Introduction
MicroRNAs (miRNAs) are a type of non-coding
small-fragment RNA with 21 basic groups, which are transcribed by
cells in order to alter gene expression, predominantly through
post-transcriptional regulation (1). With the increase of studies focusing
on this field of research, miRNAs have been confirmed to be
involved in a variety of physiological and pathological activities,
including cell proliferation, apoptosis, cell cycle regulation,
tumor formation and inflammation (2). One previous study demonstrated that
miRNA, including miR205, was closely associated with tumor
development; in addition, the involvement of miRNAs in tumor
formation can be classified into two categories according to their
different effects: Cancer-promoting and -suppressing miRNAs
(3,4).
miRNA (miR)-10a novel microRNA, which was found to
be closely associated with tumor growth, survival, invasion and
metastasis. A recent study demonstrated that miR-10a promoted tumor
growth, metastasis and invasion in cervical cancer, indicating that
miR-10a may be an important cancer-promoting miRNA (5).
Although antitumor drugs, including cisplatin, have
been highly effective and widely applied in the treatment of lung
cancer, drug-resistance has become an increasingly prominent issue,
as it is a key obstacle in the effective treatment of cancer
patients. Therefore, multidrugresistance (MDR) reversal is
significant in the development of tumor therapies. The major
mechanisms underlying tumor MDR, include incerased drug excretion,
decreased drug intracellular concentration and increased expression
of antiapoptotic factors in tumor cells (6). Furthermore, the role of miR-10a in
tumor cell apoptosis and drug resistance remains to be elucidated.
The present study aimed to explore the role and mechanisms of
miR-10a in drug-resistance reversal of non-small cell lung cancer
cells.
Materials and methods
Cell lines and cell culture
Human lung cancer A549 and human cisplatin
(DDP)-resistant lung cancer A549/DDP cells were purchased from the
Cell Bank of Chinese Academy of Science (Shanghai, China) and
cultured at 37°C with 5% CO2 in Dulbecco’s modified
Eagle’s medium (DMEM; GibcoBRL, Invitrogen Life Technologies,
Carlsbad, CA, USA) with 10% fetal bovine serum (FBS; GibcoBRL).
miR-10a antisense oligonucleotides (miR-10a inhibitor) and the
miRNA control were purchased from Shanghai GenePharma Co., Ltd
(Shanghai, China) and transfected into cells using a
LipofectamineTM 2000 assay (Invitrogen Life
Technologies, Carlsbad, CA, USA). The groups were as follows:
Parent group, A549 cells; control group, A549/DDP cells without
treatment; negative control (NC) group, A549/DDP cells transfected
with miR-10a (ASO-miR-NC); and miR-10a inhibitor group, A549/DDP
cells transfected with an miR-10a inhibitor (ASO-miR-10a).
miRNA was extracted from cells using a
TaqMan® miRNA isolation kit (Invitrogen Life
Technologies) and the expression of miR-10a was measured using a
TaqMan® Universal polymerase chain reaction (PCR) Master
Mix assay (Invitrogen Life Technologies). U6 was used as the
internal reference gene.
MTS assay
Cells (0.5 ml; 1×104/ml) were seeded onto
a 96-well microplate and incubated overnight at 37°C with 5%
CO2. Various concentrations of DDP (0, 5, 10, 20, 50 and
100 μM; SigmaAldrich, St. Louis, MO, USA) were added and cultured
for 72 h. Fresh culture medium with 20 μl MTS (SigmaAldrich) was
then added and cells were incubated for 4 h. Absorbance values were
determined using a microplate reader (Model 680; BioRad
Laboratories, Inc., Hercules, CA, USA) at 490 nm in order to
calculate the rate of cell proliferation inhibition.
Flow cytometric analysis
Flow cytometry was used to analyze the apoptotic
rate of cells. Cells (0.3 ml; 1×106/ml) were seeded onto
a six-well microplate for 24 h and exposed to 10 μM DDP for a
further 24 h. Cells were then centrifuged at 1000 × g for 5 min at
room temperature, collected and stained using a fluorescein
isothiocyanate (FITC)/propidium iodide (PI) apoptosis detection kit
(BD Biosciences, Franklin Lakes, NJ, USA) for 15 min in the dark.
Fluorescence intensity was the measured using a flow cytometer
(FACSCalibur; BD Biosciences).
For detection of intracellular rhodamine (Rh)-123
content and P-glycoprotein (P-gp) expression, cells (0.3 ml;
1×106/ml) were seeded onto a six-well microplate for 24
h and then centrifuged at 300 × g for 10 min, collected and
incubated with rabbit antihuman polyclonal Rh-123- or
P-pg-phycoerythrin antibodies (Beyotime Institute of Biotechnology,
Shanghai, China) for 30 min in the dark. Fluorescence intensity was
then measured using a flow cytometer.
