Introduction
Concanavalin A (ConA) is a lectin that induces
hepatitis through the modulation of various immune cells, including
macrophages, T cells, natural killer (NK) cells and liver
sinusoidal endothelial cells (LSECs) (1–4).
Initially, intravenously injected ConA binds predominantly to the
mannose gland of the LSEC surface and subsequently leads to the
breakdown of the LSECs (5–7). Damaged LSECs produce inflammatory
cytokines and chemokines, and LSEC detachment facilitates the
binding of ConA to macrophages. T cells recognize the ConA-modified
major histocompatibility complex class II and T cell receptor (TCR)
of macrophages and are subsequently activated (4,8,9). In
addition, these immune cells produce various inflammatory cytokines
and chemokines, including interferon-γ (IFN-γ) (10,11),
interleukin-2 (IL)-2 (11), IL-4
(12), IL-6 (12), C-C chemokine ligand 2 (CCL2)
(13) and CXC-motif chemokine
ligand 12 (9). Anti-inflammatory
cytokines, including IL-10, are also involved in ConA-induced
hepatitis. Among these biologically active substances, IL-10 has
been reported to protect the liver from ConA-induced hepatitis
(14,15).
Previous findings in glycobiology have provided
direct evidence of the involvement of oligosaccharide changes in
human diseases (16).
Oligosaccharide modification of glycoproteins is predominantly
divided into two types: N-glycans, attached to the
asparagine residues, and O-glycans, attached to the
serine/threonine residues (17)
The branching formation on N-glycans is one of the most
important factors regulating the biological functions of
oligosaccharides and terminal modifications, including sialylation
and fucosylation (17). The
branching formation on N-glycans is regulated by several
types of N-acetylglucosaminyltransferases and the
upregulation or downregulation of these glycosyltransferases may
modify the biological functions of adhesion molecules and the
signaling pathways of several growth factor receptors (18).
N-Acetylglucosaminyltransferase V (GnT-V) is
involved in the synthesis of β1–6 GlcNAc branching formation on
N-glycans (19). It is
well-established that GnT-V is one of the most important
glycosyltransferases involved in cancer metastasis (20), promoting cancer metastasis through
the enhancement of growth factor signaling, integrin function and
the expression of certain types of proteases (19–21).
GnT-V also has important functions in the immune system. Deficiency
of GnT-V in mice leads to an autoimmune disease phenotype and
GnT-V-induced TCR oligosaccharide modification suppresses TCR
signaling (22,23). These findings indicate that GnT-V
decreases inflammatory responses through suppression of T cell
activation. While the expression of GnT-V is low in the normal
liver, it is increased during the progression of chronic disease
and liver regeneration (24,25).
Our previous study indicated that the expression of GnT-V in the
normal mouse liver is higher in hepatic non-parenchymal cells,
including immune cells, compared with hepatocytes (26). These findings indicate that GnT-V
is important in the progression of liver diseases.
Considering these findings, GnT-V is expected to be
important in the progression of liver disease through modulation of
the immune system. However, the significance of changes in
GnT-V-induced glycosylation in liver diseases remains to be
elucidated. To address this issue, the present study investigated
the role of GnT-V in experimental immune hepatitis using a mouse
ConA hepatitis model.
Materials and methods
Mice
GnT-V (Mgat5) transgenic (Tg) mice (β-actin
promoter; C57BL/6J background) were produced, as previously
described (27). In the present
study, wild type (WT) litter-mates were used as control mice. The
animals were provided with unrestricted access to food and water,
housed in temperature- and humidity-controlled rooms and maintained
in a 12/12 h light/dark cycle. All experiments were performed using
8–12 week old male mice. At the end of each experimental period,
blood was drawn aseptically from the inferior vena cava and
centrifuged at 13,000 × g for 5 min at 4°C to collect the serum.
Mice were anesthetized via intraperitoneal injection of
pentobarbital (50 mg/kg), and the livers were subsequently removed
and fixed with 10% buffered paraformaldehyde (Wako Pure Chemical
Industries, Ltd., Tokyo, Japan), flash frozen in liquid nitrogen
for protein and mRNA extraction was performed as described
previously (26). All experimental
procedures described in the present study were approved by the
Ethics Review Committee for Animal Experimentation of the Osaka
University School of Medicine (Osaka, Japan).
ConA-induced hepatitis
ConA (Sigma-Aldrich, St. Louis, MO, USA) at 12.5
mg/kg body weight (BW) was dissolved in 200 μl phosphate-buffered
saline (PBS; Sigma-Aldrich) and injected into WT and GnT-V Tg mice
through the tail vein. Serum alanine aminotransferase (ALT)
concentrations were measured using a Transaminase CII-test Wako kit
(Wako Pure Chemical Industries, Tokyo, Japan). To examine the
survival rate of the rats, a relatively high dose of ConA (20
mg/kg/BW) was injected intravenously.
Hematoxylin and eosin (H&E),
terminal deoxynucleotidyl transferase dUTP nick end labeling
(TUNEL) and immunohistochemical staining
Liver sections were stained with H&E, Apop
Tag® Peroxidase in Situ Apoptosis Detection kit,
according to the manufacturer’s instructions (Merck Millipore,
Darmstadt, Germany), or monoclonal human anti-F4/80 antibody
(1:100; HCA154; Bio-Rad AbD Serotec, Oxford, UK).
Cytokine analysis
Splenic lymphocytes were collected, as previously
described (28). The cells
(1×106 cells) were cultured in flat-bottom 96-well
culture plates for 48 h in RPMI-1640 (Sigma-Aldrich) with 10% fetal
bovine serum (Sigma-Aldrich) and antibiotics/antimycotics in the
presence of anti-mouse cluster of differentiation (CD)3 (5 μg/ml)
and anti-mouse CD28 antibodies (5 μg/ml; BD Biosciences, San Jose,
CA, USA). The culture supernatant was collected and production of
the IFN-γ and IL-10 cytokines were determined by enzyme-linked
immunosorbent assay (ELISA; eBioscience, San Diego, CA, USA),
according to the manufacturer’s instructions. The levels are
expressed as the mean ± standard deviation of 1×106
cells. These cells (3×106 cells) were also cultured in
flat-bottom 96-well culture plates for 24 h in RPMI-1640 with 10%
fetal bovine serum and antibiotics/antimycotics in the presence of
ConA (5 μg/ml). The culture supernatant was collected and the
production of IFN-γ and IL-10 cytokines was determined by ELISA
(eBioscience), according to the manufacturer’s instructions. The
levels are expressed as the mean ± standard deviation of
3×106 cells.
Quantification of gene expression
levels
Total RNA was extracted from cells with a
QIAshredder and an RNeasy Mini kit, according to the manufacturer’s
instructions (Qiagen, Hilden, Germany) and transcribed into
complementary DNA with a ReverTra Ace qPCR RT kit (Toyobo, Osaka,
Japan). Reverse transcription quantitative polymerase chain
reaction was performed with a Thunderbird SYBR qPCR mix (Toyobo)
using specific primers on a LightCycler according to the
instructions provided by the manufacturer (Roche Diagnostics,
Indianapolis, IN, USA). The cycling conditions were as follows:
95°C for 30 sec, 40 cycles of 95°C for 5 sec and 60°C for 30 sec.
The primers used were as follows: Ifn-γ (cat. no.
QT01038821), Il-10 (cat. no. QT00106169), Ccl2 (cat
no. QT01747165), Ccl5 (cat. no. QT00167832) and 18s
rRNA (cat. no. QT01036875; Qiagen). The primers for T-bet,
Gata-3 and Galectin-3 were purchased from Sigma-Aldrich
and the sequences were as follows: T-bet, sense 5′-GCC AGG
GAA CCG CTT ATA TG-3′ and antisense 5′-GAC GAT CAT CTG GGT CAC ATT
GT-3′; Gata-3, sense 5′-TTA TCA AGC CCA AGC GAA G-3′ and
antisense 5′-TGG TGG TGG TCT GAC AGT TC-3′ and Galectin-3,
sense 5′-CAG GAT TGT TCT AGA TTT CAG G-3′ and antisense 5′-TTG TCC
TGC TTC GTG TTA CAC-3′. The mRNA expression levels were normalized
to the mRNA expression level of 18s and expressed in
arbitrary units.
