Introduction
Atopic dermatitis (AD) is a chronic pruritic and
inflammatory skin disease that generally occurs in children, and
the incidence of AD is increasing annually. AD is caused by a
variety of genetic and environmental factors and characterized by
inflammation and tissue damage in the skin (1,2).
Previous studies have reported that AD is associated with increased
expression of immunoglobulin E (IgE), secretion of T helper (Th) 2
cytokines and eosinophil count in the serum (3,4).
However, the pathogenesis of AD remains to be fully elucidated. As
a result, patients with AD are not treated with drugs specific to
AD, but are administered with anti-inflammatory or
immunosuppressive drugs. Certain drugs used for the long-term
treatment of AD have been reported to cause severe side effects,
including immunosuppression and dysfunction of the epidermal
barrier (5,6).
Arazyme is an extracellular metalloprotease produced
by Aranicola proteolyticus, which is an aerobic Gram
negative bacterium isolated from the intestine of the spider
Nephila clavata (7,8). A previous study demonstrated that
arazyme inhibits the secretion of inflammatory cytokines and
increases the expression of skin barrier proteins (9). In addition, arazyme has been reported
to suppress the inflammatory response induced by
lipopolysaccharides in endothelial cells (10). In the present study, the
anti-inflammatory effects of arazyme were investigated in AD-like
animal models, BALB/c and Nc/Nga mice.
Materials and methods
Enzyme purification
Arazyme was purified from extracellular fractions of
S. proteamaculan HY-3 (KCTC2390; Korean Collection for Type
Culture, Daejeon, Korea) as previously described (8). In brief, extracellular fractions were
collected by centrifugation of the Luria-Bertani (LB) culture
medium (Sigma-Aldrich Korea, Seoul, Korea) at 5,000 × g for 10 min,
or by filtration using a 0.2-μm membrane filter (Pall Life
Sciences, Port Washington, NY, USA). Chromatography was
subsequently performed on a DEAE-cellulose column (GE Healthcare
Life Sciences, Little Chalfont, UK) equilibrated with 50 mM
potassium phosphate buffer (pH 7.6; Sigma-Aldrich Korea). Bound
proteins were then eluted with a 0.1–0.5 M sodium chloride
(Sigma-Aldrich Korea) gradient at a flow rate of 400 ml/h and
following this, each fraction was concentrated using a 10-kD
cassette membrane (Pall Life Sciences). The protein solution was
loaded onto a Sephadex G-75 column (GE Healthcare Life Sciences)
equilibrated with 50 mM potassium phosphate buffer (pH 7.8) at a
flow rate of 20 ml/h and fractions with proteolytic activity were
concentrated with a 10-kD cassette membrane and stored at −20°C.
Proteolytic activity was determined spectrophotometrically by
measuring absorbance at 405 nm (SpectroQuest UV-2800, cat. no.
S90424; Thermo Fisher Scientific, Inc., Waltham, MA USA), as
previously described (8).
Induction of allergic dermatitis in
BALB/c and Nc/Nga mice
A total of 40 five-week-old female BALB/c mice