Caspase-3/8 activity was measured using an active
caspase-3/8 apoptosis kit (Xiamen Lulong Biotech Development Co.,
Ltd, Xiamen, China) according to the manufacturer’s instructions In
brief, cells (0.3 ml; 1×106/ml) were seeded onto a
six-well microplate for 24 h. Cells were then permeabilized, fixed
and incubated with active caspase-3-FITC or active caspase-8-FITC
antibodies for 30 min in the dark. Fluorescence intensity was then
measured using a flow cytometer.
Western blot analysis
Cells (0.3 ml; 1×106/ml) were seeded onto
a six-well microplate for 24 h and lysed using a lysate buffer
(Beyotime Institute of Biotechnology);100 μg total cell lysate was
the separated using 12% SDS-PAGE (Beyotime Institute of
Biotechnology). Proteins were then transferred onto a
polyvinylidene fluoride membrane (Millipore, Billerica, MA, USA)
and blocked using 5% non-fat milk for 1 h at 4°C. The membrane was
incubated with rabbit antihuman polyclonal primary antibodies
(MDR1, 1:1,000; MDR-associated protein (MRP)1, 1:1,000; RhoE,
1:1,000; B cell lymphoma 2 (Bcl-2), 1:1,000; Survivin, 1:1,000;
caspase-3, 1:1,000; caspase-8, 1:1,000; p53, 1:1,000;
phosphorylatedsignal transducer and activator of transcription
(p-STAT)3, 1:1,000; and p-STAT5, 1:1,000; Cell Signaling
Technologies, Danvers, MA, USA) and β-actin (1:5,000; SigmaAldrich)
at 4°C overnight. The membranes were then washed three times in 5%
phosphate-buffered saline with Tween-20 (Beyotime Institute of
Biotechnology) and incubated for 1 h with horseradish
peroxidase-conjugated anti-rabbit secondary antibodies (1:5,000;
Cell Signaling Technologies) at room temperature. Western blots
were visualized using an Enhanced Chemiluminescence Western
Blotting kit (GE Healthcare, Little Chalfont, UK). β-actin was as
the internal control.
Quantitative PCR (qPCR)
Cells (0.3 ml; 1×106/ml) were seeded onto
a six-well microplate for 24 h and total RNA was extracted using a
TRIzol® assay (Invitrogen Life Technologies, Carlsbad,
CA, USA). qPCR was performed using a Mastercycler Gradient (nexus
G2; Eppendorf, Hamburg, Germany). GAPDH was amplified as the
internal control. The primer sequences were as follows: MDR1
forward, 5′-CACCTTAAAGGGCCACAG-3′ and reverse,
5′-TGCCGACCGTACAAGAGT-3′; MRP1 forward,
5′-AGGTCTGCCCAGCAGACGATCCA-3′ and reverse,
5′-GGACAAGCACTGAAAGATAAGAAAGA-3′; RhoE forward,
5′-ACACATATGAAGGAGAGAA -3′ and reverse, 5′-TAAGGCGGCCGCAACATGA-3′;
Bcl-2 forward, 5′-GGTGAACTGGGGGAGGATTGT-3′ and reverse,
5′-CTTCAGAGACAGCCAGGAGAA-3′; Survivin forward,
5′-CTCTACATTCAAGAACTGGCC-3′ and reverse,
5′-TTGGCTCTTTCTCTGTCCAG-3′; GAPDH forward,
5′-TGCACCACCAACTGCTTAGC-3′ and reverse,
5′-GGCATGGACTGTGGTCATGAG-3′. Following 5 min of denaturation, 30
cycles of amplification were performed, each cycle consisted of: 55
sec at 95°C, 40 sec at 55°C and 65 sec at 72°C; the reaction was
activated at 72°C for 5 min and terminated at 4°C.
Reporter gene assay
Cells (0.3 ml; 1×106/ml) were seeded onto
a six-well microplate for 24 h. Luciferase reporter plasmids
(Beyotime Institute of Biotechnology) for hypoxia-inducible factor
(HIF) and eukaryotic translation initiation factor 4E (eIF4E) as
well as Renilla luciferase plasmids were transfected into
cells using LipofectamineTM 2000 and incubated for 24 h.
Cells were then centrifugated, collected and the fluorescence
intensity of fluorescein was detected using a dual luciferase assay
kit. Renilla luciferase was used as the internal
control.
ELISA assay
Transforming growth factor (TGF)-β detection was
performed using an ELISA kit (R&D Systems, Minneapolis, MN,
USA) according to the manufacturer’s instructions. In brief, cells
(0.3 ml; 1×106/ml) were seeded onto a six-well
microplate for 24 h, the serum-free medium was replaced for and
cells were incubated for a further 24 h. Absorbance was then
determined using a microplate reader at 490 nm.
Statistical analysis
A one-way analysis of variance was used to determine
differences between groups. Data analyses were performed using SPSS
11.0 statistical software (SPSS Inc., Chicago, IL, USA). P<0.05
was considered to indicate a statistically significant difference
between values.