Isolation of mouse LSECs
LSECs were isolated from WT and GnT-V Tg mice by
performing a two-step collagenase-pronase perfusion of their
livers, as described previously (29). Briefly, the livers were perfused
for 3 min at room temperature at a flow rate of 4 ml/min with SC-1
solution containing 8,000 mg/l NaCl, 400 mg/l KCl, 78 mg/l
NaH2PO4 2H2O, 151 mg/l
Na2HPO4 12H2O, 2380 mg/l
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), 350
mg/l NaHCO3, 190 mg/l ethylene glycol tetraacetic acid
and 900 mg/l glucose (pH 7.2), followed by digestion at 37°C for 3
min with 0.053% pronase and 0.027% collagenase dissolved in an SC-2
solution containing 8,000 mg/l NaCl, 400 mg/l KCl, 78 mg/l
NaH2PO4 2H2O, 151 mg/l
Na2HPO4 12H2O, 2,380 mg/l HEPES,
350 mg/l NaHCO3 and 735 mg/l CaCl2
2H2O (pH 7.5). Each digested liver was excised and cut
into 2-mm sections. The resulting suspension was filtered through a
100 μm cell strainer and centrifuged at 50 × g for 1 min at 4°C to
remove hepatocytes. This protocol was repeated three times and the
supernatants were centrifuged at 300 × g for 10 min at 4°C. The
pellet was washed and suspended in Dulbecco’s modified Eagle’s
medium twice. Non-parenchymal cells were further separated from
parenchymal cells by density-gradient centrifugation at 1,500 × g
for 20 min at 4°C on a 30% Histodenz cushion (Sigma-Aldrich). The
LSECs were then isolated by magnetic cell sorting using magnetic
beads (MACS; Miltenyi Biotec, Gladbach, Germany) with rat
anti-mouse CD31 antibody (11-0311-85; eBioscience), according to
the manufacturer’s instructions. The purity of the MACS-enriched
LSECs was assessed by flow cytometric analysis with rat anti-mouse
CD31 antibody (11-0311-85; eBioscience) using a FACS Canto II (BD
Biosciences).
Western and lectin blot analyses
Immunoblotting was performed, as described
previously (26). Briefly,
(2×106) isolated mouse LSECs were lysed with 1% Triton
X-100 and ~10 mg frozen liver tissue was then lysed with lysis
buffer containing 10 mM Tris-HCl (pH 7.4), 150 mM NaCl, 1% NP-40,
0.1% sodium deoxycholate and 0.1% sodium dodecyl sulfate (SDS). The
samples were then subjected to heat denaturation at 98°C for 5 min,
separated with SDS-polyacrylamide gel electrophoresis and
transferred onto a polyvinylidene fluoride membrane (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). The membrane was then
blocked with either 5% skimmed milk for western blotting or 3%
bovine serum albumin for lectin blotting. The following antibodies
were used for immunodetection: Rabbit polyclonal anti-GnT-V (24D11;
1:3,000; Fujirebio, Tokyo, Japan), leukoagglutinating
phytohemagglutinin (L4-PHA) lectin (1:1,000; cat. no.
J112; J-Oil Mills, Inc., Tokyo, Japan), ConA lectin (1:1,000; cat.
no. J103; J-Oil Mills, Inc.) and rabbit polyclonal anti-GAPDH
(1:3,000; cat. no. 2275-PC-1; Trevigen, Gaithersburg, MD, USA).
Immunoreactive bands were visualized on an GE Healthcare film using
Amersham Enhanced Chemiluminscence Western Blotting Detection
reagents (GE Healthcare, Waukesha, WI, USA).
Isolation of mouse hepatocytes and liver
mononuclear cells (MNCs)
Mouse hepatocytes and MNCs from the liver were
prepared, as previously described (30). Briefly, the mice were anesthetized
and their abdomens were opened. The inferior vena cava and portal
vein were cut to enable blood outflow. The liver was removed and
gently passed through a stainless steel mesh. The liver cell
suspension was collected and the hepatocytes were separated from
the MNCs by centrifugation at 50 × g for 1 min. This procedure was
repeated three times and the supernatants were centrifuged at 150 ×
g for 7 min at 4°C. The MNC populations were purified by
centrifugation through a Percoll gradient. The cells were
collected, washed in PBS and resuspended in 40% Percoll
(Sigma-Aldrich). The cell suspension was gently overlaid onto 70%
Percoll (Sigma-Aldrich) and centrifuged for 15 min at 1,400 × g.
The MNCs were collected from the interface and were washed twice in
PBS for use in subsequent analysis.
Flow cytometric analysis of hepatic MNCs
and LSECs
ConA (12.5 mg/kg/BW; Sigma-Aldrich) dissolved in 200
μl PBS was injected into the WT and GnT-V Tg mice through the tail
vein. After 2 h, the liver MNCs were isolated, as described above.
The liver MNCs, stained with monoclonal rat anti-mouse CD4
(4800041-82), rat anti-mouse CD8α (11-0081-82), mouse anti-mouse
CD19 (17-0191-829, rat anti-mouse CD11b (12-0112-82), hamster
anti-mouse CD11c (48-0114-82), and mouse anti-mouse NK1.1
(17-5941-82) antibodies (eBioscience) and the LSECs, stained with
anti-CD31 antibody, fluorescein isothiocyanate-labeled
L4-PHA (J512) and ConA (J503) (J-Oil Mills Inc.) were
subjected to flow cytometric analysis using a FACS Canto II™ (BD
Biosciences) flow cytometer. Data were analyzed using FlowJo
software version 7.6.1 (TreeStar Inc., Ashland, OR, USA). The
detailed procedure has been described previously (31).
Depletion of macrophages
The suicidal liposome technique has been used
previously to deplete macrophages (32). Clodronate-liposomes were purchased
from Clodronate Liposomes, (VUmc FdG, Amsterdam, Netherlands).
Briefly, the mice were injected with 200 μl clodronate-liposomes
through the tail vein. The injection was performed 2 days prior to
the ConA administration.
Statistical analysis
Statistical analysis was performed using JMP Pro
10.0 software (SAS Institute Inc., Cary, NC, USA). Kaplan-Meier
curves were used to demonstrate the survival rates. The results are
expressed as the mean ± standard deviation. Groups of data were
compared by the Wilcoxon test for non-parametric data. P<0.05
was considered to indicate a statistically significant
difference.
Results
ConA-induced hepatic injury is
exacerbated in GnT-V Tg mice compared with WT mice in vivo
ConA (12.5 mg/kg/BW) was injected intravenously into
WT and GnT-V Tg mice. Histological analyses of the liver tissue
sections indicated that the GnT-V Tg mice were more sensitive to
ConA-induced hepatic injury (Fig.
1A). Liver tissue sections in the GnT-V Tg mice exhibited more
widespread necrotic areas compared with the WT mice. In addition,
liver tissue sections from the GnT-V Tg mice exhibited increased
numbers of TUNEL-positive cells (Fig.
1B). The serum ALT levels 24 h after ConA injection were
significantly higher in the GnT-V Tg mice compared with those in
the WT mice (Fig. 1C). To compare
the survival rate of each mouse following ConA administration, a
relatively high dose of ConA (20 mg/kg/BW) was injected into each
mouse. A significantly higher mortality rate was identified in the
GnT-V Tg mice compared with the WT mice (Fig. 1D). In total, >40% of the GnT-V
Tg mice succumbed to mortality within 6 h following ConA injection,
with a survival rate of 14% observed 48 h after injection. By
contrast, a survival rate of 86% was observed in the WT mice after
48 h.
