Introduction
The mortality rate for out-of-hospital cardiac
arrest (OHCA) is as high as 89%. Even if return of spontaneous
circulation (ROSC) is successfully achieved, 50% of patients
succumb to the disease prior to hospital discharge (1). Ischemia/reperfusion (I/R) injury is a
major concern in patients with cardiac arrest (CA). Endothelial
cells are widely distributed and actively secrete multiple factors,
including nitric oxide, endothelin 1 and prostacyclin, which are
associated with vascular tension, blood coagulation and
inflammation. Therefore, the endothelium has been recognized as an
excellent target within the signal transduction mechanism of a
number of diseases, and subsequently as a key therapeutic target
for I/R injury (2). During the
reperfusion phase, the release of oxygen free radicals, cytokines,
coagulants and complement-activation products leads to marked
activation of the inflammatory response, with neutrophil adhesion
to the endothelium and the induction of whole body I/R injury
(3).
A novel approach for post-CA therapies may improve
clinical outcomes and so it has become one of the most important
areas of focus in resuscitation science. The post-resuscitation
abnormalities following CA observed are similar to the
immunological and coagulation disorders exhibited in sepsis.
Further investigations into anti-inflammation therapeutic
approaches are required for patients following successful
resuscitation. Previous studies have suggested that prostaglandin
E1 (PGE1) may have anti-inflammatory roles due to weakening of
leukocyte adhesion to the microvascular endothelium and lower
tissue expression of some inflammatory markers involved in the I/R
injury process (4–8). Currently, TTM is recommended as one
of the therapeutic strategies for patients with ROSC following CA
by the American Heart Association (9). However, it is unclear whether
anti-inflammatory effects comprise one of the main protective
mechanisms of TTM for PCAS (10–12).
The aim of the present study was to investigate the
potential benefits of PGE1 and TTM, with respect to
anti-inflammatory effects, for I/R injury to the renal
microvascular endothelium of rats with ROSC.
Materials and methods
Animals and reagents
A total of 70 specific-pathogen free healthy male
Sprague-Dawley rats (age, 12 to 14 weeks; weight, 370±20 g) were
purchased from Chengdu Dashuo Biological Technology Co., Ltd.
(Chengdu, China). The rats were housed under a 12/12 h light/dark
cycle in a temperature-(22±2°C) and humidity-(40 to 60%) controlled
room with free access to fresh water and standard laboratory food.
The procedures for animal care were approved by the Committee on
the Ethics of Animal Experiments at Sichuan University (Sichuan,
China).
The bipolar pacing electrode used for inducing
ventricular fibrillation was the SelectSecure Model 3830
(Medtronic, Inc., Minneapolis, MN, USA). The PGE1 solution (5
µg/ml) was purchased from Beijing Tide Pharmaceutical Co., Ltd.
(Beijing, China). The vascular endothelial (VE)-cadherin antibody
(cat. no. #2500) was purchased from Cell Signaling Technology, Inc.
(Danvers, MA, USA). Vascular endothelial growth factor receptor
(VEGFR) antibody (cat. no. #BS4205) was purchased from Bioworld
Technology, Inc. (St. Louis Park, MN, USA). The RNA prep pure,
Quant cDNA and SuperReal PreMix Plus (SYBR-Green) kits were
purchased from Tiangen Biotech Co., Ltd. (Beijing, China). The rat
TM ELISA kit (cat. no. #CSB-E07939r) was purchased from Cusabio
Biotech Co., Ltd. (Wuhan, Hubei, China). The rat IL-6 (cat. no.
#ab00772) and TNF-α ELISA kit (cat. no. #ab100785) were purchased
from Abcam (Shanghai, China).
Animal model and experimental
protocol
A total of 14 rats were included in each group. Rats
without VF were considered to be the Sham group (S). VF was induced
in the experimental rats by transesophageal cardiac pacing with an
alternating current (50 Hz, 6 mA, 4 msec) for 150 s (13,14).