Results
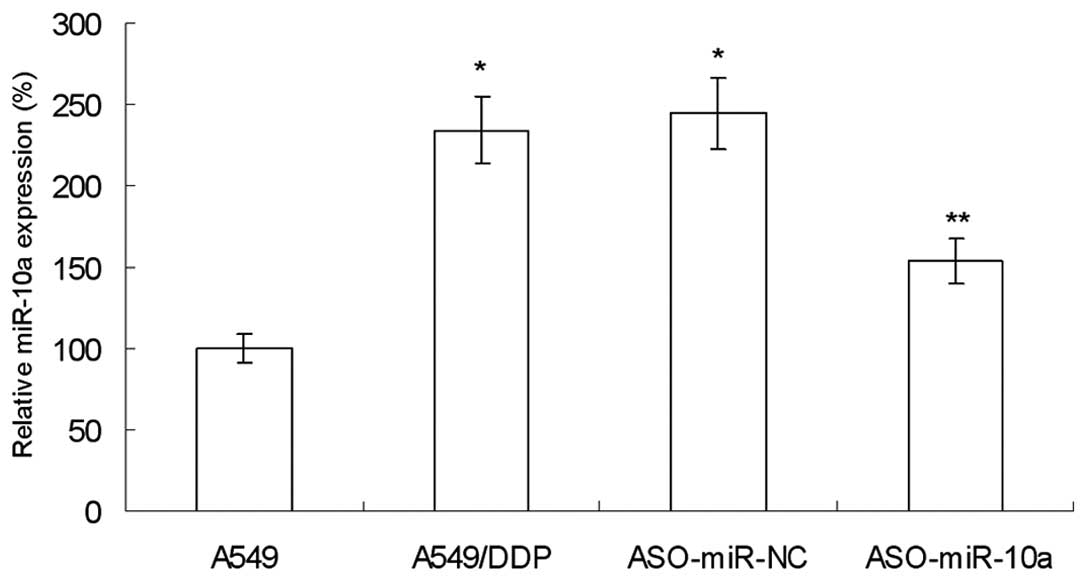
Increased expression of miR-10a in lung
cancer DDP-resistant A549/DDP cells
In order to study the drug-resistance reversal
effect of miR-10a, the expression of miR-10a was examined in A549
and DDP-resistant A549/DDP cells. As shown in Fig. 1, the expression of miR-10a in
A549/DDP cells was significantly increased compared with that of
A549 cells, indicating that miR-10a may be closely associated with
DDP-resistance.
MiR-10a silencing increases DDP
sensitivity in A549/DDP cells
An miR-10a antisense oligonucleotide (ASO-miR-10a)
was used to silence the expression of miR-10a in A549/DDP cells.
Cell viability was the assess in miR-10a cells treated with various
concentrations of DDP (Fig. 2A).
The results showed that following silencing miR-10a, the
sensitivity of A549/DDP cells to DDP was significantly enhanced. In
addition, the effect of miR-10a silencing on DDP-induced apoptosis
was investigated (Fig. 2B). The
results showed that miR-10a silencing increased the apoptotic rate
of A549/DDP cells. This therefore suggested that miR-10a had an
important role in DDP-resistance inA549/DDP cells.
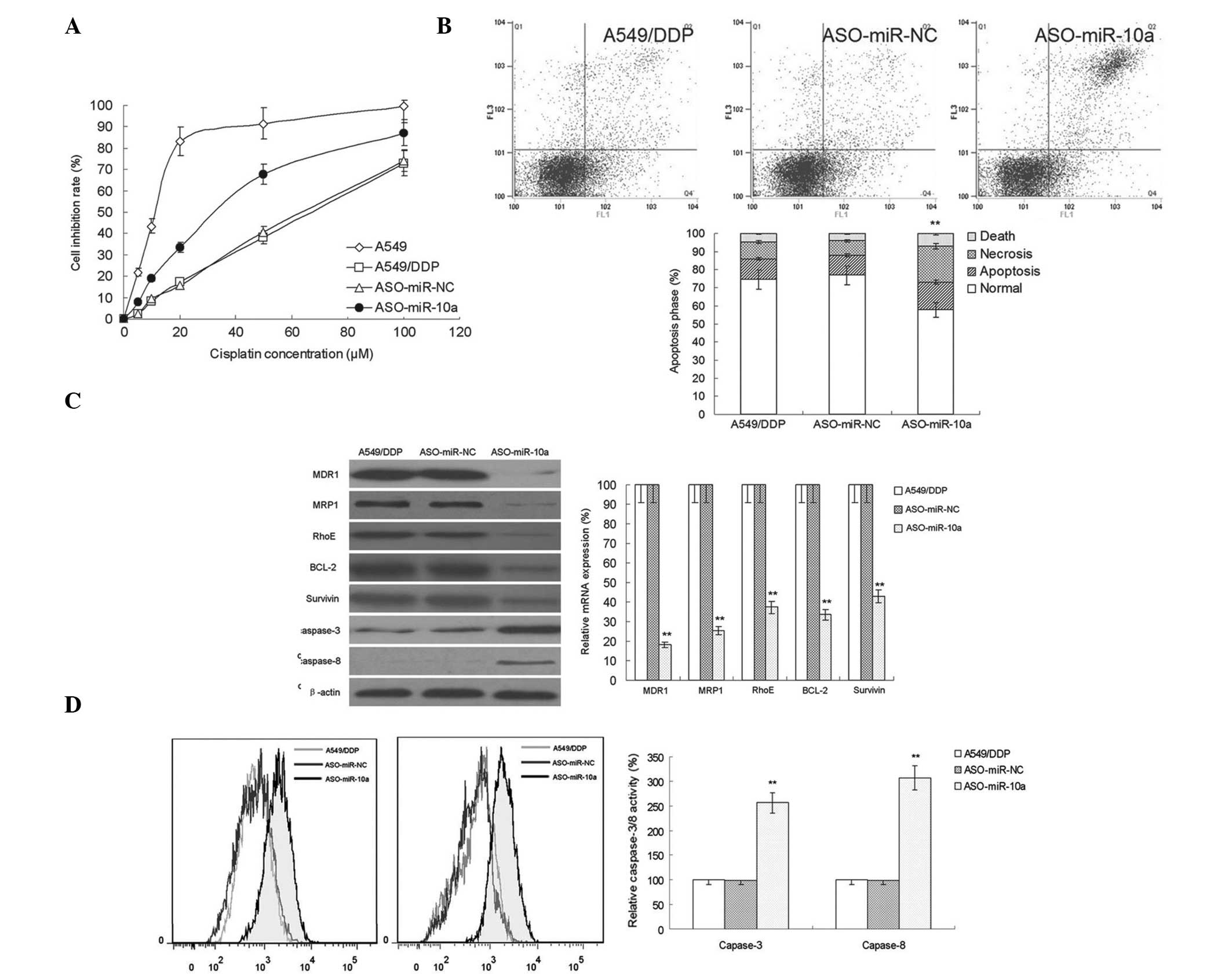 | Figure 2miR-10a silencing reverses DDP
resistance in A549/DDP cells. (A) MTS assay for cell inhibition
demonstrated the effect of miR-10a silencing on DDP sensitivity in
cells in each group (n=10). (B) Flow cytometric analysis of the
apoptotic rate of A549/DDP cells following transfection of
ASO-miR-NC or the miR-10a inhibitor ASO-miR-10a (n=3). (C) Western
blot analysis and quantitative polymerase chain reaction were used
to detect the protein and mRNA expression, respectively, of MDR1,
MRP1, RhoE, Bcl-2 and survivin in A549/DDP cells following