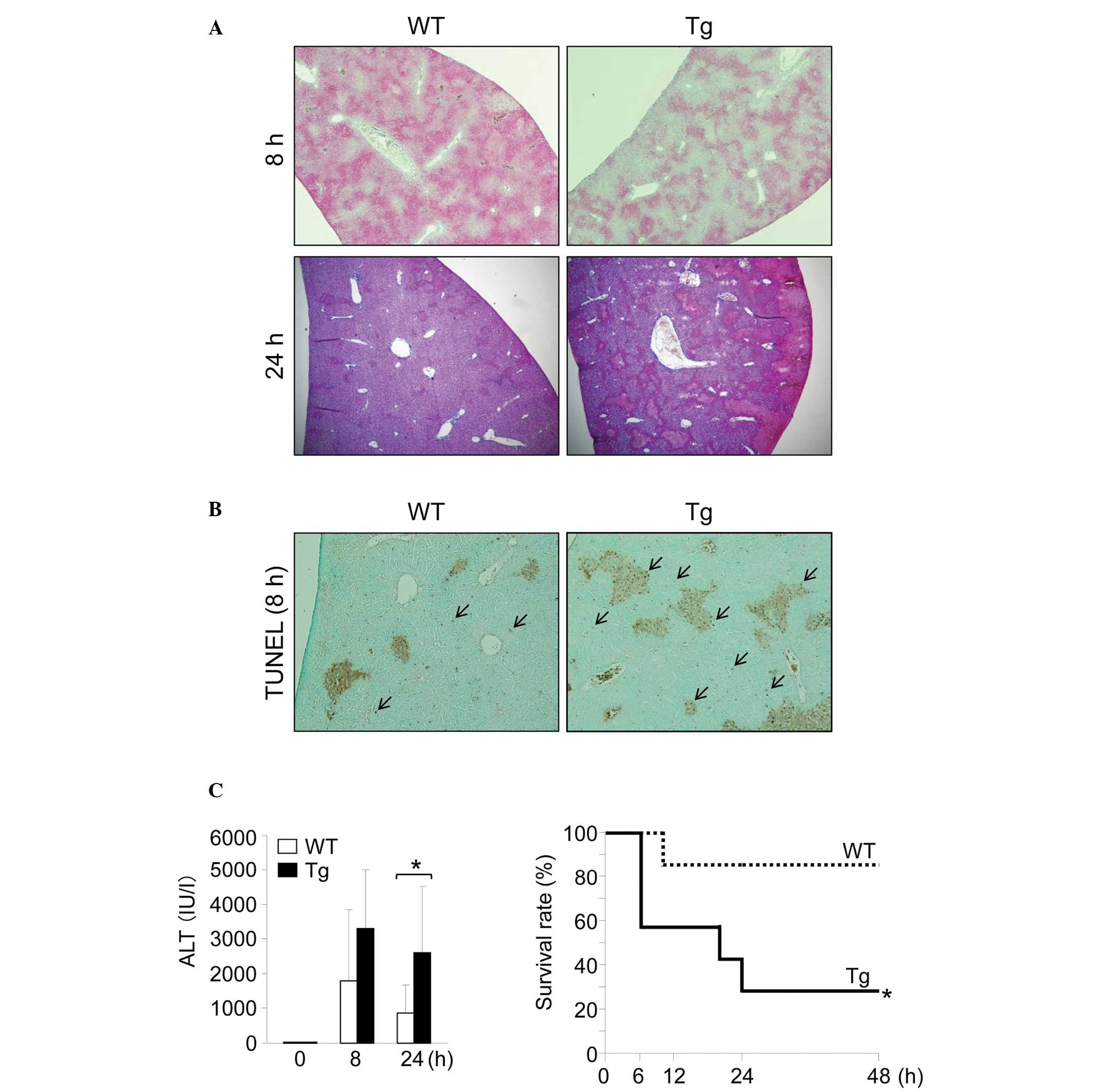 | Figure 1GnT-V Tg mice are sensitive to
ConA-induced hepatic injury. (A) Photomicrographs of representative
hematoxylin and eosin-stained mouse livers 8 or 24 h after the
injection of ConA at 12.5 mg/kg BW (magnification, ×40). (B)
Photomicrographs of representative TUNEL-stained mouse livers 8 h
after the injection of ConA at 12.5 mg/kg BW. Arrows indicate
apoptotic hepatocytes (magnification, ×40). (C) Serum ALT levels in
mice 0 (n=3), 8 (n=8–9) and 24 (n=7–8) h after the injection of
ConA at 12.5 mg/kg BW. Results are expressed as the mean ± standard
deviation; *P<0.05. The survival rate following ConA
injection. Mice were injected intravenously with ConA at 20 mg/kg
BW and followed for 48 h (n=7); *P<0.05. GnT-V,
N-acetylglucosaminyltransferase V; WT, wild-type; Tg,
transgenic; ConA, concanavalin A; BW, body weight; ALT, alanine
aminotransferase TUNEL, terminal deoxynucleotidyl transferase dUTP
nick end labeling. |
Subsequently, the gene expression of
inflammation-associated cytokines and transcription factors in the
mouse livers were investigated. No significant differences were
identified in the expression levels of the Th1-associated
(Ifn-γ and T-bet) or Th2-associated (Il-10 and
Gata-3) genes in the mouse livers prior to or following
injection of ConA (Fig. 2A). The
serum levels of IFN-γ and IL-10 in the GnT-V Tg mice 8 h after the
injection of ConA were significantly lower compared with those in
the WT mice (Fig. 2B).
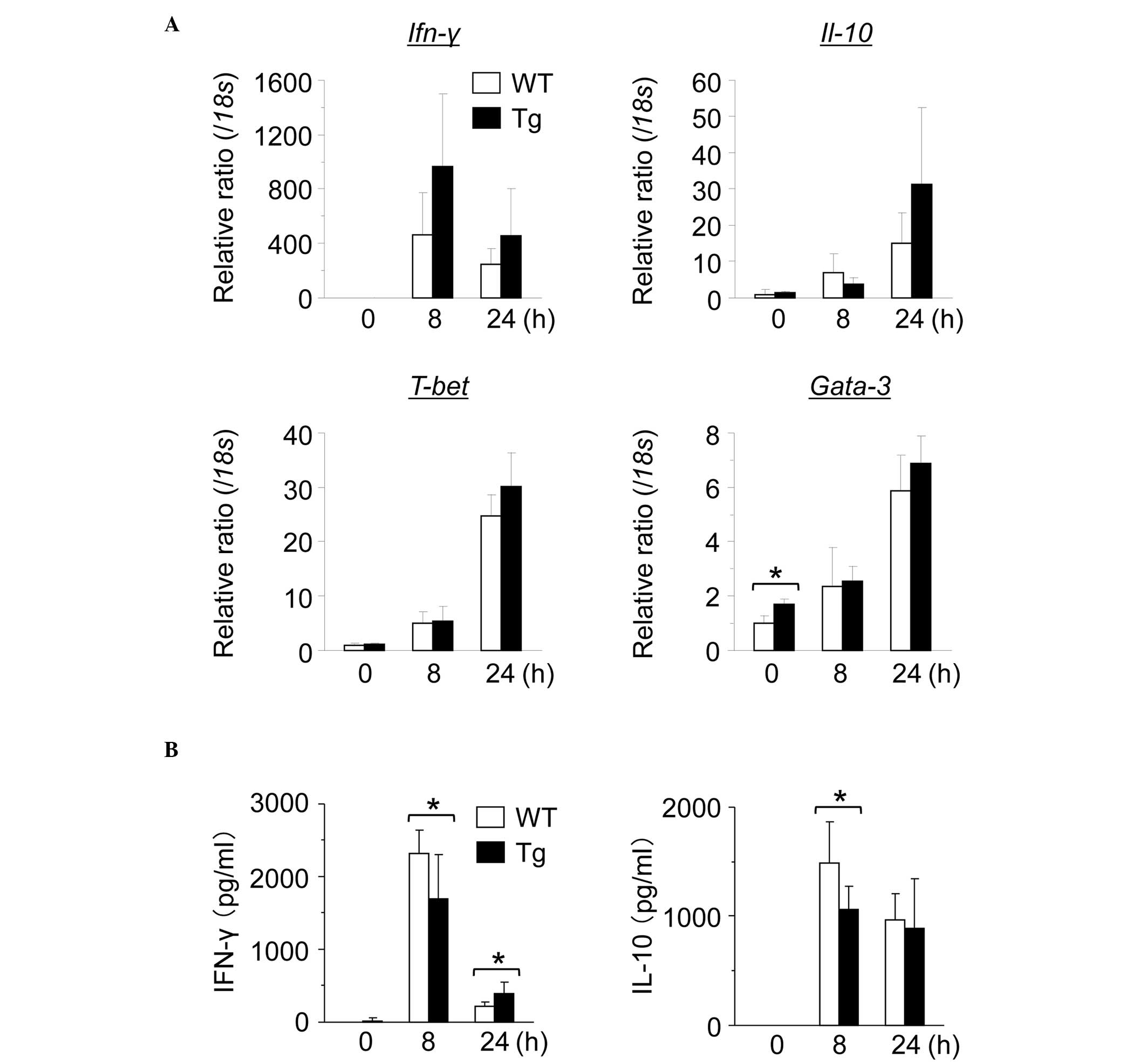 | Figure 2Expression of in
vivo-activated cytokines demonstrates no significant changes.
Hepatic gene expression in WT and GnT-V Tg mice 0, 8, or 24 h after
the injection of ConA at 12.5 mg/kg BW. Gene expression of (A)
Ifn-γ, Il-10, T-bet and Gata-3 in mouse livers was
evaluated using reverse transcription quantitative polymerase chain
reaction. The mRNA expression levels were normalized relative to
the mRNA expression of 18s and are expressed in arbitrary
units. The results are expressed as the mean ± standard deviation;
*P<0.05. (B) Serum cytokine levels in the WT and
GnT-V Tg mice 0, 8, or 24 h after the injection of ConA at 12.5
mg/kg BW. Serum levels of IFN-γ and IL-10 were measured by ELISA at
the indicated time points. Results are expressed as the mean ±
standard deviation; *P<0.05. GnT-V,
N-acetylglucosaminyltransferase V; WT, wild-type; Tg,
transgenic; ConA, concanavalin A; BW, body weight; Ifn, interferon;
IL, interleukin. |
Th1 to Th2 cytokine shift in GnT-V Tg
mouse splenic lymphocytes in vitro
T cells are important in ConA-induced hepatitis
(1,4,5). To
investigate function of splenic lymphocytes from the WT and GnT-V
Tg mice in cytokine production, the mouse splenic lymphocytes were
stimulated with anti-CD3/CD28 antibodies in vitro. Levels of
the IFN-γ Th1 cytokine were significantly lower in the GnT-V Tg
compared with the WT mouse splenic lymphocytes (Fig. 3A), whereas levels of the IL-10 Th2
cytokine were higher in the GnT-V Tg mouse splenic lymphocytes
compared with the WT lymphocytes.