After 4 min, the rats with cardiac arrest were resuscitated with
200 times/min chest compression using a home-made animal
cardiopulmonary resuscitator. ROSC for the rats was defined as the
return of supraventricular rhythm with a mean aortic pressure of
≥60 mmHg for a minimum of 10 min (15). A total of 56 rats with successful
cardiopulmonary resuscitation (CPR) were established as the ROSC
model and then randomly divided into 4 groups: ROSC control group
(R), PGE1 group [P; 1 ml PGE1 solution was administered
intravenously with a syringe pump in 1 min (high injection speed
would decrease the success rate of resuscitation)], TTM group (T;
body temperature was decreased to 33±1°C within 30 min by placing
ice around the rats) and PGE1/TTM group (PT; combined treatment
with PGE1 and mild hypothermia). Blood samples were drawn from the
left femoral vein of 5 rats from each group at 0.5, 4 and 8 h to
evaluate the concentration of TM, TNF-α and IL-6. In every group, 3
rats were sacrificed at each time point (0.5, 4 and 8 h) for
VE-cadherin and VCAM-1 mRNA expression analysis with the renal
tissue homogenate. At 8 h post treatment, hematoxylin and eosin
(H&E) and VE-cadherin/VEGFR double fluorescent
immunohistochemistry (IHC) staining was performed (Fig. 1).
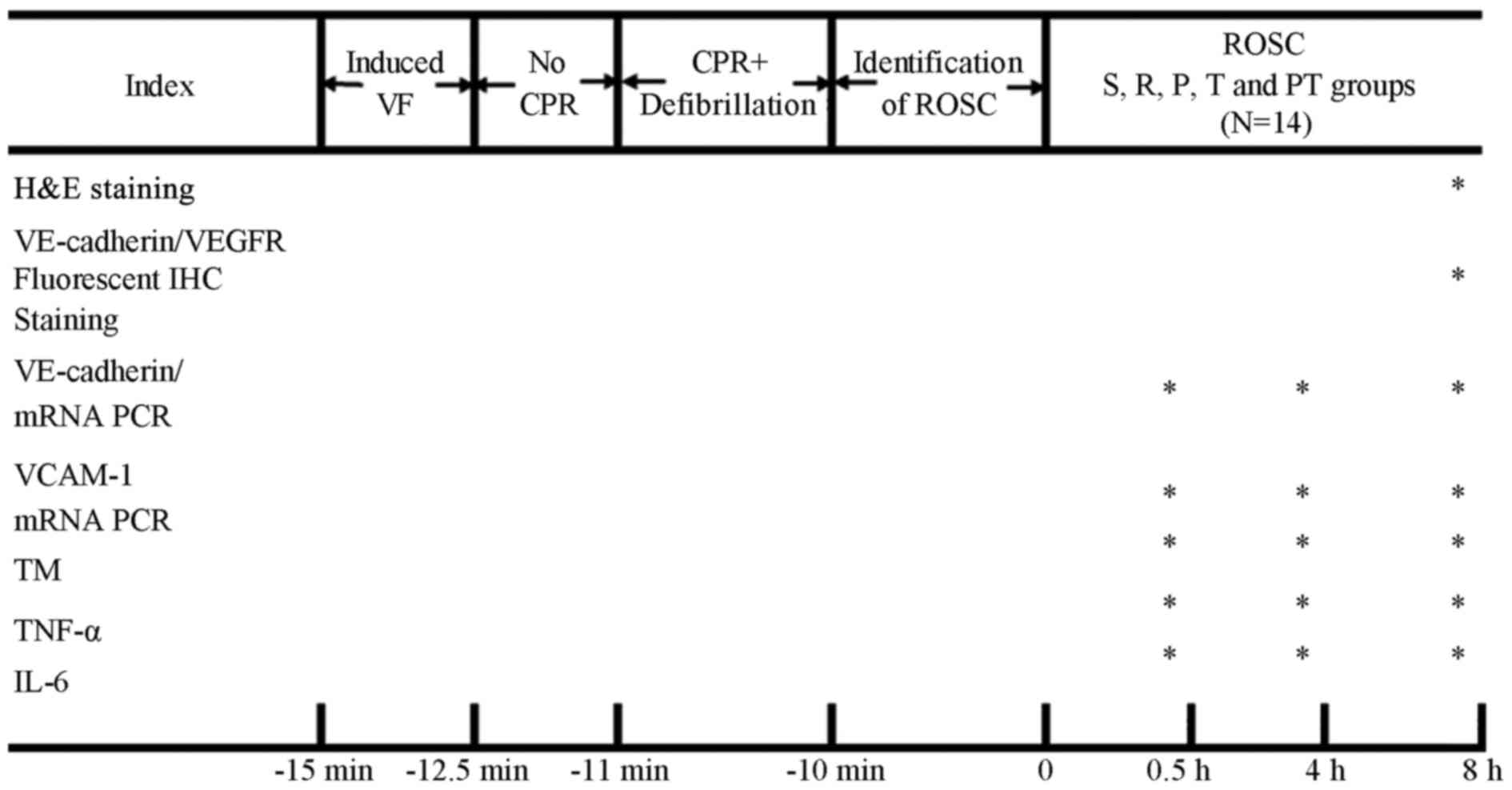 | Figure 1.Schematic of the protocol and
evaluation indexes. The asterisks (*) indicate which experimental
analyses were performed at different time point. H&E staining
and VE-cadherin/VEGFR fluorescent IHC staining were evaluated at 8
h following ROSC. The expression of VE-cadherin and VCAM-1 mRNA,
and the levels of TM, IL-6 and TNF-α in the plasma were analyzed at
0.5, 4 and 8 h following the induction of ROSC. ROSC, return of
spontaneous circulation; R, ROSC control group; P, prostaglandin E1
group; T, target temperature management group; PT, prostaglandin
E1/target temperature management group; S, sham group; VF,
ventricular fibrillation; CPR, cardiopulmonary resuscitation;
H&E, hematoxylin and eosin staining; VE, vascular endothelial;
VEGFR, vascular endothelial growth factor receptor; IHC,
immunohistochemistry; PCR, polymerase chain reaction; TM,
thrombomodulin; TNF-α, tumor necrosis factor-α; IL-6,
interleukin-6. |
H&E staining
H&E staining was performed on tissue slides as
described previously (16). Cell
edema, inflammatory cell infiltration and micro-thrombus formation
were examined by microscopy using the blind method. Tubular injury
was scored according to Pallor's method (17): In total, 100 tubules from 10
different high power fields were scored. Higher scores represented
more severe damage (maximum score/tubule was 10), with points given
for the presence and extent of tubular epithelial cell flattening