transfection of ASO-miR-NC or ASO-miR-10a (n=5) (D) Flow cytometric
analysis of caspase-3/8 activity in A549/DDP cells following
transfection of ASO-miR-NC or ASO-miR-10a (n=3). Values are
presented as the mean ± standard deviation. **P<0.05
vs. the A549/DDP group. DDP, cisplatin; A549 group, control;
A549/DDP group, DDP-resistant A549 cells without treatment;
ASO-miR-NC group, negative control group of A549/DDP cells
transfected with miR-10a; ASO-miR-10a group, A549/DDP cells
transfected with an miR-10a inhibitor; miR, microRNA; MDR1,
multidrug resistance protein 1; MRP1, multidrug
resistance-associated protein 1; Bcl-2, B-cell lymphoma 2. |
Bcl-2 and survivin are two important families
involved in the regulation of cell apoptosis (7,8); in
addition, RhoE also has an important role in tumor drug resistance
(9). In the present study, the
effect of miR-10a silencing on the protein and messenger (m) RNA
expression of Bcl-2, survivin and RhoE was investigated in
DDP-resistant A549/DDP cells (Fig.
2C). Compared with that of the control group and
ASO-NC-transfected cells, the expression of RhoE was significantly
reduced in the ASO-miR-10-transfected cells. In addition, the
expression of multi-drug resistance-associated genes MDR1 and MRP1
was investigated and the results showed that the mRNA and protein
expression levels of MDR1 and MRP1 were significantly decreased
following miR-10a silencing. Furthermore, as shown in Fig. 2D, there was an significant increase
in the expression and activity of caspase-3/8 in miR-10a-silenced
A549/DDP cells. These results indicated that miR-10a regulated the
expression of apoptosis-associated genes in order to enhance drug
resistance; therefore, Bcl-2, Survivin, RhoE and caspase-3/8 may be
potential targets for the inhibition of apoptosis in A549/DDP
cells.