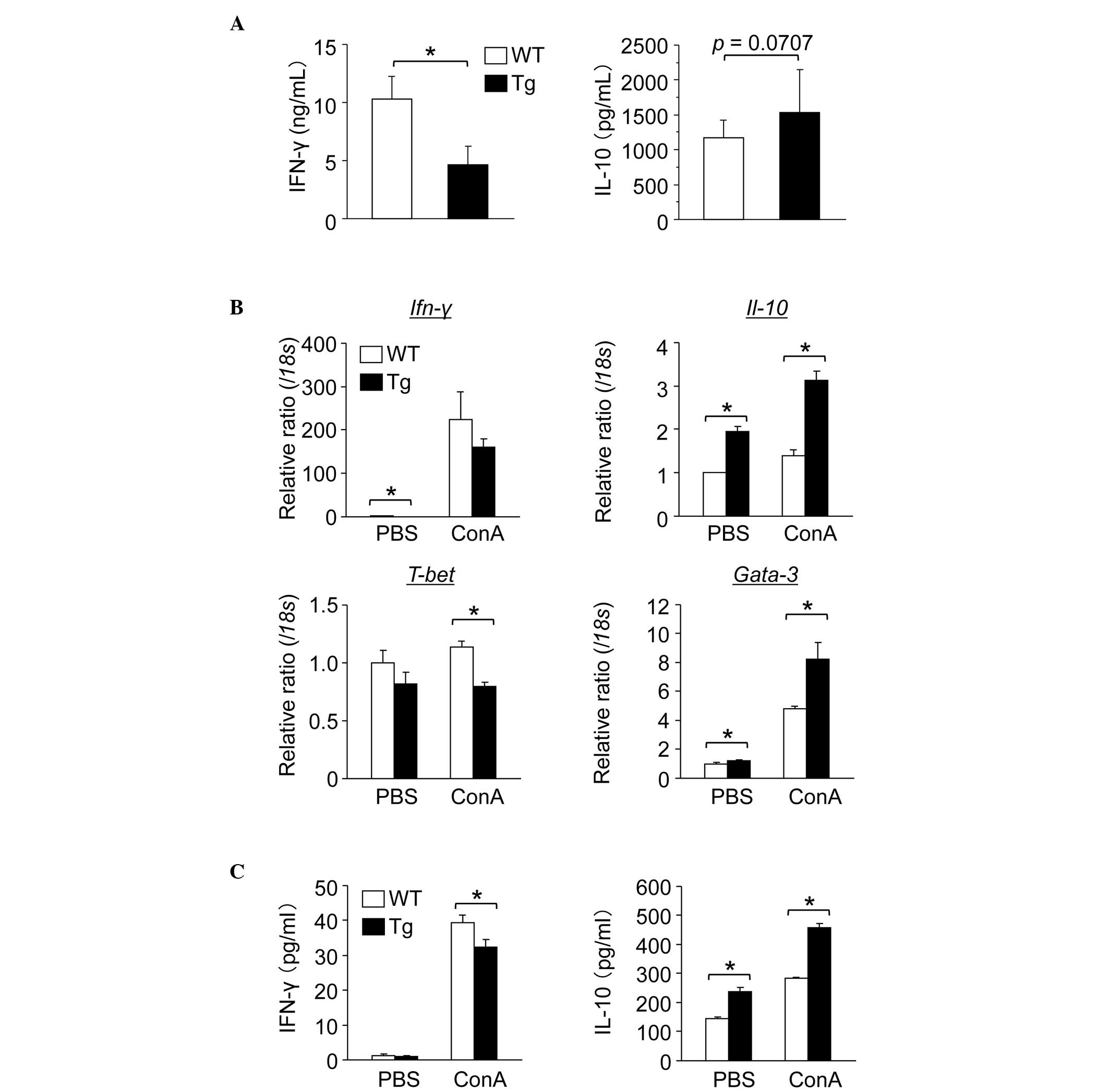 | Figure 3GnT-V Tg mouse splenic lymphocytes
produce increased Th2 cytokines. (A) Anti-CD3/CD28 antibody-induced
cytokine production levels secreted into the medium from splenic
lymphocytes. Mononuclear cells of the spleen, isolated from WT and
GnT-V Tg mice, were cultured for 48 h in the presence of anti-CD3
(5 μg/ml) and anti-CD28 (5 μg/ml) antibodies and levels of IFN-γ
and IL-10 were measured using ELISA. The results are expressed as
the mean ± standard deviation (n=3–8); *P<0.05. (B)
Gene expression of cytokinines in the splenic lymphocytes following
ConA stimulation is shown. Splenic lymphocytes from WT and GnT-V Tg
mice were cultured for 24 h in the presence of ConA (5 μg/ml) and
the gene expression levels of Ifn-γ, Il-10, T-bet and
Gata-3 were measured by reverse transcription quantitative
polymerase chain reaction. The mRNA expression levels were
normalized relative to that of 18s and are expressed in
arbitrary units. The results are expressed as the mean ± standard
deviation (n=3); *P<0.05. (C) ConA-induced cytokine
production levels secreted into the medium from splenic
lymphocytes. Splenic lymphocytes from WT and GnT-V Tg mice were
cultured for 24 h in the presence of ConA (5 μg/ml) and levels of
IFN-γ and IL-10 were measured using ELISA. Results are expressed as
the mean ± standard deviation (n=3); *P<0.05. WT,
wild-type mouse splenic lymphocytes; GnT-V,
N-acetylglucosaminyltransferase V; Tg, GnT-V transgenic
mouse splenic lymphocytes; ConA, concanavalin A; BW, body weight;
Ifn, interferon; IL, interleukin; PBS, phosphate-buffered saline;
Th, T helper; CD, cluster of differentiation. |
ConA activates lymphocyte function,
including cytokine production (1,4,5)
Subsequently, mouse splenic lymphocytes were
stimulated with ConA in vitro. As was observed following
stimulation with the anti-CD3/CD28 antibodies, the levels of gene
expression and the production of IFN-γ in the GnT-V Tg mouse
splenic lymphocytes were lower compared with those in the WT,
whereas the levels of IL-10 were higher (Fig. 3B and C). IFN-γ is considered a
typical pro-inflammatory cytokine (10,11)
and IL-10 is known to protect the liver from ConA-induced hepatitis
(14,15). Therefore, the exacerbated
ConA-induced hepatitis in the GnT-V Tg mice in vivo was not
explained by these in vitro results.