(1 point), brush border loss (1 point), cell membrane bleb
formation (1 or 2 points), interstitial edema (1 point),
cytoplasmic vacuolization (1 point), cell necrosis (1 or 2 points)
and tubular lumen obstruction (1 or 2 points). Two technicians
calculated these scores and the average values of the two sets of
scores were recorded.
Fluorescent IHC staining
VE-cadherin/VEGFR double fluorescent IHC staining
was performed according to the manufacturer's instructions
(18). I/R injury to the RMEC was
assessed by the extent of the damage exhibited by VE-cadherin
immunostaining in the majority of the renal microvascular
endothelium.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Renal total RNA was extracted using the RNA prep
pure kit according to manufacturer's instructions. The quantity of
RNA product was determined using a UV-Visible spectrophotometer
(Thermo Scientific Evolution 201; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) and the ratio of 28S, 18S and 5S bands on a 2%
agarose gel. Then, total RNA was reverse transcribed to cDNA using
the Quant cDNA kit (Tiangen Biotech Co., Ltd.), according to the
manufacturer's instructions. cDNA was used as a template for
quantification of the genes of interest by their primers on the
CFX-96 Touch Real-time System (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). The Super Real PreMix Plus (SYBR-Green) kit
(Tiangen Biotech Co., Ltd.) was used for qPCR, according to the
manufacturer's instructions. The thermocycling conditions for qPCR
were as follows: 95°C for 15 min, then 40 cycles of 95°C for 10
sec, 55°C for 20 sec and 72°C for 30 sec. Actin served as the
internal control. The primers used were as follows: VE-cadherin,
forward, 5′-CATCCGCAAGACCAGTGAC-3′ and reverse,
5′-ACCACGTCCTTGTCTGTTGC-3′; VACM-1, forward,
5′-TACATTGGCACCATCTCA-3′ and reverse, 5′-GTTCAGCATCAGGGAGTT-3′;
β-actin, forward, 5′-CCCATCTATGAGGGTTACGC-3′ and reverse,
5′-TTTAATGTCACGCACGATTTC-3′.
ELISA
ELISA kits were used for the evaluation of TM, TNF-α
and IL-6 concentrations in the blood collected at 0.5, 4 and 8 h
for each group and were performed according to manufacturer's
instructions.
Statistical analysis
Data are presented as mean ± standard deviation.
Comparisons between groups were analyzed by one-way analysis of
variance. Comparisons between 2 groups with P<0.05 were further
analyzed by Student-Newman-Keuls analysis. Using Pearson's
correlation analysis, the correlation between TM peak concentration
at 8 h and the concentration of TNFa, and between TM peak
concentration and the concentration of IL-6 in each group was
analyzed. P<0.05 was considered to indicate a statistically
significant difference. All statistical analyses were performed
using SPSS 19.0 software (IBM SPSS, Armonk, NY, USA).