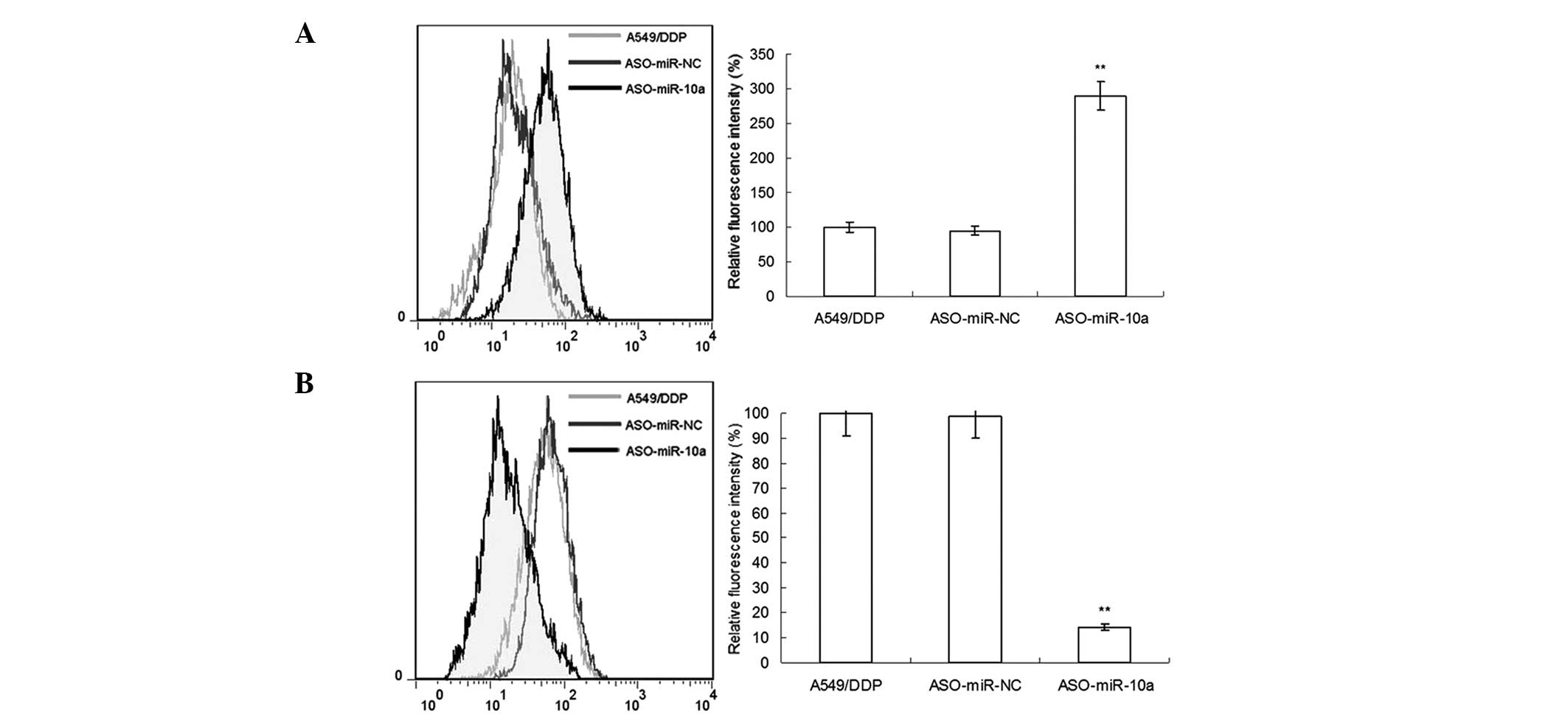
miR-10a silencing suppresses the drug
efflux of A549/DDP cells
The drug-resistance mechanisms of tumors were
reported to be established due to the efflux function of cells
(10,11). It was therefore hypothesized that
the effect of miR-10a silencing on DDP sensitivity may, at least in
part, be due to the suppression of the efflux ability of cells. In
the present study, the efflux ability of cistplatin-resistant
A549/DDP cells on rhodamine-123 dye was evaluated following miR-10a
silencing. As shown in Fig. 3A,
the concentration of rhodamine-123 in A549/DDP cells following
ASO-miR-10a transfection was significantly increased compared with
that of the A549/DDP and ASO-NC groups, indicating that the efflux
capacity was significantly reduced. Furthermore, the expression of
P-gp on the cell surface of A549/DDP cells was examined using flow
cytometry; as shown in Fig. 3B,
miR-10a silencing markdely reduced the concentration of P-gp
compared to that of the A549/DDP and ASO-NC groups. These
experimental results indicated that drug efflux had an important
role in increasing the drug sensitivity of A549/DDP cells following
miR-10a silencing.
MiR-10a silencing regulates drug
resistance associated signaling pathway
Tumor cells are able to enhance their proliferation,
survival, invasion and metastasis abilities via the secretion of
cytokines (12). In the present
study, the secretion of TGF-β was evaluated using an ELISA assay
and the results demonstrated that miR-10a silencing decreased the
secretion of TGF-β (Fig. 4A).