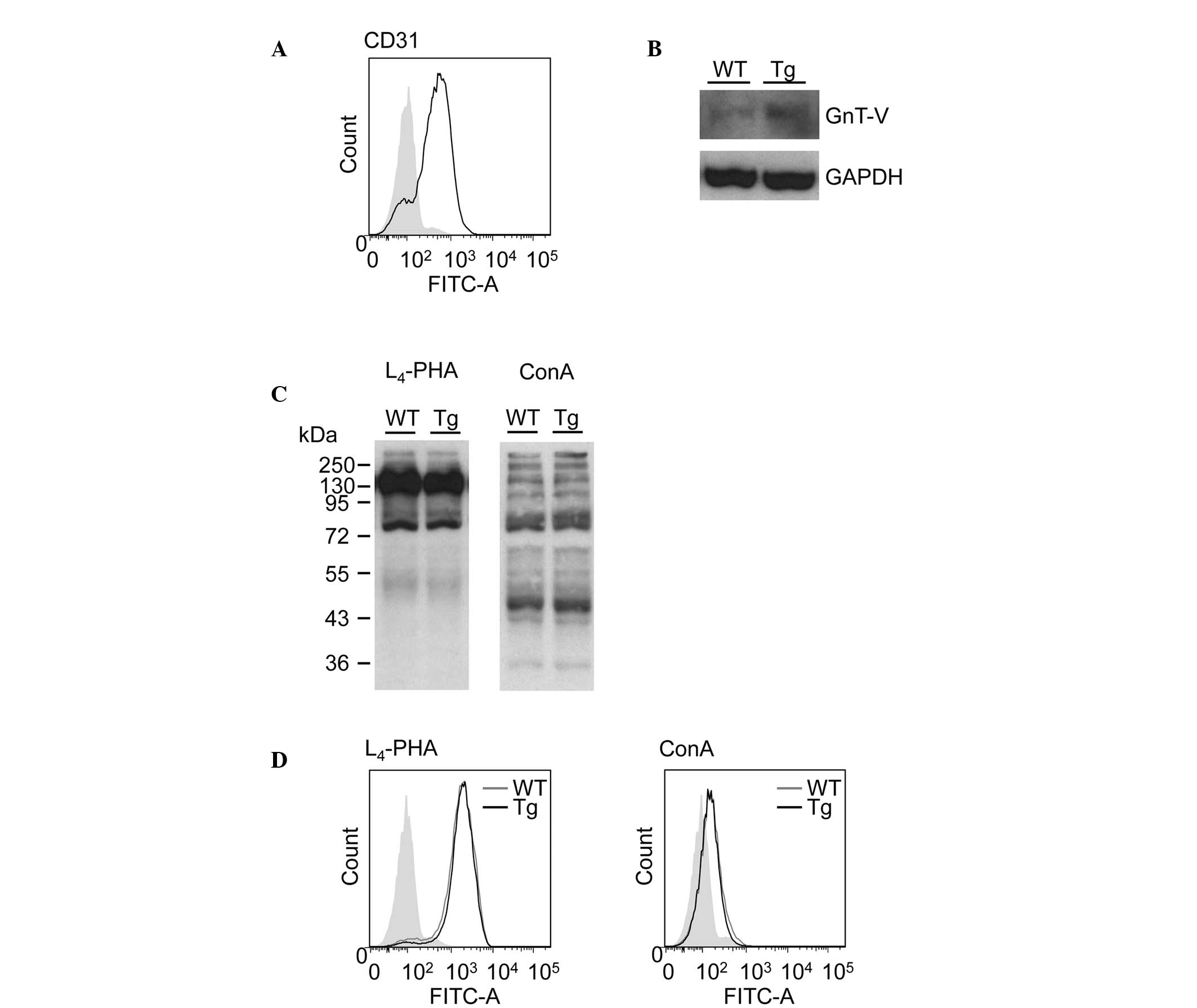
No difference is observed in the binding
affinity of ConA to LSECs between WT and GnT-V Tg mice
The binding of ConA to the mannose gland of the LSEC
surface, which is followed by LSEC damage, recruits T lymphocytes
from the sinusoidal circulation and is an early event in T
cell-mediated liver injury (5).
The subsequent loss of function of the LSEC barrier exposes the
underlying hepatocytes to further attack from activated T
lymphocytes (5–7). To examine the binding affinity of
ConA to LSECs from the WT and the GnT-V Tg mice, ConA lectin
blotting and flow cytometric analysis were performed. The purity of
the isolated LSECs was >75% (Fig.
4A).
To investigate the difference in the GnT-V and β1–6
GlcNAc branching of N-glycans expression in LSECs from the
WT and GnT-V Tg mice, GnT-V immunoblotting, L4-PHA
lectin blotting and flow cytometric analysis were performed
(Fig. 4B–D). L4-PHA
binds to the β1–6 GlcNAc branching of N-glycans, which is
the product of GnT-V. Although the protein expression of GnT-V was
increased in the GnT-V Tg mouse LSECs compared with the WT mouse
LSECs (Fig. 4B), the expression of
β1–6 GlcNAc branching of N-glycans, determined by
L4-PHA lectin blotting and flow cytometric analysis, did
not differ between the WT and GnT-V Tg mouse LSECs (Fig. 4C, left panel and 4D, left panel).
ConA lectin blotting and flow cytometric analysis also revealed no
difference in the binding affinity of ConA to the LSECs between the
WT and GnT-V Tg mouse LSECs (Fig.
4Cand D).
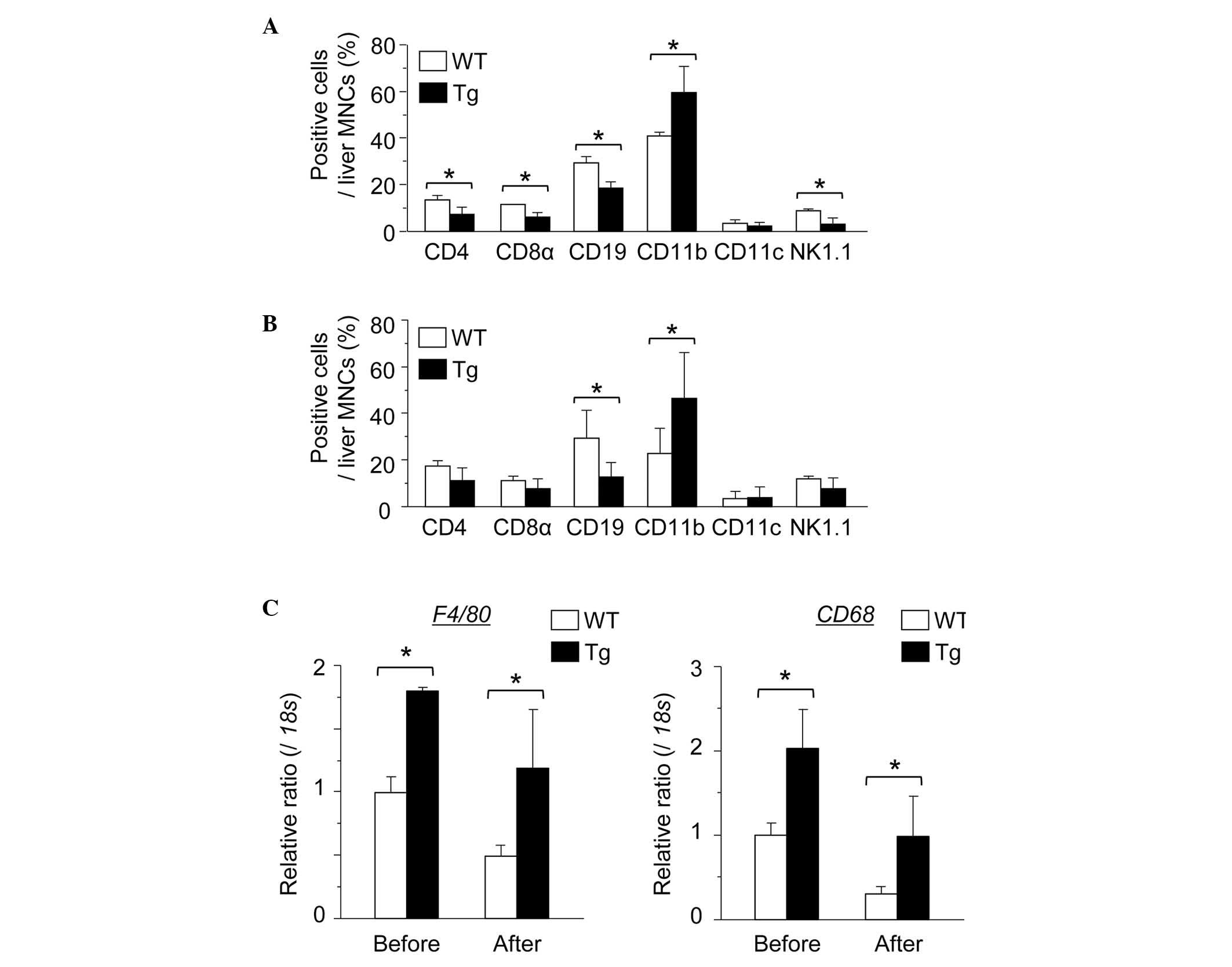
Number of hepatic macrophages is
significantly increased in GnT-V Tg compared with WT mice
Subsequently, the hepatic immune cells were examined
and the liver MNC subset was investigated prior to or following the
injection of ConA. Notably, the number of CD11b-positive cells was
significantly increased in the GnT-V Tg liver compared with the WT
mouse liver prior to and 2 h after ConA injection (Fig. 5A and B). In addition, the gene
expression of the macrophage markers F4/80 and CD68 were
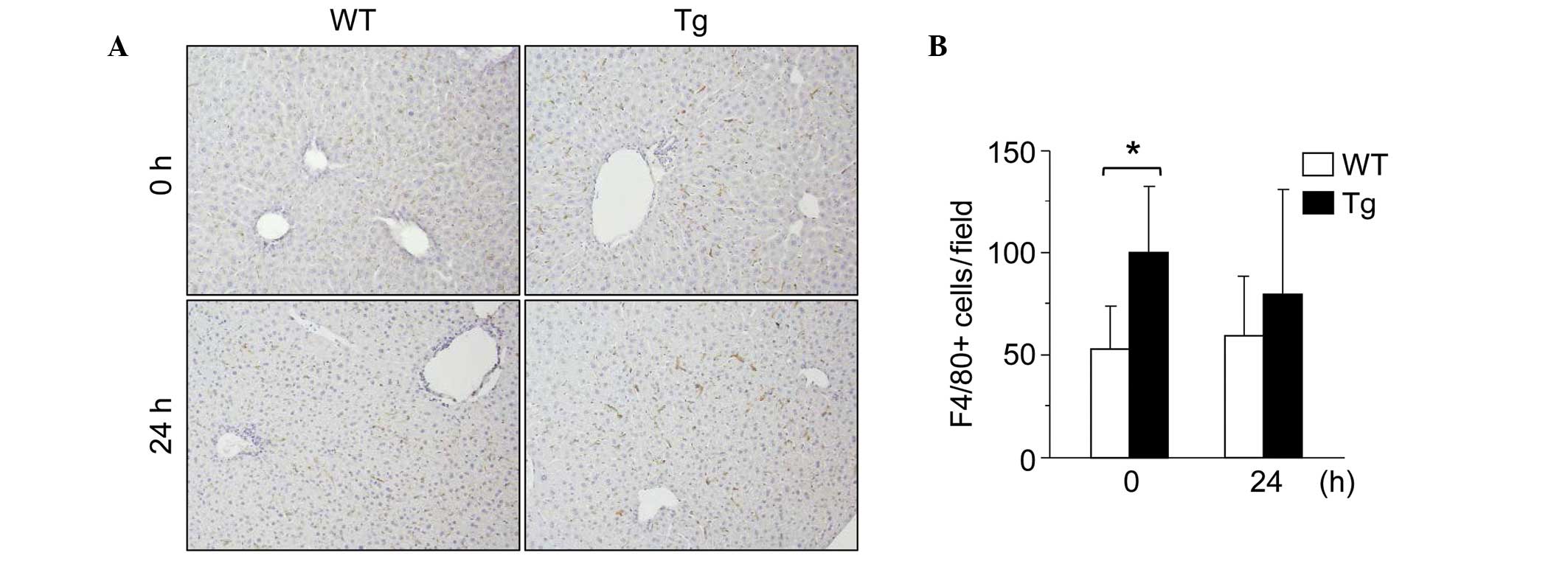
significantly increased in the Tg mouse MNCs (Fig. 5C). F4/80 immunohistochemical
staining of the livers also revealed that the number of hepatic
F4/80-positive macrophages was significantly increased in the GnT-V
Tg mice compared with the WT mice (Fig. 6A and B). These results indicated
that the number of hepatic macrophages was higher in the GnT-V Tg
mice than in the WT mice.