(weight, 17–19 g) and 30 NC/Nga mice (weight, 19–21 g) were
purchased from Japan SLC, Inc. (Hamamatsu, Japan) and acclimated
for one week prior to the start of the experiments. Animals were
housed in an air-conditioned animal unit at 23±2°C and a humidity
of 50±10%. Mice were provided with solid feed (Rodfeed; Daehan
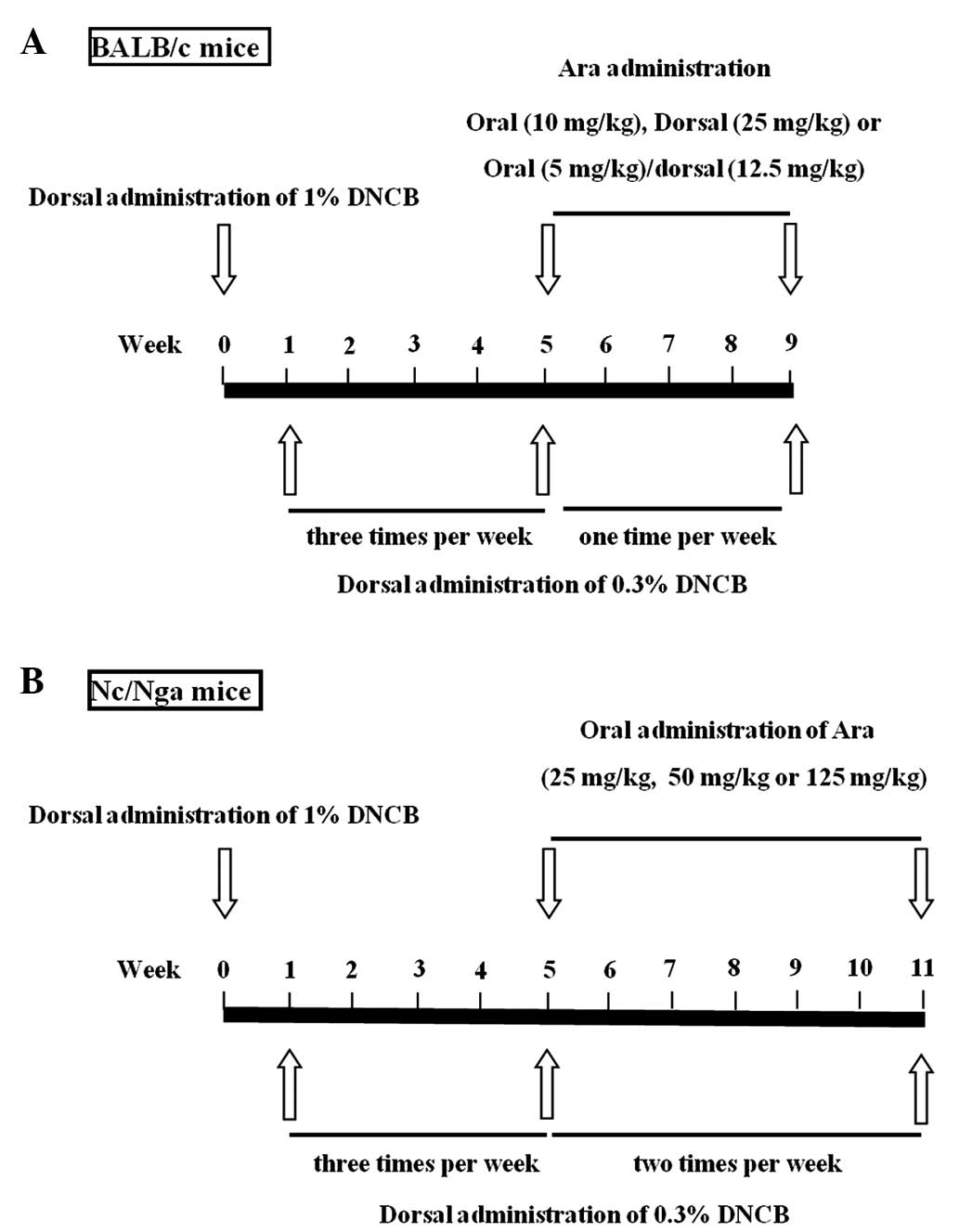
Biolink Co., Ltd., Eumsung, Korea). A schematic diagram of the
experimental procedure is provided in Fig. 1. Induction of AD was performed with
2,4-dinitrochlorobenzene (DNCB; Sigma-Aldrich, St. Louis, MO, USA)
in BALB/c and NC/Nga mice. In brief, 1% DNCB was dissolved in an
acetone-olive oil mixture (acetone/olive oil, 3:1; Sigma-Aldrich
Korea). The dorsal hair of the mice was removed with an electric
razor and no skin damage (e.g. chafed skin or hemorrhage) was
observed. A total of 0.15 ml 1% DNCB solution was applied to the
same area of dorsal skin. Following sensitization with 1% DNCB, the
BALB/c mice were dorsally treated with 0.3% DNCB three times/week
for 4 weeks, then once/week for 4 weeks. Nc/Nga mice were treated
with 0.3% DNCB three times/week for 4 weeks and then twice per week
for 6 weeks. The protocol for the care and treatment of the mice
was approved by the Institutional Animal Care and Use Committee of
Eulji University (Daejeon, Republic of Korea).
Arazyme administration
BALB/c mice were divided into the following eight
groups (n=5 in each group): Untreated; DNCB; oral arazyme (10
mg/kg); 25 mg/kg dorsal arazyme; combined treatment with 5 mg/kg
oral and 12.5 mg/kg dorsal arazyme; 5 mg/kg oral dexamethasone; 5
mg/kg dorsal dexamethasone; and combined treatment with 2.5 mg/kg
oral and 2.5 mg/kg dorsal dexamethasone, which were all purchased
from Sigma-Aldrich Korea. Nc/Nga mice were divided into the
following six groups (n=5 in each group): Untreated; DNCB; 25 mg/kg
arazyme; 50 mg/kg arazyme; 125 mg/kg arazyme; 5 mg/kg
dexamethasone. The DNCB, arazyme and dexamethasone groups were
dorsally administered with 1% DNCB and subsequently dorsally
treated with 0.3% DNCB. The DNCB, arazyme and dexamethasone groups
were treated with phosphate-buffered saline (PBS; Sigma-Aldrich
Korea), arazyme and dexamethasone via gastric inoculation with a
mouse-feeding needle (Cadence, Inc., Staunton, VA, USA) and/or
application to the same area of the dorsal skin. The untreated
group was treated with PBS without administration of DNCB, arazyme
or dexamethasone.
Histological analysis
Subsequent to sacrifice of the mice by
CO2 asphyxiation, the dorsal skin was removed, fixed in
Carnoy’s solution (Sigma-Aldrich Korea), embedded in paraffin
(Sigma-Aldrich Korea) and sectioned (5 μm-thick). The sections were
then stained with hematoxylin-eosin solution (Sigma-Aldrich Korea)
and subsequently examined by light microscopy (Leica Microsystems,
Wetzlar, Germany) for histological evaluation. Specifically, the
epidermis was evaluated for hypertrophy and infiltration by
inflammatory cells, while the dermis was evaluated for infiltration
by inflammatory cells.