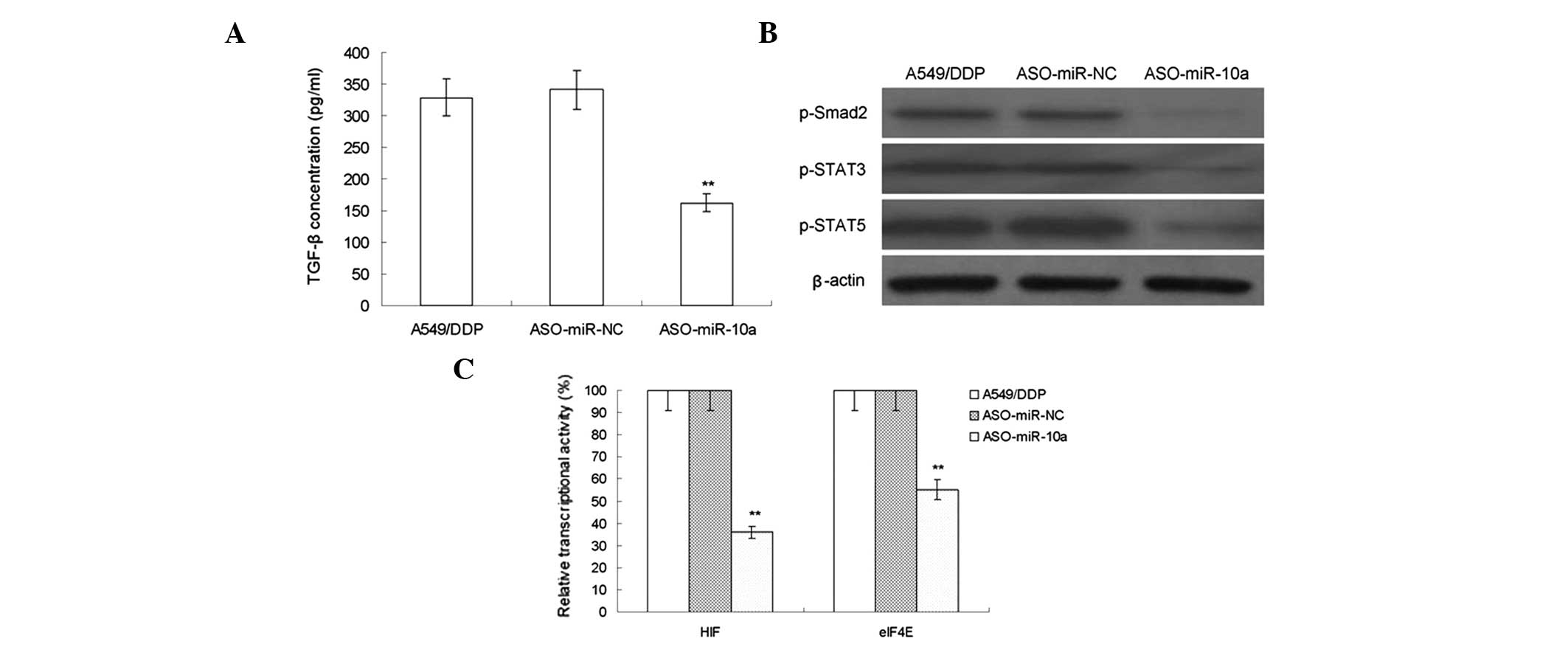 | Figure 4The effect of MiR-10a silencing on
regulating the drug resistance-related signaling pathway of
A549/DDP cell lines. (A) ELISA assays were used to evaluate the
secretion of TGF-β in A549/DDP cells following transfection of
ASO-miR-NC or the miR-10a inhibitor ASO-miR-10a (n=10). (B) Western
blot analysis of phosphorylated levels of Smad2, STAT3 and STAT5 in
in A549/DDP cells following transfection of ASO-miR-NC or the
miR-10a inhibitor ASO-miR-10a. (C) Luciferase gene report assay was
used to detect the activity of HIF and eIF4E in A549/DDP cells
following transfection of ASO-miR-NC or the miR-10a inhibitor
ASO-miR-10a (n=10). Values are presented as the mean ± standard
deviation **P<0.05 vs. the A549/DDP group. DDP,
cisplatin; miR, microRNA; TGF-β, transforming growth factor β;
Smad, Sma- and Mad-related protein; STAT, signal transducer and
activator of transcription; HIF, hypoxia-inducible factor; eIF4E,
eukaryotic translation initiation factor 4E; p-, phosphorylated;
A549/DDP group, DDP-resistant A549 cells without transfection;
ASO-miR-NC group, negative control group of A549/DDP cells
transfected with miR-10a; ASO-miR-10a group, A549/DDP cells
transfected with an miR-10a inhibitor. |
The activation of drug resistance-associated
signaling pathways was reported to be an important indicator of
tumor drug resistance (13). In
the present study, the phosphorylation levels of Sma and Madrelated
protein (Smad)2, STAT3 and STAT5 in A549/DDP cells was determined
following miR-10a silencing. As shown in Fig. 4B, the phosphorylation levels of
Smad2, STAT3 and STAT5 were significantly reduced compared with
those of the control group. Furthermore, the luciferase reporter
gene method was used to detect the activity of the transcription
factors HIF and eIF4E in A549/DDP cells. As shown in Fig. 4C, following miR-10a silencing, the
activities of HIF and eIF4E were significantly reduced. These
experimental results indicated that miR-10a regulated the
TGF-β/Smad2/STAT3/STAT5 signaling pathway and downstream
transcription factors HIF and eIF4E in order to induce DDP
resistance in A549 cells.