Depletion of hepatic macrophages reduces
the degree of difference in ConA-induced hepatitis between WT and
GnT-V Tg mice
To investigate the effect of hepatic macrophages in
ConA-induced hepatitis in GnT-V Tg mice, macrophages were depleted
from the WT and GnT-V Tg mice using clodronate-liposomes, and the
severity of ConA-induced hepatitis was determined. The depletion of
hepatic macrophages was confirmed by immunohistochemical staining
with anti-F4/80 antibody (Fig.
7A). Macrophage depletion significantly suppressed ConA-induced
liver injury in the WT and GnT-V Tg mice and it reduced the
differences in liver injury between the WT and GnT-V Tg mice
(Fig. 7B and C).
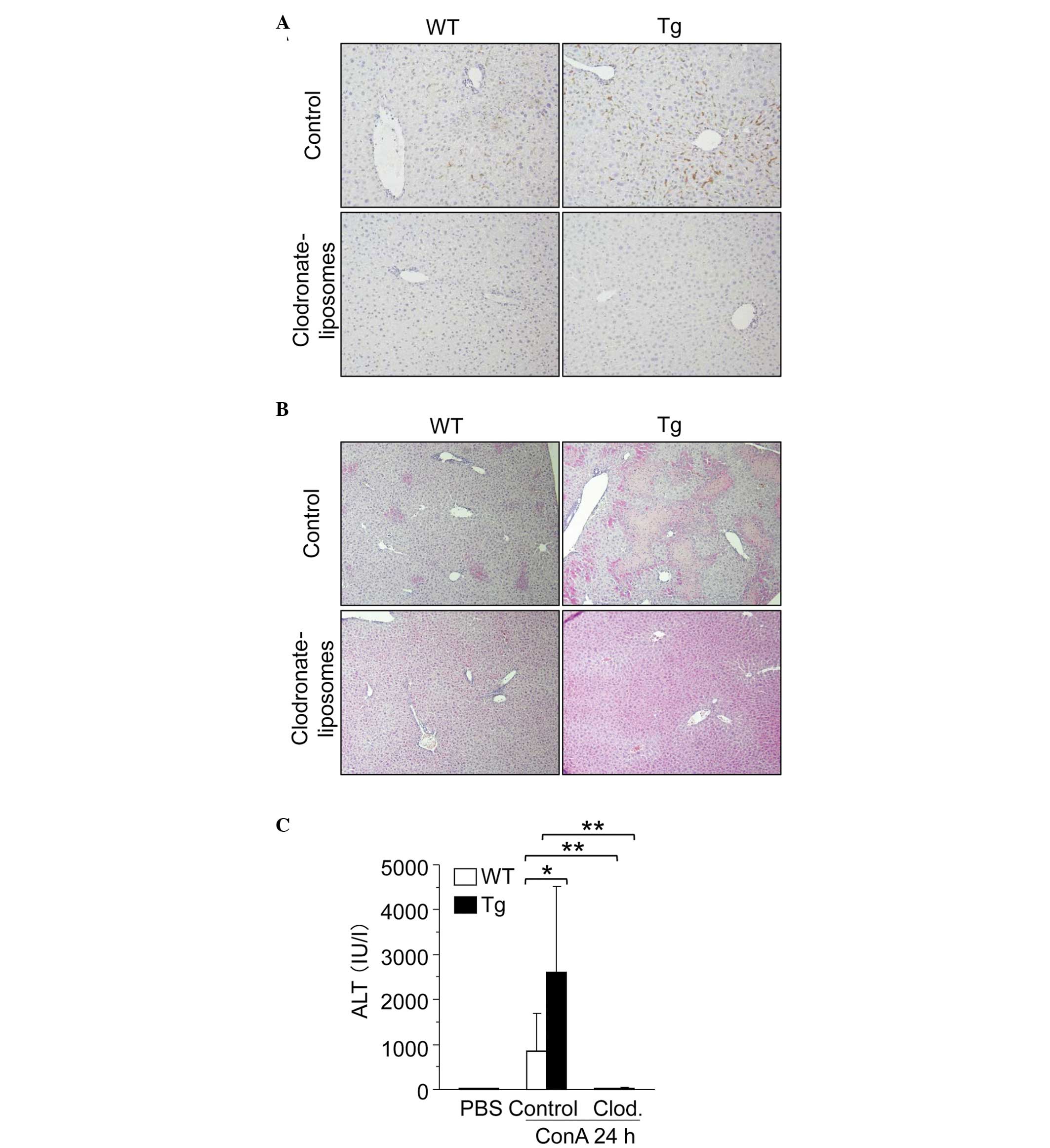 | Figure 7Hepatic macrophage depletion reduces
the difference in the degree of hepatitis between WT and GnT-V Tg
mice. (A) Representative photomicrographs mouse livers stained with
anti-F4/80 antibody 24 h after the injection of ConA at 12.5 mg/kg
BW (magnification, ×200). Clodronate-liposomes were injected 2 days
prior to ConA administration. (B) Representative photomicrographs
of hematoxylin and eosin stained mouse livers 24 h after the
injection of ConA at 12.5 mg/kg BW (magnification, ×40). (C) Levels
of serum ALT in mice (n=6) 24 h after the injection of ConA at 12.5
mg/kg BW. Results are expressed as the mean ± standard deviation;
*P<0.05 and **P<0.01. WT, wild-type
mice; GnT-V, N-acetylglucosaminyltransferase V; Tg, GnT-V
transegenic mice; Clod, clodronate-liposomes; ALT, alanine
aminotransferase; PBS, phosphate-buffered saline; ConA,
concanavalin A; BW, body weight. |
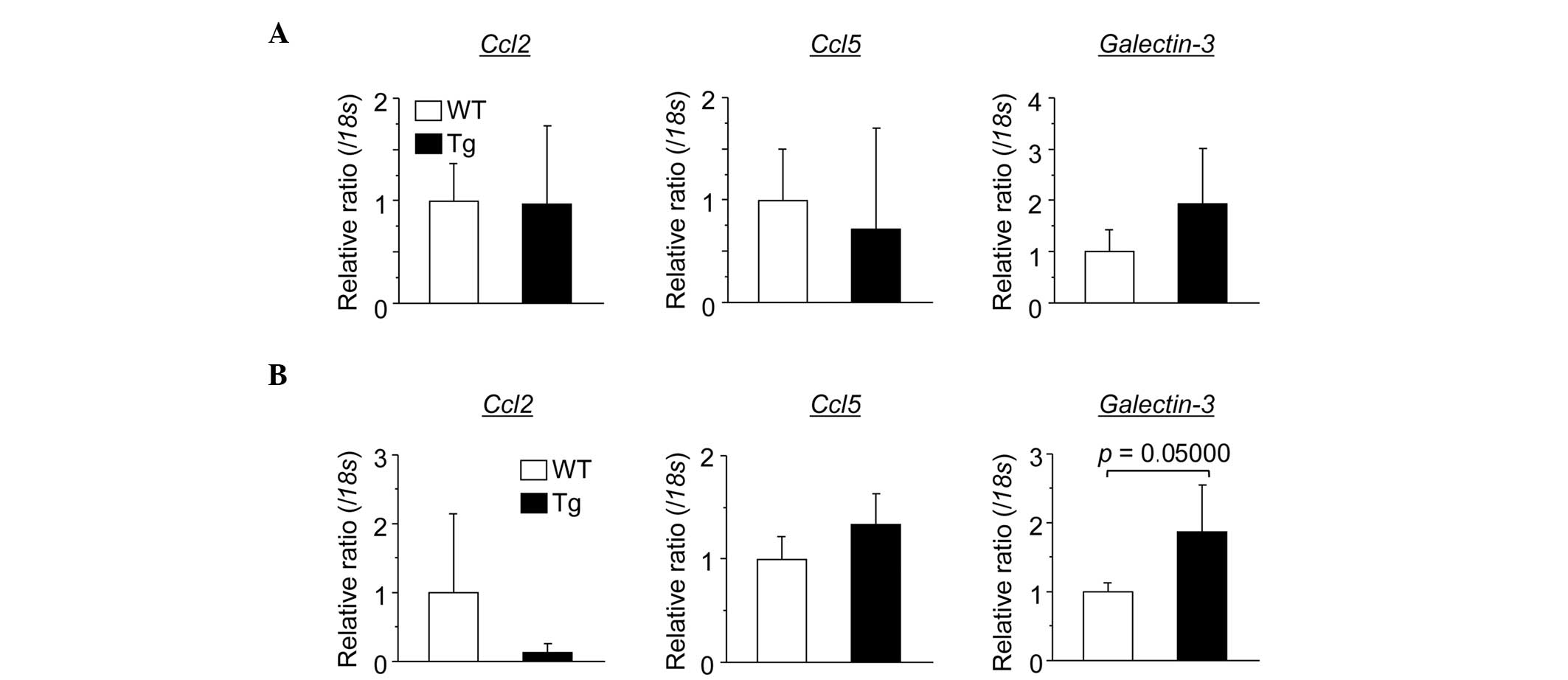
Expression of galectin-3 is elevated in
Tg mouse hepatocytes
To elucidate the reason why the number of liver
macrophages in the Tg mice was elevated compared with the number in
the WT mice, the expression of macrophage chemoattractant genes
(Ccl2, Ccl5 and Galectin-3) were investigated in
mouse hepatocytes (Fig. 8). The
results demomnstrated that the gene expression of galectin-3
was elevated in the Tg mouse hepatocytes compared with the WT mouse
hepatocytes.
Discussion
In the present study, it was initially observed that
ectopic expression of GnT-V exacerbated ConA-induced hepatitis
despite the Th1 to Th2 cytokine shift observed in the GnT-V Tg
mouse splenic lymphocytes. A relatively high dose of ConA induced a
significantly higher mortality rate in the GnT-V Tg mice compared
with the that in WT mice. The binding affinity of ConA to LSEC,
which occurs in the first phase of ConA hepatitis, did not differ
between the WT and GnT-V Tg mice. The results also revealed
significant increases in hepatic macrophage infiltration in the
GnT-V Tg mice liver compared with the WT mouse liver, prior to or
following ConA injection. Notably, the gene expression of
galectin-3, a hepatocyte and one of the major chemoattractants of
macrophage, was increased in the Tg mice. These findings indicated
that GnT-V-induced galectin-3 elevation recruited monocytes to the
liver and resulted in an increased number of hepatic macrophages,
ultimately leading to enhanced ConA-induced hepatitis in the Tg
mice. The present study also observed that the depletion of
macrophages inhibited and reduced the difference in the degree of
ConA-induced hepatitis between the WT and GnT-V Tg mice.
Considering these findings, the present study demonstrated that
aberrant glycosylation, induced by GnT-V, increased hepatic
macrophage infiltration and resulted in enhanced ConA-induced
hepatitis.
In the liver, LSECs, Kupffer cells (hepatic
macrophages), lymphocytes (T cells) and NK cells are involved in
the immune response of ConA-induced hepatitis (5). Among these immune cells, T cells are
critical in ConA-induced hepatitis (1,4,5).
Therefore, to investigate the roles of GnT-V on T cell activation,