Measurement of serum IgE
Blood was collected from the tail of the mice every
week. The serum was obtained by centrifugation and then stored at
−70°C until required. Total IgE levels in the serum were measured
using sandwich ELISA kits (BD Biosciences, San Jose, CA, USA)
according to the manufacturer’s instructions.
Splenocyte preparation
The BALB/c mice were sacrificed and subsequently
their spleens were removed under aseptic conditions. Splenocytes
were then isolated from the spleens as previously described
(11), after which the red blood
cells were hemolyzed using red blood cell lysis solution
(Sigma-Aldrich). Splenocytes were seeded in a 24-well plate at a
concentration of 5×106 cells/ml in RPMI-1640 medium with
1% penicillin-streptomycin and 10% fetal bovine serum (Gibco-BRL,
Grand Island, NY, USA).
ELISA
Splenocytes were pretreated in the absence or
presence of arazyme and then stimulated with 1 μg/ml concanavalin A
(Sigma-Aldrich Korea) for 24 h. The cell supernatants were
collected and the concentrations of interleukin (IL)-4, IL-5 and
IL-13 were measured in the supernatant by a sandwich ELISA [OptEIA™
Human IL-4 and IL-5 sets; BD Biosciences; and Human IL-13 DuoSet
kit, R&D Systems, Inc. (Minneapolis, MN, USA)] according to the
manufacturer’s instructions. The concentration of each protein was
calculated from the standard curves.
Evaluation of skin severity
The severity of dermatitis was assessed
macroscopically in a blinded experiment. The four indicators of
skin lesions were: i) Erythema/hemorrhage, ii) edema/swelling, iii)
excoriation/erosion and iv) dryness. Scoring was performed as
follows: 0 (no symptoms), 1 (mild), 2 (moderate) and 3 (severe)
(12).
Measurement of alanine aminotransferase
(ALT) and aspartate aminotransferase (AST)
The concentrations of ALT and AST were measured
using the Reitman-Frankel method (13) in the serum of BALB/c mice using ALT
and AST assay kits (Asan Pharm Co., Seoul, Korea) according to the
manufacturer’s instructions.
Statistical analysis
Values are expressed as the mean ± standard
deviation. Data were analyzed using Student’s t-test using SPSS
software, version 10.0 (SPSS Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Arazyme reduces the aggravation of
atopic-like skin lesions but has no effect on the serum IgE levels
in DNCB-induced BALB/c mice
The therapeutic effects of arazyme were investigated
in mice with DNCB-induced dermatitis by histological evaluation.
Histological analysis of the skin of mice in the untreated group
revealed that the tissue was normal, whereas mice in the DNCB group
exhibited epidermal hypertrophy, hyperkeratosis of the epidermis
and infiltration of inflammatory cells (Fig. 2A). Oral and oral/dorsal
administration of arazyme markedly ameliorated the
histopathological alterations as compared with those in the
dexamethasone group, while dorsal administration of arazyme
resulted in a small reduction in these alterations. Mice in the
group administered oral/dorsal dexamethasone exhibited atrophy of
the skin. Since IgE is known to act as an important pathogenic
factor in AD (3,4), it was investigated whether the anti-inhibitory
effects of arazyme were involved in the alteration of IgE
production. Although the serum levels of IgE in the DNCB group were
markedly upregulated compared with those in the untreated group,
arazyme was not observed to effectively reduce these elevated IgE
levels (Fig. 2B). The body weight
of mice in the arazyme group was comparable to that of mice in the
untreated and DNCB groups (Fig.
2C), while a reduction in body weight was observed in the
dexamethasone group after 5 weeks.