Results
PGE1, TTM and PGE1/TTM combined
interventions demonstrate different levels of protective effects on
the renal tissue of rats with ROSC
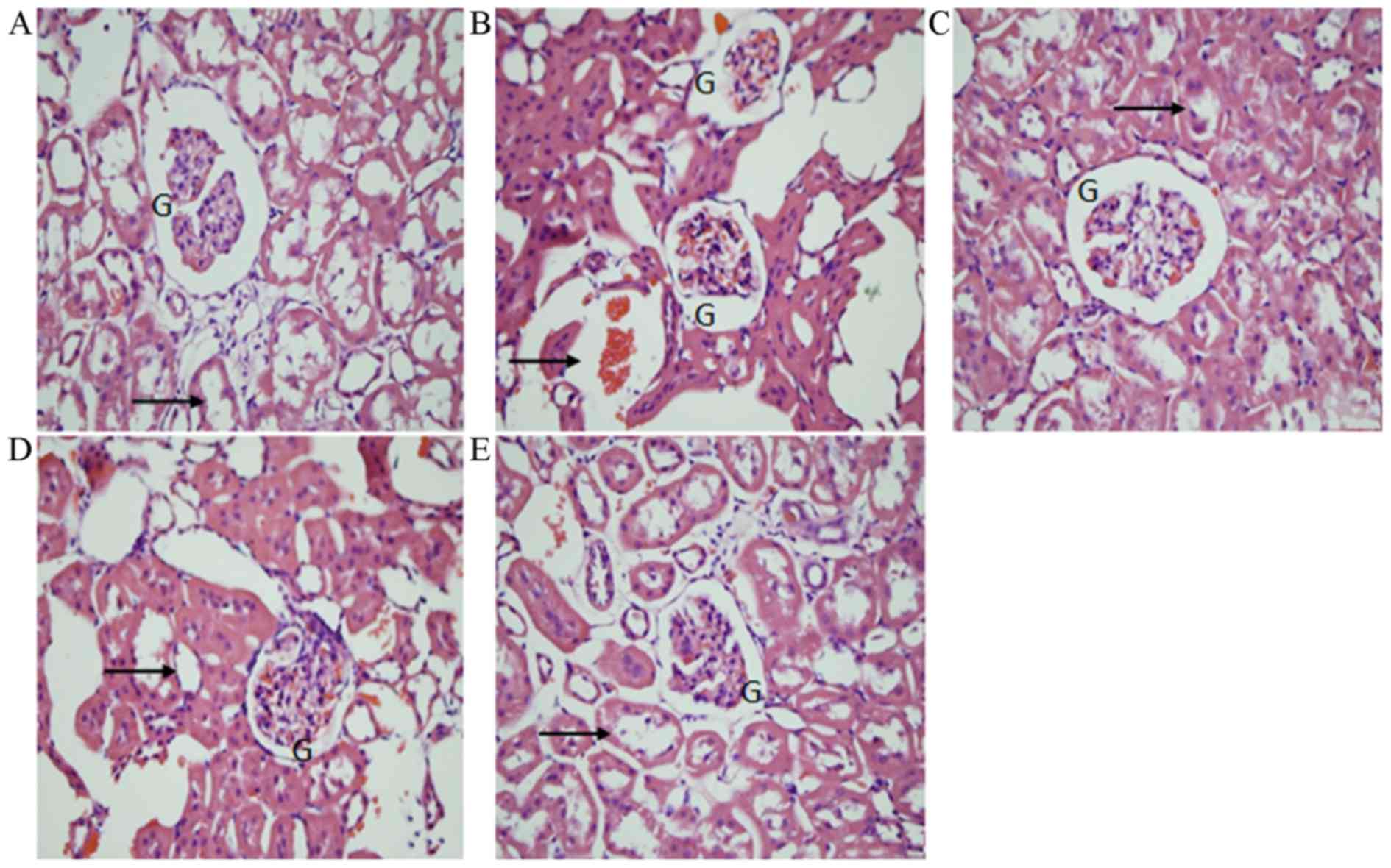
Rat kidney sections were stained using H&E and
examined using a light microscope (magnification, ×400; Fig. 1). The morphology of the glomeruli
and tubules was assessed. The normal structure was preserved in the
S group, while a decrease in glomerulus size and widespread
degeneration of the tubular architecture, intratubular cast
formation and luminal congestion with extensive loss of the brush
border were observed in the R group. The P and T groups exhibited
marked improvements in histological features associated with renal
injury. In the PT group, there was no significant difference when
compared with the sham group (Fig.
2).