splenic lymphocytes from WT and GnT-V Tg mice were stimulated with
anti-CD3/CD28 antibodies or ConA in vitro. The GnT-V Tg
mouse splenic lymphocytes produced lower levels of the IFN-γ Th1
cytokine and higher levels of the IL-10 Th2 cytokine compared with
the WT mouse splenic lymphocytes. These findings were consistent
with those of previous studies, which demonstrated that
GnT-V-induced TCR oligosaccharide modification suppresses TCR
signaling (22,23). However, these findings indicate
that GnT-V tends to suppress inflammatory responses through
suppression of T cell activation, which differ from the results of
the present in vivo study.
ConA binds predominantly to LSECs within 15 min
following intravenous injection. After 4 h, ConA begins to bind to
hepatic macrophages (33), and
activated lymphocytes are then trafficked towards hepatocytes,
leading to inflammation (5,32).
The present study hypothesized that the glycosylation differences
between the LSECs of WT and GnT-V Tg mice may be important in the
progression of ConA-induced hepatitis. To examine this hypothesis,
differences in the lectin affinities of each mouse LSEC were
investigated. It was found that ConA and L4-PHA lectin
bound equally to the WT and GnT-V Tg mouse LSECs, however, the
expression of GnT-V was increased in the GnT-V Tg mice. Since
levels of β1–6 GlcNAc branching are regulated by UDP-GlcNAc, a
donor substrate of GnT-V (33), a
change in the expression of GnT-V alone were insufficient to
increase levels of β1–6 GlcNAc branching in the Tg mouse LSECs.
In response to these T cell and LSEC findings, the
present study investigated hepatic macrophages as the main effector
cell in the second phase of ConA-induced hepatitis (33,35).
The hepatic macrophages were significantly increased in the GnT-V
Tg mice compared with the WT mice prior to and following ConA
administration. It is understood that β1–6 GlcNAc branching extends
repeated glycans of GlcNAc and galactose and results in a
polylactosamine structure (36).
Endogenous galectin-3 binds to this polylactosamine structure.
Galectin-3 is a chemoattractant for monocytes and macrophages,
which induces macrophage infiltration into the organs (35,37,38)
and promotes inflammatory changes in various organs (35). Therefore, the polylactosamine
structure induced in GnT-V Tg mice may increase the quantity of
hepatic galectin-3. The present study also observed that the gene
expression of galectin-3 was increased in the GnT-V Tg hepatocytes
compared with the WT mouse hepatocytes. Therefore, GnT-V Tg mouse
hepatocytes may produce higher quantities of galectin-3 than WT
mouse hepatocytes. Increased hepatic galectin-3 in the GnT-V Tg
mice may result in the elevated proportion of macrophages among the
hepatic MNCs. In the present study, depletion of hepatic
macrophages by clodronate-liposome infusion decreased the severity
of ConA-induced hepatitis in the WT and GnT-V Tg mice and reduced
the differences in liver injury between these mice. These results
indicated that aberrant glycosylation by GnT-V elevated hepatic
macrophage infiltration via an increase in hepatic galectin-3,
exacerbating ConA-induced hepatitis. The reason for GnT-V-induced
increases in hepatic galectin-3 and target glycoproteins for GnT-V
in macrophages remains to be elucidated and its mechanisms require
further investigation.
In conclusion, aberrant glycosylation by GnT-V led
to increases in hepatic macrophage infiltration and enhanced
ConA-induced hepatitis in mice. These findings indicate that the
modulation of glycosylation may be a novel therapeutic target for
immunity-associated acute hepatitis.
Acknowledgements
The present study was supported by Grants-in-Aid for
Scientific Research (no. 21249038) and the Japan Society for the
Promotion of Science (no. 24590972),.