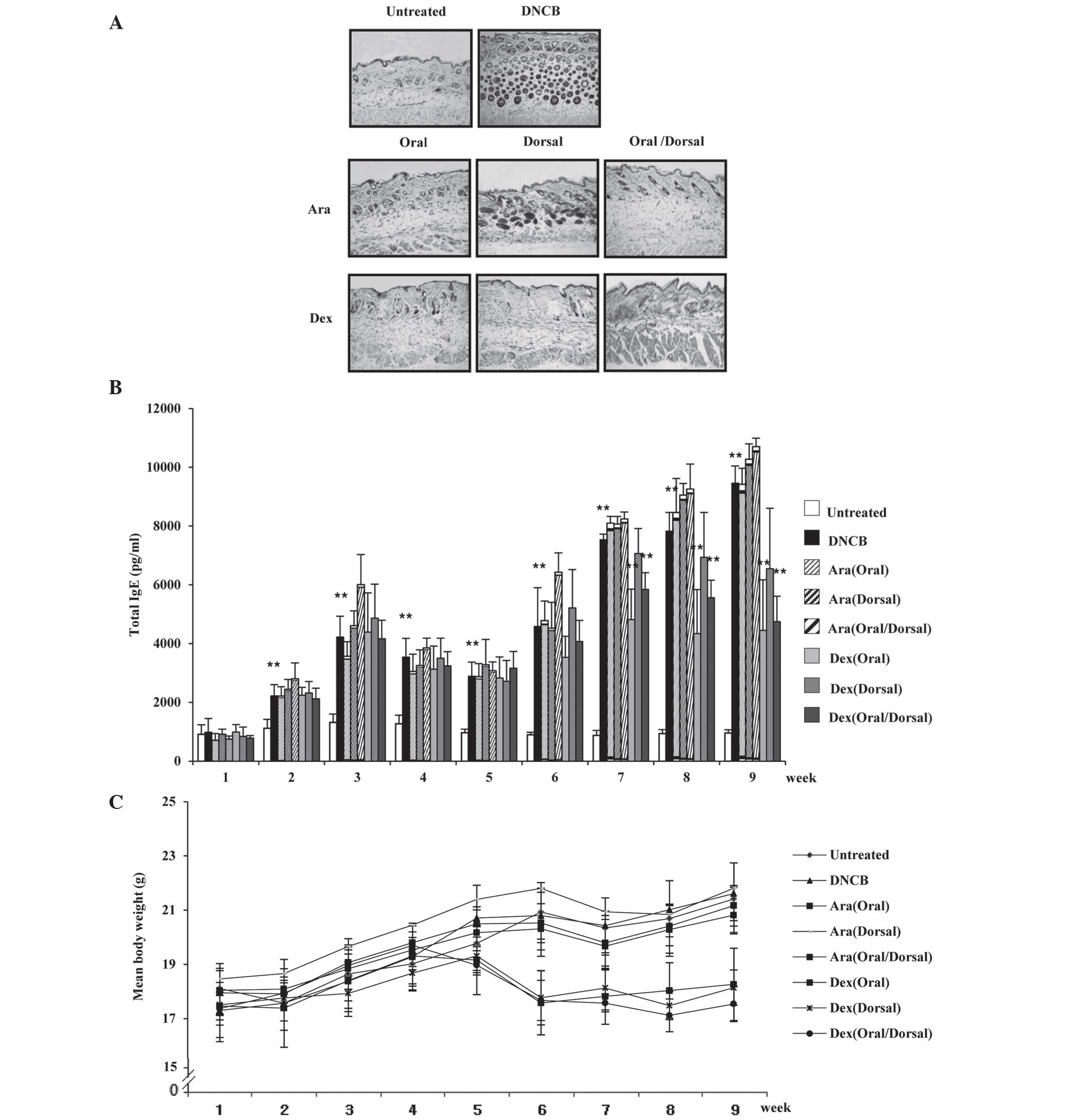 | Figure 2Arazyme reduces the aggravation of
atopic-like skin lesions, but has no effect on the serum levels of
IgE in DNCB-induced BALB/c mice. Mice were divided into the
following four groups: Untreated, DNCB, Ara and Dex. (A) For
histological analysis, the dorsal skin was fixed and embedded in
paraffin, sectioned, stained with hematoxylin and eosin solution
and examined by light microscopy (magnification, ×200). (B) Serum
was collected from the blood of the NC/Nga mice weekly and total
IgE levels in the serum were measured using sandwich ELISA kits.
(C) The mean body weight of mice was measured using an electric
scale. Values are presented as the mean ± standard deviation.
**P<0.01, untreated vs. DNCB group and DNCB vs.
drug-treated group. IgE, immunoglobulin E; DNCB,
2,4-dinitrochlorobenzene; Ara, arazyme; Dex, dexamethasone. |
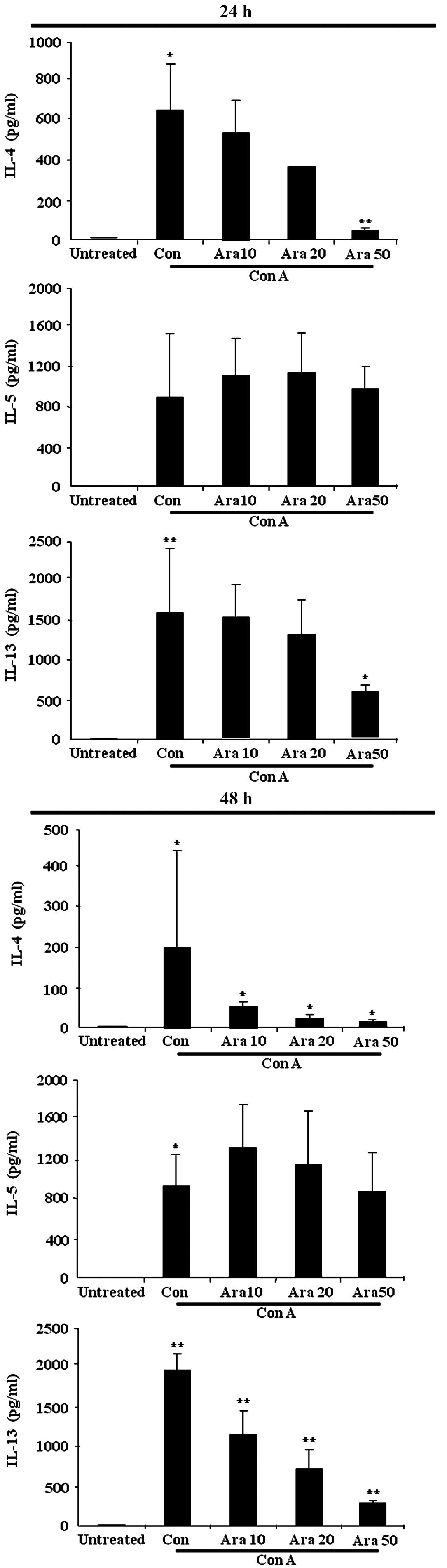
Arazyme suppresses cytokine levels in
mouse splenocytes
Inflammatory cytokines, particularly Th2 cytokines,
serve essential roles in allergic diseases such as AD (3,4); thus,
the effects of arazyme on cytokine production were investigated.