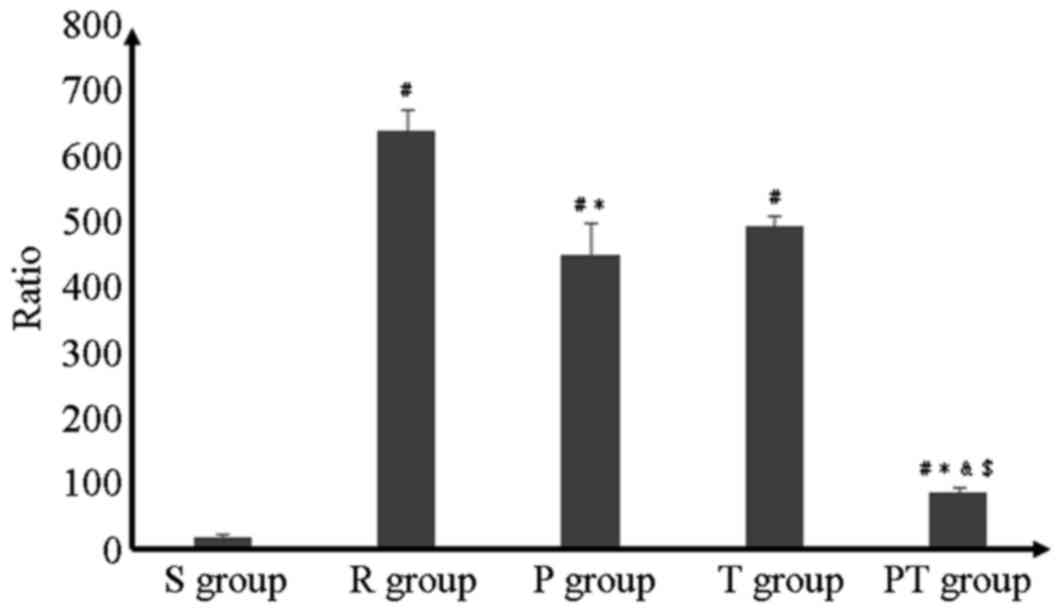
Blind review of the specimens from different groups
revealed greater tubular injury in the R group. The P and PT groups
exhibited a significant protective effect; the cytoplasmic
vacuolization, cellular necrosis, tubular luminal debris and
obstruction were much more marked in the R group (P<0.05).
Statistical analysis revealed that the PT group experienced greater
benefits with respect to reductions in injury than the P and T
groups (P<0.05; Fig. 3).
Benefits of PGE1, TTM and PGE1/TTM
combined interventions for I/R injury to the renal microvascular
endothelium in rats with ROSC
The kidney is a blood vessel-rich organ. The
microvascular endothelial cells are primarily present in the
glomerular and peritubular capillary bed. To determine the extent
of the damage caused by I/R injury to the RMEC, the pattern of
VE-cadherin immunostaining was examined. Under physiological
conditions, VE-cadherin immunostaining was noted along the renal
microvascular endothelium. Following I/R injury, the loss of
VE-cadherin immunostaining in the majority of the renal
microvascular endothelium suggested a disruption of the normal
junctional complex. The destruction of VE-cadherin in the P, T and
PT groups was markedly decreased compared with the R group, while
the PT group was similar to the sham operation group under
physiological conditions (Fig.
4).
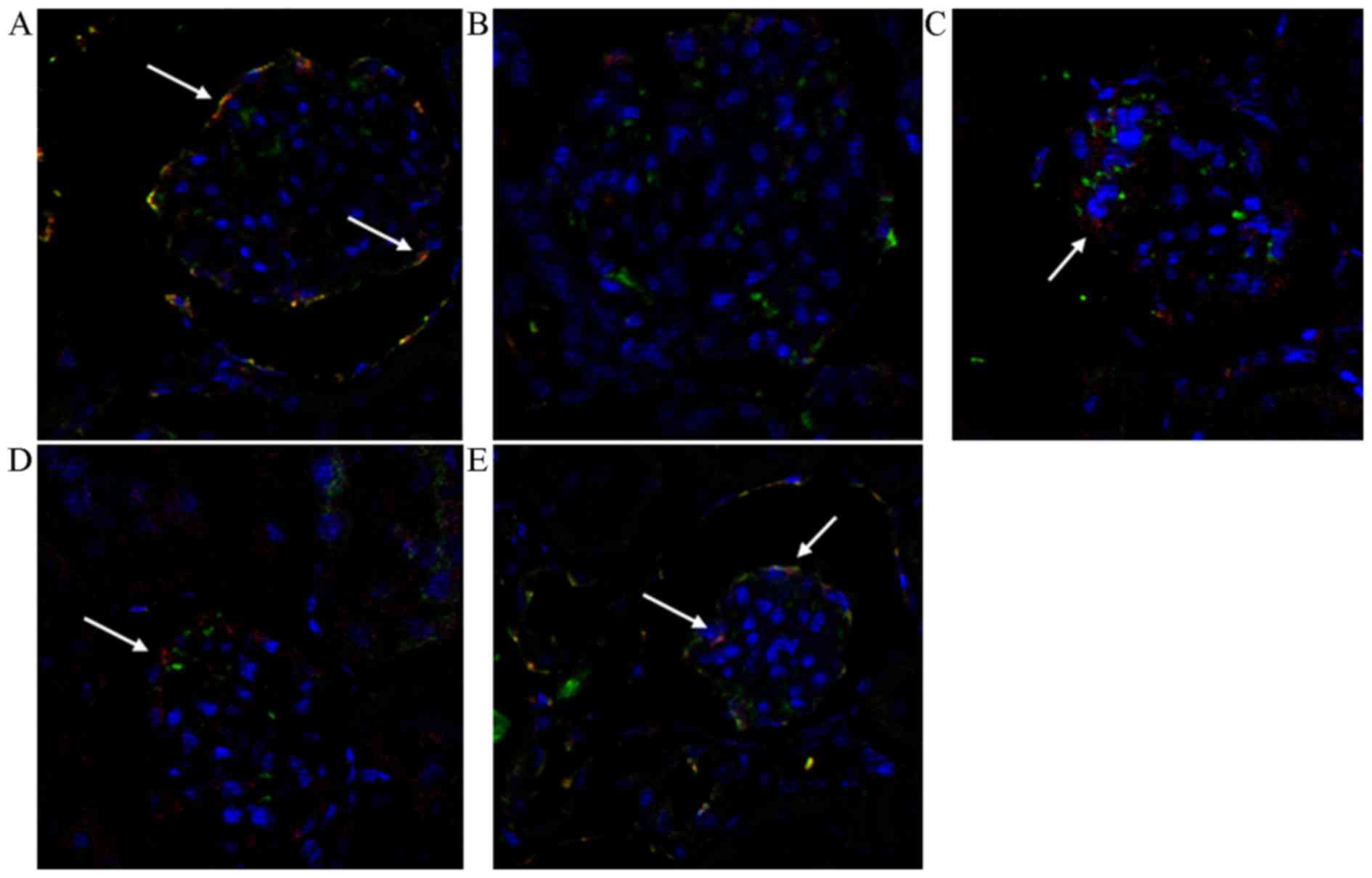 | Figure 4.Double fluorescent
immunohistochemistry staining in VEGFR/VE-cadherin (magnification,