Abbreviations:
|
CCL2
|
C-C chemokine ligand 2
|
|
ConA
|
concanavalin A
|
|
GAPDH
|
glyceraldehyde 3-phosphate
dehydrogenase
|
|
GnT-V
|
N-acetylglucosaminyltransferase
V
|
|
IFN-γ
|
interferon-γ
|
|
IL
|
interleukin
|
|
LSEC
|
liver sinusoidal endothelial cell
|
|
NK cell
|
natural killer cell
|
|
TCR
|
T cell receptor
|
|
TNF-α
|
tumor necrosis factor-α
|
References
|
1
|
Gantner F, Leist M, Lohse AW, Germann PG
and Tiegs G: Concanavalin A-induced T-cell-mediated hepatic injury
in mice: the role of tumor necrosis factor. Hepatology. 21:190–198.
1995.PubMed/NCBI
|
|
2
|
Margalit M, Abu Gazala S, Alper R, et al:
Glucocerebroside treatment ameliorates ConA hepatitis by inhibition
of NKT lymphocytes. Am J Physiol Gastrointest Liver Physiol.
289:G917–G925. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Takeda K, Hayakawa Y, Van Kaer L, Matsuda
H, Yagita H and Okumura K: Critical contribution of liver natural
killer T cells to a murine model of hepatitis. Proc Natl Acad Sci
USA. 97:5498–5503. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tiegs G, Hentschel J and Wendel A: A T
cell-dependent experimental liver injury in mice inducible by
concanavalin A. J Clin Invest. 90:196–203. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang HX, Liu M, Weng SY, et al: Immune
mechanisms of Concanavalin A model of autoimmune hepatitis. World J
Gastroenterol. 18:119–125. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yang MC, Chang CP and Lei HY: Endothelial
cells are damaged by autophagic induction before hepatocytes in Con
A-induced acute hepatitis. Int Immunol. 22:661–670. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tsui TY, Obed A, Siu YT, et al: Carbon
monoxide inhalation rescues mice from fulminant hepatitis through
improving hepatic energy metabolism. Shock. 27:165–171. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Racanelli V and Rehermann B: The liver as
an immunological organ. Hepatology. 43:S54–S62. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schrage A, Wechsung K, Neumann K, et al:
Enhanced T cell transmigration across the murine liver sinusoidal
endothelium is mediated by transcytosis and surface presentation of
chemokines. Hepatology. 48:1262–1272. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gove ME, Rhodes DH, Pini M, et al: Role of
leptin receptor-induced STAT3 signaling in modulation of intestinal
and hepatic inflammation in mice. J Leukoc Biol. 85:491–496. 2009.
View Article : Google Scholar :
|
|
11
|
Takahashi K, Murakami M, Kikuchi H, Oshima
Y and Kubohara Y: Derivatives of Dictyostelium
differentiation-inducing factors promote mitogen-activated IL-2
production via AP-1 in Jurkat cells. Life Sci. 88:480–485. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Miller ML, Sun Y and Fu YX: Cutting edge:
B and T lymphocyte attenuator signaling on NKT cells inhibits
cytokine release and tissue injury in early immune responses. J
Immunol. 183:32–36. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Boring L, Gosling J, Chensue SW, et al:
Impaired monocyte migration and reduced type 1 (Th1) cytokine
responses in C-C chemokine receptor 2 knockout mice. J Clin Invest.
100:2552–2561. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kato M, Ikeda N, Matsushita E, Kaneko S
and Kobayashi K: Involvement of IL-10, an anti-inflammatory
cytokine in murine liver injury induced by Concanavalin A. Hepatol
Res. 20:232–243. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Di Marco R, Xiang M, Zaccone P, et al:
Concanavalin A-induced hepatitis in mice is prevented by
interleukin (IL)-10 and exacerbated by endogenous IL-10 deficiency.
Autoimmunity. 31:75–83. 1999. View Article : Google Scholar
|
|
16
|
Ohtsubo K and Marth JD: Glycosylation in
cellular mechanisms of health and disease. Cell. 126:855–867. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rademacher TW, Parekh RB and Dwek RA:
Glycobiology. Annu Rev Biochem. 57:785–838. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhao Y, Sato Y, Isaji T, et al: Branched
N-glycans regulate the biological functions of integrins and
cadherins. FEBS J. 275:1939–1948. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Taniguchi N, Miyoshi E, Ko JH, Ikeda Y and
Ihara Y: Implication of N-acetylglucosaminyltransferases III and V
in cancer: gene regulation and signaling mechanism. Biochim Biophys
Acta. 1455:287–300. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lau KS and Dennis JW: N-Glycans in cancer
progression. Glycobiology. 18:750–760. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Taniguchi N, Ihara S, Saito T, Miyoshi E,
Ikeda Y and Honke K: Implication of GnT-V in cancer metastasis: a
glycomic approach for identification of a target protein and its
unique function as an angiogenic cofactor. Glycoconj J. 18:859–865.
2001. View Article : Google Scholar
|
|
22
|
Demetriou M, Granovsky M, Quaggin S and
Dennis JW: Negative regulation of T-cell activation and
autoimmunity by Mgat5 N-glycosylation. Nature. 409:733–739. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li D, Li Y, Wu X, et al: Knockdown of
Mgat5 inhibits breast cancer cell growth with activation of CD4+ T
cells and macrophages. J Immunol. 180:3158–3165. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Miyoshi E, Nishikawa A, Ihara Y, Gu J, et
al: N-acetylglucosaminyltransferase III and V messenger RNA levels
in LEC rats during hepatocarcinogenesis. Cancer Res. 53:3899–3902.
1993.PubMed/NCBI
|
|
25
|
Miyoshi E, Ihara Y, Nishikawa A, et al:
Gene expression of N-acetylglucosaminyltransferases III and V: a
possible implication for liver regeneration. Hepatology.
22:1847–1855. 1995.PubMed/NCBI
|
|
26
|
Kamada Y, Mori K, Matsumoto H, et al:
N-Acetylglucosaminyltransferase V regulates TGF-beta response in
hepatic stellate cells and the progression of steatohepatitis.
Glycobiology. 22:778–787. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Terao M, Ishikawa A, Nakahara S, et al:
Enhanced epithelial-mesenchymal transition-like phenotype in
N-acetylglucosaminyltransferase V transgenic mouse skin promotes
wound healing. J Biol Chem. 286:28303–28311. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Aicher WK, Fujihashi K, Yamamoto M, Kiyono
H, Pitts AM and McGhee JR: Effects of the lpr/lpr mutation on T and
B cell populations in the lamina propria of the small intestine, a
mucosal effector site. Int Immunol. 4:959–968. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kristensen DB, Kawada N, Imamura K, et al:
Proteome analysis of rat hepatic stellate cells. Hepatology.
32:268–277. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Trobonjaca Z, Leithauser F, Moller P,
Schirmbeck R and Reimann J: Activating immunity in the liver. I
Liver dendritic cells (but not hepatocytes) are potent activators
of IFN-gamma release by liver NKT cells. J Immunol. 167:1413–1422.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shinzaki S, Iijima H, Fujii H, et al:
Altered oligosaccharide structures reduce colitis induction in mice
defective in beta-1,4-galactosyltransferase. Gastroenterology.
142:1172–1182. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Van Rooijen N and Sanders A: Liposome
mediated depletion of macrophages: mechanism of action, preparation
of liposomes and applications. J Immunol Methods. 174:83–93. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Knolle PA, Gerken G, Loser E, et al: Role
of sinusoidal endothelial cells of the liver in concanavalin
A-induced hepatic injury in mice. Hepatology. 24:824–829. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sasai K, Ikeda Y, Fujii T, Tsuda T and
Taniguchi N: UDP-GlcNAc concentration is an important factor in the
biosynthesis of beta1,6-branched oligosaccharides: regulation based
on the kinetic properties of N-acetylglucosaminyltransferase V.
Glycobiology. 12:119–127. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bacigalupo ML, Manzi M, Rabinovich GA and
Troncoso MF: Hierarchical and selective roles of galectins in
hepatocarcinogenesis, liver fibrosis and inflammation of
hepatocellular carcinoma. World J Gastroenterol. 19:8831–8849.
2013. View Article : Google Scholar :
|
|
36
|
Lee RT and Lee YC: Affinity enhancement by
multivalent lectin-carbohydrate interaction. Glycoconj J.
17:543–551. 2000. View Article : Google Scholar
|
|
37
|
Sano H, Hsu DK, Yu L, et al: Human
galectin-3 is a novel chemoattractant for monocytes and
macrophages. J Immunol. 165:2156–2164. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Volarevic V, Milovanovic M, Ljujic B, et
al: Galectin-3 deficiency prevents concanavalin A-induced hepatitis
in mice. Hepatology. 55:1954–1964. 2012. View Article : Google Scholar : PubMed/NCBI
|