Splenocytes isolated from the spleens of BALB/c mice were treated
with arazyme for 1 h and subsequently with concanavalin A for 24 h.
The synthesis of IL-4, IL-5 and IL-13 was observed to increase in
the supernatant of splenocytes following stimulation with
concanavalin A for 24 and 48 h (Fig.
3). Pretreatment with arazyme inhibited the increased secretion
of IL-4 and IL-13. IL-5 release remained unchanged in the
arazyme-treated splenocytes.
Arazyme suppresses the severity of
dermatitis and IgE levels in DNCB-induced AD using NC/Nga mice
As the oral treatment of arazyme proved effective at
inhibiting histopathological features in AD-like BALB/c mice, the
effects of oral administration of arazyme on Nc/Nga mice were
investigated as an additional AD-like model. The DNCB group
exhibited epidermal hyperplasia, hyperkeratosis and inflammation
(Fig. 4A). Oral administration of
arazyme resulted in suppression of the histological phenomena
associated with AD at low concentrations (25 mg/kg), while
treatment with high concentrations (50 and 100 mg/kg) of arazyme
resulted in either a weak or absent effect. The severity of
dermatitis was evaluated every week. Clinical signs and symptoms of
AD developed subsequent to dorsal treatment with DNCB and these
symptoms were observed to worsen with time following the initial