×400); VE-cadherin protein is shown in red and VEGFR (VEGFR
positive, green) was used to label the RMEC. The white arrows
indicate areas where the VE-cadherin protein is expressed normally
at RMEC junctions (VEGFR/VE-cadherin positive, pink). (A) In the
sham group, VE-cadherin protein was expressed normally in RMEC
junctions. (B) In the ROSC control group, the expression
significantly decreased. (C) Though the damage to VE-cadherin
protein expression in the PGE1 group was still apparent, it was
relatively lessened when compared with the ROSC group. (D) The TTM
group exhibited similar results to the PGE1 group. (E) The PGE1/TTM
group exhibited the highest expression among interventions. ROSC,
return of spontaneous circulation; PGE1, prostaglandin E1; TTM,
target temperature management; PT, PGE1/TTM group; VEGFR, vascular
endothelial growth factor receptor; VE, vascular endothelial; RMEC,
renal microvascular endothelium cells. |
Relative quantitative analysis of mRNA expression of
VE-cadherin and VCAM-1 genes in renal tissue with different
interventions was performed using RT-qPCR. In I/R injury, enhanced
VE-cadherin mRNA expression was observed in the rat kidney within 8
h following ROSC. When compared to the ROSC control group, at the
0.5 and 4 h time points, the 3 intervention groups significantly
slowed the rise of VE-cadherin mRNA expression (P<0.05).
However, no statistical differences were identified between these 3
intervention groups (Fig. 5).