Discussion
miRNA has been the subject of studies worldwide and
as a results of the continuous advancements of the associated
research, the important roles of miRNA in human physiological and
pathological processes have been gradually elucidate; most notably,
its roles in cancer (14).
DDP has been widely used in the clinical treatment
of cancer; however, the occurrence of DDP resistance has hindered
its application (15). The present
study demonstrated that miR-10a had an important role in the
DDP-resistant mechanisms of non-small cell lung cancer A549/DDP
cells. The expression of miR-10a in DDP-resistant A549/DDP cells
was found to be significantly increased compared to that of normal
A549 cells. Following miR-10a silencing in A549/DDP cells, DDP
sensitivity was significantly improved, suggesting that the
increase of miR-10a is a key mechanism of DDP resistance in lung
cancer. In addition, the results of the present study demonstrated
that miR-10a silencing increased the apoptotic rate of A549/DDP
cells; furthermore, the expression levels of Bcl-2 and Survivin
were markedly reduced, indicating that miR-10a inhibited apoptosis
via the inhibition of apoptosis-associated gene expression. RhoE is
a member of the small guanine triphosphatase protein superfamily
and previous studies have reported that RhoE had an important role
in the drug resistance of tumors (16). The present study found that miR-10a
regulated the expression of RhoE; following miR-10a silencing in
A549/DDP cells, the expression of RhoE was significantly decreased,
confirming the association between miR-10a and RhoE.
The most common mechanism of drug resistance in
tumor cells is the decrease of intracellular drug concentration
caused by increased drug efflux of associated proteins; MDR1 and
MRP1 were reported to be the most important genes involved in
enhancing drug efflux (17,18).
In the present study, inhibition of miR-10a was found to
significantly reduce the expression of MDR1 and MRP1, indicating
that miR-10a promoted their expression and therefore promoted DDP
resistance through the enhancement of drug efflux.
The secretion of cancer-promoting cytokines was
reported to be another important mechanism for tumor cells to
maintain proliferation and vitality through affecting the tumor
microenvironment and signal pathways (19). TGF-β has been shown to be an
important cytokines involved in tumor promotion (20). The present study demonstrated that
miR-10a silencing suppressed the expression of TGF-β in tumor
cells, suggesting that miR-10a may enhance drug resistance through
affecting the expression of TGF-β.
Tumor cells may maintain their survival abilities
through activating certain signaling pathways. The present study
demonstrated that miR-10a was able to regulate the
TGF-β/Smad2/STAT3/STAT5 signaling pathway, which has been reported
to be one of the most important drug resistance-associated
signaling pathways involved in drug efflux and inhibiting apoptosis
(21). The results of the present
study showed that miR-10a promoted the activity of Smad2, STAT3 and
STAT5 as well as the downstream transcriptional factors of HIF and
eIF4E in order to induce DDP resistance in A549 cells.
In conclusion, the present study demonstrated that
miR-10a had an important role in promoting drug resistance in
tumors through enhancing drug efflux and inhibiting apoptosis via
upregulation of MDR1, MRP1 and RhoE expression. In addition,
miR-10a promoted the expression of TGF-β as wells as regulated the
activity of the Smad2/STAT3/STAT5 pathway and its downstream
transcriptional factors of HIF and eIF4E, which may be the
potential mechanism of drug resistance in A549 cells. Therefore,
miR-10a may be an important drug target for improving cancer
treatment; however, further studies are required to explore the
clinical applications of miR-10a inhibitors.
References
|
1
|
Joshi P, Middleton J, Jeon YJ and Garofalo
M: MicroRNAs in lung cancer. World J Methodol. 2:59722014.
|
|
2
|
Liu R, Liu X, Zheng Y, Gu J, Xiong S,
Jiang P, Jiang X, Huang E, Yang Y, Ge D and Chu Y: MicroRNA7
sensitizes nonsmall cell lung cancer cells to paclitaxel. Oncol
Lett. 5:219322002014.
|
|
3
|
Cai J, Fang L, Huang Y, Li R, Yuan J, Yang
Y, Zhu X, Chen B, Wu J and Li M: miR-205 targets PTEN and PHLPP2 to
augment AKT signaling and drive malignant phenotypes in non-small
cell lung cancer. Cancer Res. 73:5402–5415. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ayaz L, Görür A, Yaroğlu HY, Ozcan C and
Tamer L: Differential expression of microRNAs in plasma of patients
with laryngeal squamous cell carcinoma: potential early-detection
markers for laryngeal squamous cell carcinoma. J Cancer Res Clin
Oncol. 139:1499–1506. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Köhler CU, Bryk O, Meier S, Lang K,
Rozynek P, Brüning T and Käfferlein HU: Analyses in human
urothelial cells identify methylation of miR-152, miR-200b and
miR-10a genes as candidate bladder cancer biomarkers. Biochem
Biophys Res Commun. 438:48–53. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhu Y, Liu XJ, Yang P, Zhao M, Lv LX,
Zhang GD, Wang Q and Zhang L: Alkylglyceronephosphate synthase
(AGPS) alters lipid signaling pathways and supports chemotherapy
resistance of glioma and hepatic carcinoma cell lines. Asian Pac J
Cancer Prev. 7:321932262014.