treatment. As demonstrated in Fig.
4B, the DNCB group exhibited thick skin with severe erythema,
hemorrhage, edema, erosion and excoriation. However, oral
application of arazyme at low concentrations inhibited the
development of these skin conditions. In addition, 25 mg/kg arazyme
was observed to significantly reduce (**P<0.01) the
elevated IgE levels in the serum at weeks 9, 10 and 11 and the
suppressive effect of arazyme was comparable to that produced by
treatment with dexamethasone (Fig.
4C). However, high concentrations of arazyme had no inhibitory
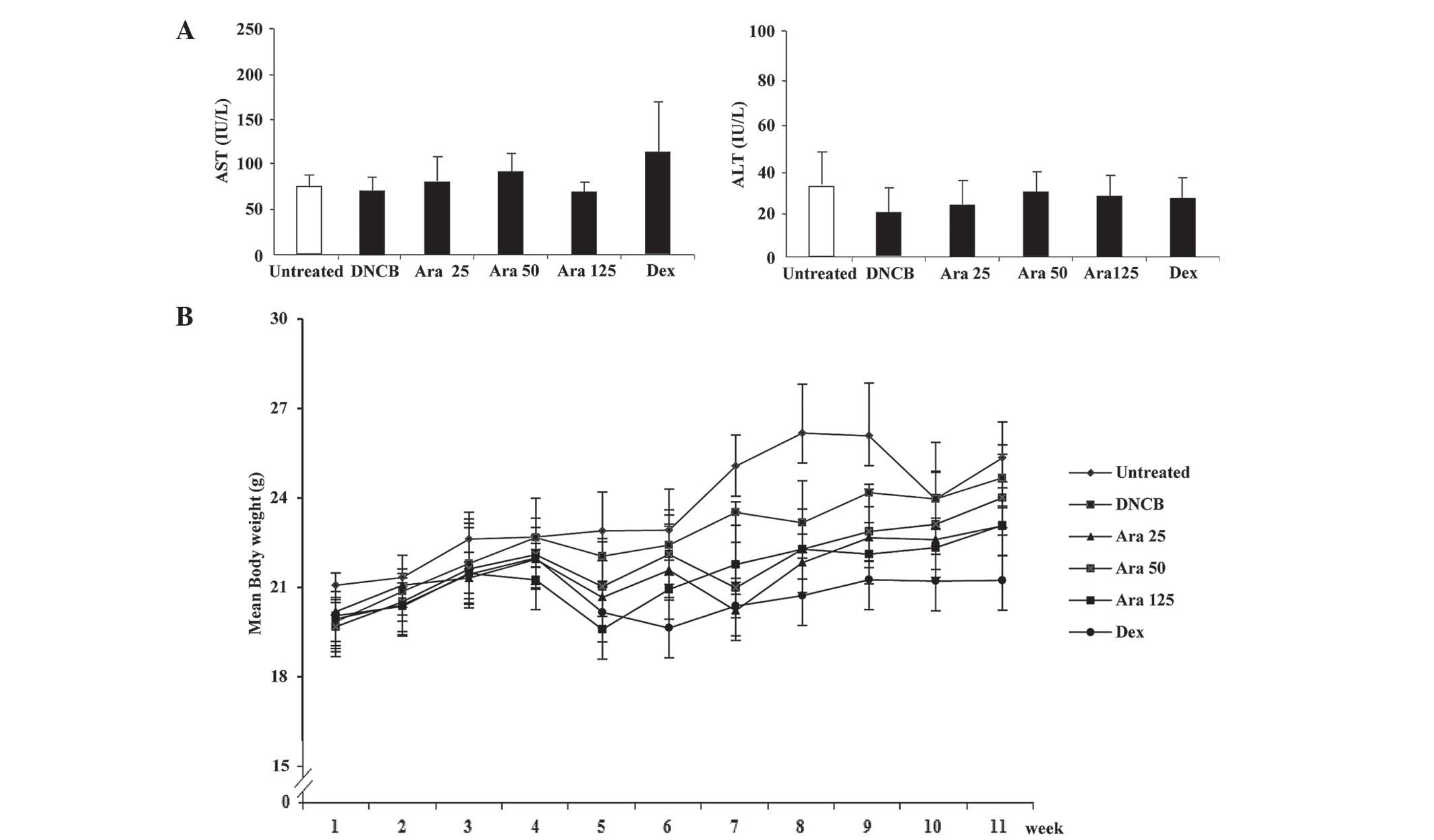
effect on the alterations in IgE levels. To confirm the
cytotoxicity of arazyme, the alterations in body weight, ALT and
AST levels in the serum were investigated. The levels of ALT and
AST were unchanged by administration of arazyme (Fig. 5A). Body weight was only identified
to be altered in the dexamethasone group (Fig. 5B).
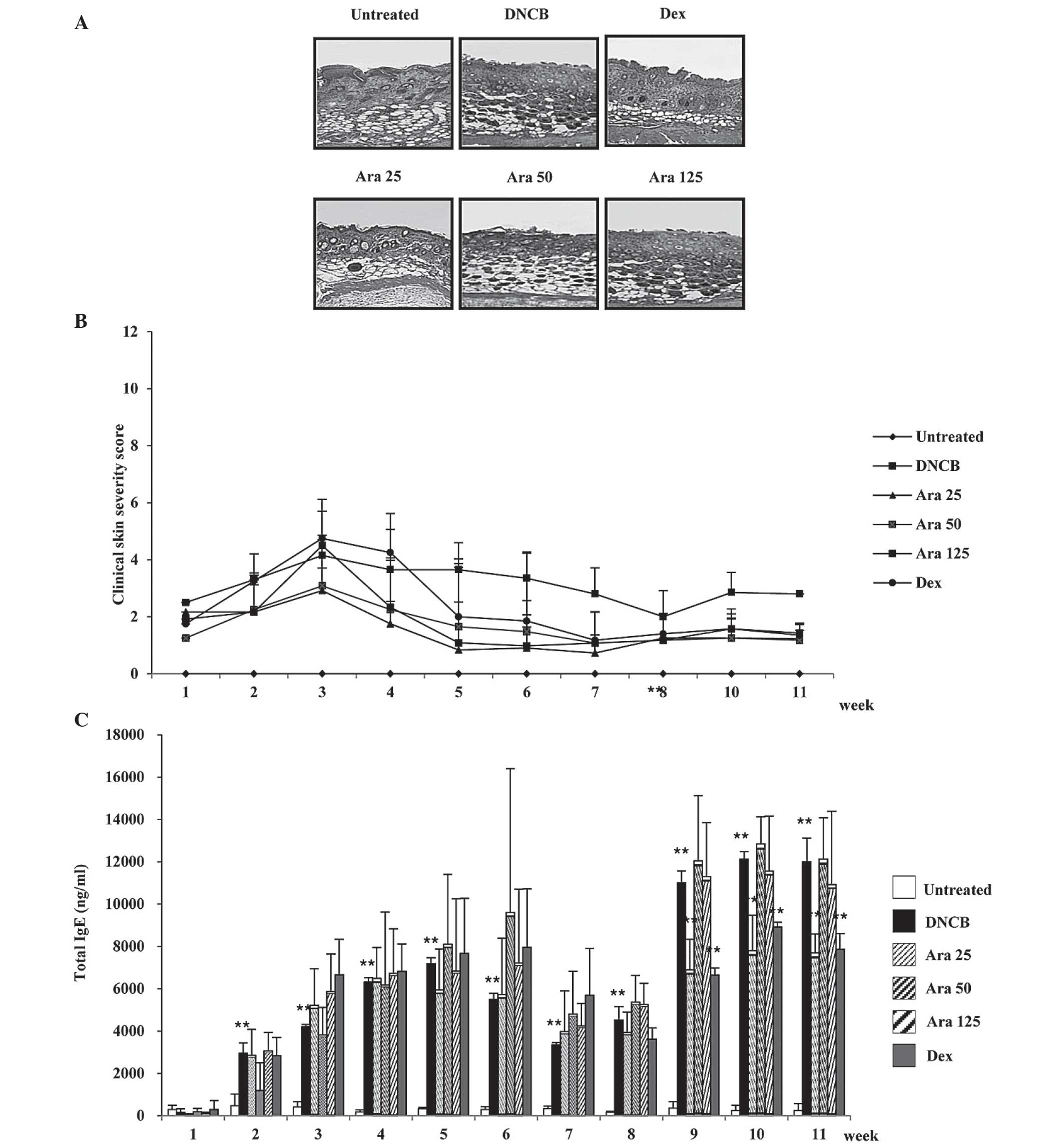 | Figure 4Arazyme suppresses the severity of
dermatitis and IgE levels in DNCB-induced AD using NC/Nga mice. The
mice were divided into the following four groups: Untreated, DNCB,
Ara and Dex. The DNCB, Ara and Dex groups were dorsally
administered with 1% DNCB and then dorsally treated with 0.3% DNCB.