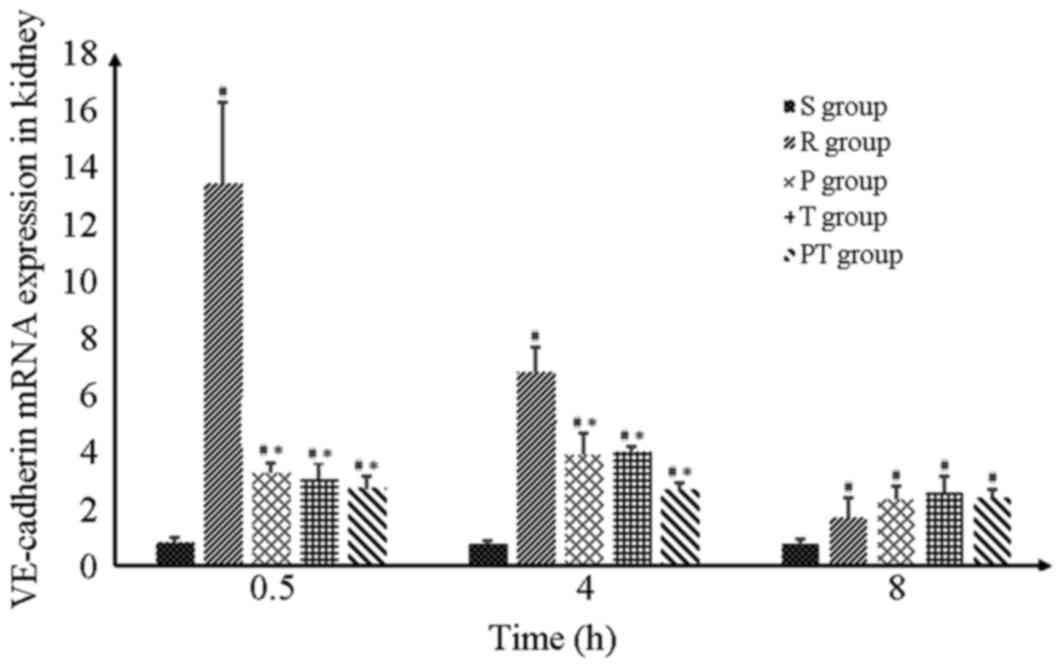 | Figure 5.VE-cadherin mRNA expression.
VE-cadherin mRNA levels markedly increased following return of
spontaneous circulation induction and gradually declined within 8 h
(P<0.05). At 0.5 and 4 h, there was a significant difference
between the R group and the P, T and PT groups (P>0.05).
However, there was no statistical difference between the R group
and the 3 intervention groups at 8 h, although the VE-cadherin mRNA
expression levels were still higher when compared to the S group.
#P<0.05, vs. S group; *P<0.05 vs. R group. Data
are presented as the mean ± standard deviation. S, sham group; R,
return of spontaneous circulation control group; P, prostaglandin
E1 group; T, target temperature management group; PT, prostaglandin
E1/target temperature management group; VE, vascular
endothelial. |
VCAM-1 mRNA expression was also examined to assess
I/R injury to the RMEC following ROSC. When compared to the S
group, all of the other groups exhibited significantly upregulated
expression of VCAM-1 mRNA within 8 h (P<0.05). When VCAM-1 mRNA
expression rose to the highest level at 4 h, the 3 different
interventions demonstrated a marked ability to decrease the
expression (P<0.05). There was no marked difference between the
R group and P or T group at 8 h; however, the PT treatment did
significantly reduce the mRNA expression of VCAM 1 (P<0.05;
Fig. 6).
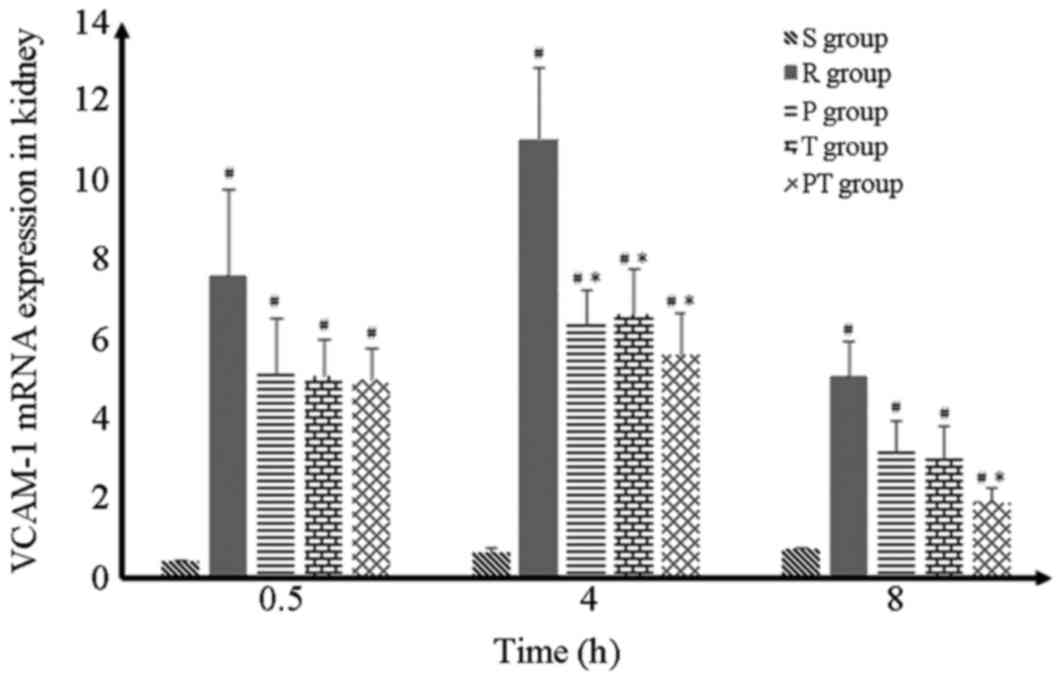 | Figure 6.VCAM-1 mRNA expression. VCAM-1 mRNA
expression increased at each time period. Following 4 h post return
of spontaneous circulation induction, it peaked and, at the same
time point, 3 interventions resulted in a significantly smaller
increase in VCAM-1 mRNA expression. However, only the PT group
exhibited a marked effect at 8 h. #P<0.05 vs. S
group; *P<0.05 vs. R group. Data are presented as the mean ±
standard deviation. VCAM-1, vascular cell adhesion molecule-1; S,
sham group; R, return of spontaneous circulation control group; P,
prostaglandin E1 group; T, target temperature management group; PT,
prostaglandin E1/target temperature management group. |
Circulating TM, a common early indicator of
endothelial injury, was used as a cell marker in the present study.
I/R injury induced a rapid increase in circulating TM
concentration. All 3 of the interventions provided a protective
