation of water transport in regions of high expression,
including the spinal cord, resulting in edema. Spinal cord edema is
one of the most important factors contributing to SCII and the
degree of edema is associated with SCII prognosis (9,33).
Previous studies have found that the expression of AQP4 is
decreased in spinal cord injury, which may result in acute spinal
cord edema, ultimately affecting the normal function of the spinal
cord (9,34). While the exact mechanism of SCII
remains to be elucidated, spinal cord edema occurs at an early
phase and therefore the effects of AQP4 expression in the
development of edema and SCII are uncertain. However, it is known
that without early intervention, water imbalance and cell edema may
lead to cell necrosis and apoptosis (35). Therefore the maintenance of proper
cellular water balance is crucial and the identification of
mechanisms contributing to this is of importance. Therefore,
alterations in the expression of AQP4 may impart a mechanism for
the development of SCII and thus provide a novel target for
therapeutic intervention.
The present study found that AQP4 mRNA and protein
expression was reduced following SCII in rats. The levels of AQP4
expression increased over time, but remained significantly lower
than blank and sham control groups up to 7 days after the insult.
Concomitant with the initial reduction in AQP4 expression was a
loss of nerve function as evidenced by locomotor scores. In
addition, as recovery of nerve function was accompanied by an
increase in AQP4 protein expression, an association between AQP4
expression and normal nerve function was indicated. Therefore, it
was suggested that manipulations or treatments resulting in an
increase in AQP4 expression may provide a viable therapeutic
intervention to increase functional recovery and prognosis
following SCII.
As treatment with ginsenoside Rb1 has previously
been demonstrated to have therapeutic potential in the treatment of
ischemic injuries, its effect on SCII was investigated. Treatment
with ginsenoside Rb1 significantly increased locomotor scores in
rats subjected to SCII. In addition, morphological analysis of
spinal cords revealed that ginsenoside Rb1 treatment significantly
reduced signs of cellular damage caused by SCII, as well as
significantly reducing levels of neuronal apoptosis. Finally, the
current results demonstrated that SCII markedly reduced AQP4
expression and treatment with ginsenoside Rb1 attenuated this
reduction, promoting recovery to near-normal levels by 7 days
post-SCII. Therefore, the reduction in AQP4 expression caused by
SCII may be partially corrected with ginsenoside Rb1 treatment.
In conclusion, the results demonstrate that SCII
involves the abnormal expression of AQP4 and that treatment with
ginsenoside Rb1 inhibits this change, improves impaired nerve
function and prevents abnormalities in spinal cord nerve cell
morphology. The present results provide a mechanism and new
therapeutic option for the clinical treatment of SCII. Further
investigation into the safety and efficacy of ginsenoside Rb1
treatment is required, including investigation into other clinical
therapeutic approaches based on Traditional Chinese Medicine.
Acknowledgements
This study was sponsored by a grant awarded to Dr
Fei Huang from the National Natural Science Foundation of China
(grant no. 81301034).
References
|
1
|
Wang JY, Shen J, Gao Q, et al: Ischemic
postconditioning protects against global cerebral
ischemia/reperfusion-induced injury in rats. Stroke. 39:983–990.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Omura A and Okita Y: Surgical treatment of
thoracoabdominal aortic aneurysm. Kyobu Geka. 65:67–79.
2012.PubMed/NCBI
|
|
3
|
Xing B, Chen H, Zhang M, et al: Ischemic
postconditioning inhibits apoptosis after focal cerebral
ischemia/reperfusion injury in the rat. Stroke. 39:2362–2369. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Park ES, Gao X, Chung JM and Chung K:
Levels of mitochondrial reactive oxygen species increase in rat
neuropathic spinal dorsal horn neurons. Neurosci Lett. 391:108–111.
2006. View Article : Google Scholar
|
|
5
|
Varija D, Kumar KP, Reddy KP and Reddy VK:
Prolonged constriction of sciatic nerve affecting oxidative
stressors & antioxidant enzymes in rat. Indian J Med Res.
129:587–592. 2009.PubMed/NCBI
|
|
6
|
Ning N, Dang X, Bai C, Zhang C and Wang K:
Panax notoginsenoside produces neuroprotective effects in rat model
of acute spinal cord ischemia-reperfusion injury. J Ethnopharmacol.
139:504–512. 2012. View Article : Google Scholar
|
|
7
|
Oshio K, Binder DK, Yang B, Schecter S,
Verkman AS and Manley GT: Expression of aquaporin water channels in
mouse spinal cord. Neuroscience. 127:685–693. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rash JE, Yasumura T, Hudson CS, Agre P and
Nielsen S: Direct immunogold labeling of aquaporin-4 in square
arrays of astrocyte and ependymocyte plasma membranes in rat brain
and spinal cord. Proc Natl Acad Sci USA. 95:11981–11986. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nesic O, Lee J, Ye Z, et al: Acute and
chronic changes in aquaporin 4 expression after spinal cord injury.
Neuroscience. 143:779–792. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Saadoun S, Bell BA, Verkman AS and
Papadopoulos MC: Greatly improved neurological outcome after spinal
cord compression injury in AQP4-deficient mice. Brain.
131:1087–1098. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hinson SR, McKeon A and Lennon VA:
Neurological autoimmunity targeting aquaporin-4. Neuroscience.
168:1009–1018. 2010. View Article : Google Scholar
|
|
12
|
Nicaise C, Soyfoo MS, Authelet M, et al:
Aquaporin-4 overexpression in rat ALS model. Anat Rec (Hoboken).