Ara was administered orally at the following concentrations: Ara
25, 25 mg/kg; Ara 50, 50 mg/kg; Ara 125, 125 mg/kg. Dex was
administerd orally at 5 mg/kg. (A) For histological analysis, the
dorsal skin was fixed and embedded in paraffin, sectioned, stained
with hematoxylin and eosin solution and examined by light
microscopy (magnification, ×200). (B) The severity of dermatitis
was assessed macroscopically in a blinded experiment and (C) total
IgE levels in the serum were measured using sandwich ELISA kits.
Values are presented as the mean ± standard deviation.
**P<0.01, untreated vs. DNCB group and DNCB vs.
drug-treated group. Ara, arazyme; IgE, immunoglobulin E; DNCB,
2,4-dinitrochlorobenzene; AD, atopic dermatitis; Dex,
dexamethasone. |
Discussion
Arazyme isolated from Aranicola proteolyticus
has been previously observed to serve a protective role in hepatic
injury (8,14). Arazyme has been identified to exert
an inhibitory effect on the inflammatory response in human
umbilical vein endothelial cells induced by lipopolysaccharides and
on cytokine expression in inflammatory cells; furthermore, an
arazyme-induced upregulation of skin barrier protein levels has
been observed in keratinocytes (9,10).
Thus, the present study investigated whether arazyme alleviates the
clinical features of AD. Mouse models of AD are commonly classified
into three groups: i) Those that require epicutaneous
administration of sensitizers, ii) skin gene-defective transgenic
models and iii) models involving the spontaneous development of AD
(4,15). In the present study, BALB/c and
Nc/Nga mice were selected for use as AD-like mouse models and DNCB
was employed as the sensitizer. In BALB/c mice, arazyme was
identified to inhibit the histological features associated with AD.
Furthermore, oral administration of arazyme was observed to be more
effective than dorsal treatment. Arazyme additionally suppressed
the histopathological appearance and clinical severity score in
Nc/Nga mice. In contrast to BALB/c mice, arazyme lowered the serum
IgE levels in Nc/Nga mice. This difference may have been due to the
use of different mouse species. It is well known that immunological
responses of humans and mice are distinctly different; therefore,
caution should be taken prior to conducting a clinical trial. In
the present study, the effects of arazyme on the severity of AD
were not dose-dependent. To determine an appropriate concentration
of arazyme and overcome the different results obtained using
different animal models, future studies employing another model
such as a mite-induced or transgenic model would be beneficial.
The pathogenesis and progression of AD is caused by
various factors associated with immune dysregulation or
hypersensitivity and the balance of T helper (Th) 1/Th2 cytokines
(16–18). IL-4 results in the differentiation
of naive Th0 cells to Th2 cells and induces chronic inflammation
(15). Additionally, it was
recently reported that IL-4 regulates alternative macrophage
activation (19). The function of
IL-13 is similar to that of IL-4 (15). In the present study, arazyme
suppressed the secretion of IL-4 and IL-13, but had no effect on
that of IL-5. Inhibition of IL-4 and IL-13 may be correlated with
alleviation of histopathological features associated with AD.
Although increased circulation of IgE in AD is positively
correlated to IL-4 and IL-13 expression in CD4+ T cells (20), arazyme was not observed to be
effective at reducing the serum IgE levels in AD-like BALB/c mice.
In contrast to the effects of arazyme on IgE expression in BALB/c
mice, arazyme downregulated the serum levels of IgE and inhibited
histological inflammation in Nc/Nga mice.
To elucidate this difference, future studies are
required in order to determine how arazyme inhibits the
pathophysiological mechanisms of AD. Arazyme induces anti-oxidant
signaling in addition to the inhibition of Th2 cytokine release
(9,14). Additionally, arazyme induces
expression of filaggrin and involucrin included in skin barrier
proteins (9). As arazyme is a
metalloprotease, it may exert its function via a protease-activated
receptor. Further studies are required to examine these complex
inhibitory mechanisms in greater detail.
In conclusion, arazyme alleviated the clinical
features in BALB/c and Nc/Nga models of AD and diminished the
synthesis of Th2 cytokines, including IL-4 and IL-13, in addition
to the serum IgE levels. These observations indicated that arazyme
may be valuable for the treatment of allergic diseases such as
AD.
Acknowledgements
This research was supported by a grant from the
Korea Research Institute of Bioscience & Biotechnology Research
Initiative Program.