|
|
7
|
Ulasli SS, Celik S, Gunay E, Ozdemir M,
Hazman O, Ozyurek A, Koyuncu T and Unlu M: Anticancer Effects of
Thymoquinone, Caffeic Acid Phenethyl Ester and Resveratrol on A549
Non-small Cell Lung Cancer Cells Exposed to Benzo(a)pyrene. Asian
Pac J Cancer Prev. 14:6159–6164. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sun PL, Jin Y, Kim H, Seo AN, Jheon S, Lee
CT and Chung JH: Survivin expression is an independent poor
prognostic marker in lung adenocarcinoma but not in squamous cell
carcinoma. Virchows Arch. 463:427–436. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang C, Zhou F, Li N, Shi S, Feng X, Chen
Z, Hang J, Qiu B, Li B, Chang S, et al: Overexpression of RhoE has
a prognostic value in non-small cell lung cancer. Ann Surg Oncol.
14:2628–2635. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Prochazka L, Koudelka S, Dong LF, Stursa
J, Goodwin J, Neca J, Slavik J, Ciganek M, Masek J, Kluckova K, et
al: Mitochondrial targeting overcomes ABCA1-dependent resistance of
lung carcinoma to α-tocopheryl succinate. Apoptosis. 18:286–299.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Minami T, Kijima T, Otani Y, Kohmo S,
Takahashi R, Nagatomo I, Hirata H, Suzuki M, Inoue K, Takeda Y, et
al: HER2 as therapeutic target for overcoming ATP-binding cassette
transporter-mediated chemoresistance in small cell lung cancer. Mol
Cancer Ther. 11:830–841. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang YS, Miao LY, Liu L, Cai HR, Ding JJ,
Ren SX, Zhou CC and Schmid-Bindert G: Serum cytokine levels in
patients with advanced non-small cell lung cancer: correlation with
clinical outcome of erlotinib treatment. Chin Med J (Engl).
126:3931–3935. 2013.
|
|
13
|
Cha Y, Kim DK, Hyun J, Kim SJ and Park KS:
A3 binds to TGF-beta receptor I and induces Smad-independent,
JNK-dependent apoptosis in ovarian cancer cells. Cell Signal.
25:1245–1251. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang K, Zhang Y, Liu C, Xiong Y and Zhang
J: MicroRNAs in the diagnosis and prognosis of breast cancer and
their therapeutic potential. Int J Oncol. 3:9509582014.
|
|
15
|
Li Y, Li L, Guan Y, Liu X, Meng Q and Guo
Q: MiR-92b regulates the cell growth, cisplatin chemosensitivity of
A549 non small cell lung cancer cell line and target PTEN. Biochem
Biophys Res Commun. 440:604–610. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Avasarala S, Bikkavilli RK, Van Scoyk M,
Zhang W, Lapite A, Hostetter L, Byers JT, Heasley LE, Sohn JW and
Winn RA: Heterotrimeric g-protein, gα16, is a critical downstream
effector of non-canonical wnt signaling and a potent inhibitor of
transformed cell growth in non small cell lung cancer. PLoS One.
8:e768952013. View Article : Google Scholar
|
|
17
|
Surowiak P, Pawełczyk K, Maciejczyk A,
Pudełko M, Kołodziej J, Zabel M, Murawa D, Drag M, Gansukh T,
Dietel M and Lage H: Positive correlation between cyclooxygenase 2
and the expression of ABC transporters in non-small cell lung
cancer. Anticancer Res. 28(5B): 2967–2974. 2008.PubMed/NCBI
|
|
18
|
Ak Y, Demirel G and Gülbas Z: MDR1, MRP1
and LRP expression in patients with untreated acute leukaemia:
correlation with 99mTc-MIBI bone marrow scintigraphy. Nucl Med
Commun. 28:541–546. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang L, Deng Q, Wang J, Bai X, Xiao X, Lv
HR, Zhao MF and Liu PJ: Effect of CIK on multidrugresistance
reversal and increasing the sensitivity of ADR in K562/ADR cells.
Oncol Lett. 4:177817822014.
|
|
20
|
Kin R, Kato S, Kaneto N, Sakurai H,
Hayakawa Y, Li F, Tanaka K, Saiki I and Yokoyama S: Procyanidin C1
from Cinnamomi Cortex inhibits TGF-β-induced
epithelial-to-mesenchymal transition in the A549 lung cancer cell
line. Int J Oncol. 43:1901–1906. 2013.PubMed/NCBI
|
|
21
|
Tu B, Peng ZX, Fan QM, Du L, Yan W and
Tang TT: Osteosarcoma cells promote the production of pro-tumor
cytokines in mesenchymal stem cells by inhibiting their osteogenic
differentiation through the TGF-β/Smad2/3 pathway. Exp Cell Res.
320:164–173. 2014. View Article : Google Scholar
|