292:207–213. 2009. View
Article : Google Scholar
|
|
13
|
Radad K, Gille G, Liu L and Rausch WD: Use
of ginseng in medicine with emphasis on neurodegenerative
disorders. J Pharmacol Sci. 100:175–186. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhao J, Su C, Yang C, et al: Determination
of ginsenosides Rb1, Rb2 and Rb3 in rat plasma by a rapid and
sensitive liquid chromatography tandem mass spectrometry method:
Application in a pharmacokinetic study. J Pharm Biomed Anal.
64–65:94–97. 2012. View Article : Google Scholar
|
|
15
|
Wu L, Jin Y, Yin C and Bai L:
Co-transformation of Panax major ginsenosides Rb1 and
Rg1 to minor ginsenosides C-K and F1 by
Cladosporium cladosporioides. J Ind Microbiol Biotechnol.
39:521–527. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee JG, Baek SH, Lee YY, Park SY and Park
JH: Anti-complementary ginsenosides isolated from processed
ginseng. Biol Pharm Bull. 34:898–900. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kwok HH, Guo GL, Lau JK, et al:
Stereoisomers ginsenosides-20(S)-Rg3 and
-20(R)-Rg3 differentially induce angiogenesis through
peroxisome proliferator-activated receptor-gamma. Biochem
Pharmacol. 83:893–902. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yuan HD, Kim do Y, Quan HY, Kim SJ, Jung
MS and Chung SH: Ginsenoside Rg2 induces orphan nuclear receptor
SHP gene expression and inactivates GSK3β via AMP-activated protein
kinase to inhibit hepatic glucose production in HepG2 cells. Chem
Biol Interact. 195:35–42. 2012. View Article : Google Scholar
|
|
19
|
Ha SE, Shin DH, Kim HD, et al: Effects of
ginsenoside Rg2 on the ultraviolet B-induced DNA damage responses
in HaCaT cells. Naunyn Schmiedebergs Arch Pharmacol. 382:89–101.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jiang JW, Chen XM, Chen XH and Zheng SS:
Ginsenoside Rg3 inhibit hepatocellular carcinoma growth via
intrinsic apoptotic pathway. World J Gastroenterol. 17:3605–3613.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Poon PY, Kwok HH, Yue PY, et al:
Cytoprotective effect of 20S-Rg3 on benzo[a]pyrene-induced DNA
damage. Drug Metab Dispos. 40:120–129. 2012. View Article : Google Scholar
|
|
22
|
Lee KT, Jung TW, Lee HJ, Kim SG, Shin YS
and Whang WK: The antidiabetic effect of ginsenoside Rb2 via
activation of AMPK. Arch Pharm Res. 34:1201–1208. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Quan LH, Min JW, Sathiyamoorthy S, Yang
DU, Kim YJ and Yang DC: Biotransformation of ginsenosides Re and
Rg1 into ginsenosides Rg2 and Rh1 by recombinant β-glucosidase.
Biotechnol Lett. 34:913–917. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cheng B, Li J, Du J, Lv X, Weng L and Ling
C: Ginsenoside Rb1 inhibits osteoclastogenesis by modulating NF-κB
and MAPKs pathways. Food Chem Toxicol. 50:1610–1615. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tan S, Zhou F, Yu Z, et al: Study on
characteristics of energy metabolism in skeletal muscle of rats
with postoperative fatigue syndrome and interventional effect of
ginsenoside Rb1. Zhongguo Zhong Yao Za Zhi. 36:3489–3493. 2011.(In
Chinese).
|
|
26
|
Liu DH, Chen YM, Liu Y, et al: Rb1
protects endothelial cells from hydrogen peroxide-induced cell
senescence by modulating redox status. Biol Pharm Bull.
34:1072–1077. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lin ZY, Chen LM, Zhang J, et al:
Ginsenoside Rb1 selectively inhibits the activity of L-type
voltage-gated calcium channels in cultured rat hippocampal neurons.
Acta Pharmacol Sin. 33:438–444. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhu J, Jiang Y, Wu L, Lu T, Xu G and Liu
X: Suppression of local inflammation contributes to the
neuroprotective effect of ginsenoside Rb1 in rats with cerebral
ischemia. Neuroscience. 202:342–351. 2012. View Article : Google Scholar
|
|
29
|
Xia R, Zhao B, Wu Y, et al: Ginsenoside
Rb1 preconditioning enhances eNOS expression and attenuates
myocardial ischemia/reperfusion injury in diabetic rats. J Biomed
Biotechnol. 2011:7679302011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bowes MP, Masliah E, Otero DA, Zivin JA
and Saitoh T: Reduction of neurological damage by a peptide segment
of the amyloid beta/A4 protein precursor in a rabbit spinal cord
ischemia model. Exp Neurol. 129:112–119. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Basso DM, Beattie MS, Bresnahan JC, et al:
MASCIS evaluation of open field locomotor scores: effects of
experience and teamwork on reliability. Multicenter Animal Spinal
Cord Injury Study. J Neurotrauma. 13:343–359. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
33
|
Nesic O, Guest JD, Zivadinovic D, et al:
Aquaporins in spinal cord injury: the janus face of aquaporin 4.
Neuroscience. 168:1019–1035. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bloch O, Papadopoulos MC, Manley GT and
Verkman AS: Aquaporin-4 gene deletion in mice increases focal edema
associated with staphylococcal brain abscess. J Neurochem.
95:254–262. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li L, Zhang H, Varrin-Doyer M, Zamvil SS
and Verkman AS: Proinflammatory role of aquaporin-4 in autoimmune
neuroinflammation. FASEB J. 25:1556–1566. 2011. View Article : Google Scholar : PubMed/NCBI
|