References
|
1
|
Bieber T: Atopic dermatitis. N Engl J Med.
358:1483–1494. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Barnes KC: An update on the genetics of
atopic dermatitis: scratching the surface in 2009. J Allergy Clin
Immunol. 125:16–29. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Habu Y, Seki S, Takayama E, Ohkawa T,
Koike Y, Ami K, Majima T and Hiraide H: The mechanism of a
defective IFN-gamma response to bacterial toxins in an atopic
dermatitis model, NC/Nga mice, and the therapeutic effect of
IFN-gamma, IL-12, or IL-18 on dermatitis. J Immunol. 166:5439–5447.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jin H, He R, Oyoshi M and Geha RS: Animal
models of atopic dermatitis. J Invest Dermatol. 129:31–40. 2009.
View Article : Google Scholar
|
|
5
|
Shiohara T, Hayakawa J and Mizukawa Y:
Animal models for atopic dermatitis: are they relevant to human
disease? J Dermatol Sci. 36:1–9. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Berke R, Singh A and Guralnick M: Atopic
dermatitis: an overview. Am Fam Physician. 86:35–42.
2012.PubMed/NCBI
|
|
7
|
Bersanetti PA, Park HY, Bae KS, Son KH,
Shin DH, Hirata IY, Juliano MA, Carmona AK and Juliano L:
Characterization of arazyme, an exocellular metalloprotease
isolated from Serratia proteamaculans culture medium. Enzyme Microb
Technol. 37:574–581. 2005. View Article : Google Scholar
|
|
8
|
Kwak J, Lee K, Shin DH, Maeng JS, Park DS,
Oh HW, Son KH, Bae KS and Park HY: Biochemical and genetic
characterization of arazyme, an extracellular metalloprotease
produced from Serratia proteamaculans HY-3. J Microbiol Biotechnol.
17:761–768. 2007.PubMed/NCBI
|
|
9
|
Kim IS, Kim MJ, Shin DH, Son KH, Park HY
and Lee JS: Arazyme inhibits cytokine expression and upregulates
skin barrier protein expression. Mol Med Rep. 8:551–556.
2013.PubMed/NCBI
|
|
10
|
Kim IS, Yang EJ, Shin DH, Son KH, Park HY
and Lee JS: Effect of arazyme on the lipopolysaccharide-induced
inflammatory response in human endothelial cells. Mol Med Rep.
10:1025–1029. 2014.PubMed/NCBI
|
|
11
|
Lee JS, Kim IS, Ryu JS, Kim JH, Kim JS,
Kim DH and Yun CY: The inhibitory effect of Duchesnea chrysantha
extract on the development of atopic dermatitis-like lesions by
regulating IgE and cytokine production in Nc/Nga mice. Phytother
Res. 26:284–290. 2012. View
Article : Google Scholar
|
|
12
|
Kim IS, Kim DH, Yun CY and Lee JS: A
(S)-(+)-decursin derivative,
(S)-(+)-3-(3,4-dihydroxy-phenyl)-acrylic acid
2,2-dimethyl-8-oxo-3,4-dihydro-2H,8H-pyrano[3,2-g]-chromen-3-yl-ester,
attenuates the development of atopic dermatitis-like lesions in
NC/Nga mice. Mol Biol Rep. 40:2541–2548. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Witter RF and Grubbs LM: An evaluation of
the Reitman-Frankel method for the determination of serum glutamic
oxalacetic transaminase. Clin Chim Acta. 13:524–527. 1966.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Park JK, Jeong DH, Park HY, Son KH, Shin
DH, Do SH, Yang HJ, Yuan DW, Hong IH, Goo MJ, Lee HR, Ki MR,
Ishigami A and Jeong KS: Hepatoprotective effect of Arazyme on
CCl4-induced acute hepatic injury in SMP30 knock-out mice.
Toxicology. 246:132–142. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tanaka A, Amagai Y, Oida K and Matsuda H:
Recent findings in mouse models for human atopic dermatitis. Exp
Anim. 61:77–84. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Romagnani S: Lymphokine production by
human T cells in disease states. Annu Rev Immunol. 12:227–257.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Suto H, Matsuda H, Mitsuishi K, Hira K,
Uchida T, Unno T, Ogawa H and Ra C: NC/Nga mice: a mouse model for
atopic dermatitis. Int Arch Allergy Immunol. 120(Suppl 1): 70–75.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang EJ, Lee JS, Yun CY, Kim JH, Kim JS,
Kim DH and Kim IS: Inhibitory effects of Duchesnea chrysantha
extract on ovalbumin-induced lung inflammation in a mouse model of
asthma. J Ethnopharmacol. 118:102–107. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Luzina IG, Keegan AD, Heller NM, Rook GA,
Shea-Donohue T and Atamas SP: Regulation of inflammation by
interleukin-4: a review of “alternatives”. J Leukoc Biol.
92:753–764. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yamanaka K and Mizutani H: The role of
cytokines/chemokines in the pathogenesis of atopic dermatitis. Curr
Probl Dermatol. 41:80–92. 2011. View Article : Google Scholar : PubMed/NCBI
|