effect, however, the PT group appeared to have a much greater
advantage (Table I).
 | Table I.Circulating thrombomodulin
concentration at different time points following the return of
spontaneous circulation. |
Table I.
Circulating thrombomodulin
concentration at different time points following the return of
spontaneous circulation.
|
| TM concentration at
indicated time point (ng/ml) |
|---|
|
|
|
|---|
| Group | 0.5 h | 4 h | 8 h |
|---|
| S | 6.728±0.679 | 7.218±0.832 | 8.229±0.795 |
| R |
15.753±0.509a |
17.877±0.432a |
20.364±0.504a |
| P |
11.653±1.153a,b |
12.655±1.268a,b |
13.503±0.357a,b |
| T |
10.952±0.119a,b |
11.866±0.722a,b |
14.376±0.550a–c |
| PT |
9.622±0.909a–d |
9.298±0.314a–d |
9.316±0.529b–d |
Possible association between the
benefits from 3 interventions and anti-inflammatory mechanism
TNF-α and IL-6 concentrations were chosen as the
parameters for the study of the anti-inflammatory mechanism. The
concentrations did not alter significantly immediately following
the I/R injury and ROSC. However, they gradually increased over
time and peaked at 8 h. The 3 interventions provided beneficial
effects by preventing the elevation of TNF-α concentration
following 4 h. However, only the PT group demonstrated a much
greater protective effect in TNF-α and IL-6 when compared with the
other groups at 8 h (Table
II).
 | Table II.Concentration of TNF-α and IL-6 at
different time points following the return of spontaneous
circulation. |
Table II.
Concentration of TNF-α and IL-6 at
different time points following the return of spontaneous
circulation.
|
|
| Concentration at
indicated time point (ng/ml) |
|---|
|
|
|
|
|---|
| Parameters | Group | 0.5 h | 4 h | 8 h |
|---|
| TNF-α | S | 222.198±6.903 | 219.646±5.926 | 219.506±3.552 |
|
| R | 219.986±5.679 |
275.932±10.654a |
282.702±32.965a |
|
| P | 216.464±9.078 |
242.682±9.678a,b |
232.376±11.187b |
|
| T | 220.503±6.154 |
257.123±10.215a,b |
251.128±6.215a |
|
| PT | 219.622±15.341 |
233.776±6.094a–c |
214.594±12.955b,c |
| IL-6 | S | 78.968±8.840 | 80.842±7.140 | 83.852±4.217 |
|
| R | 85.094±7.719 |
100.892±3.838a |
116.112±8.102a |
|
| P | 84.868±4.713 | 95.736±7.831 |
97.686±10.606a |
|
| T | 85.820±6.217 | 93.432±5.429 |
103.770±8.632a |
|
| PT | 86.244±11.589 | 89.946±5.476 |
90.404±6.455b |
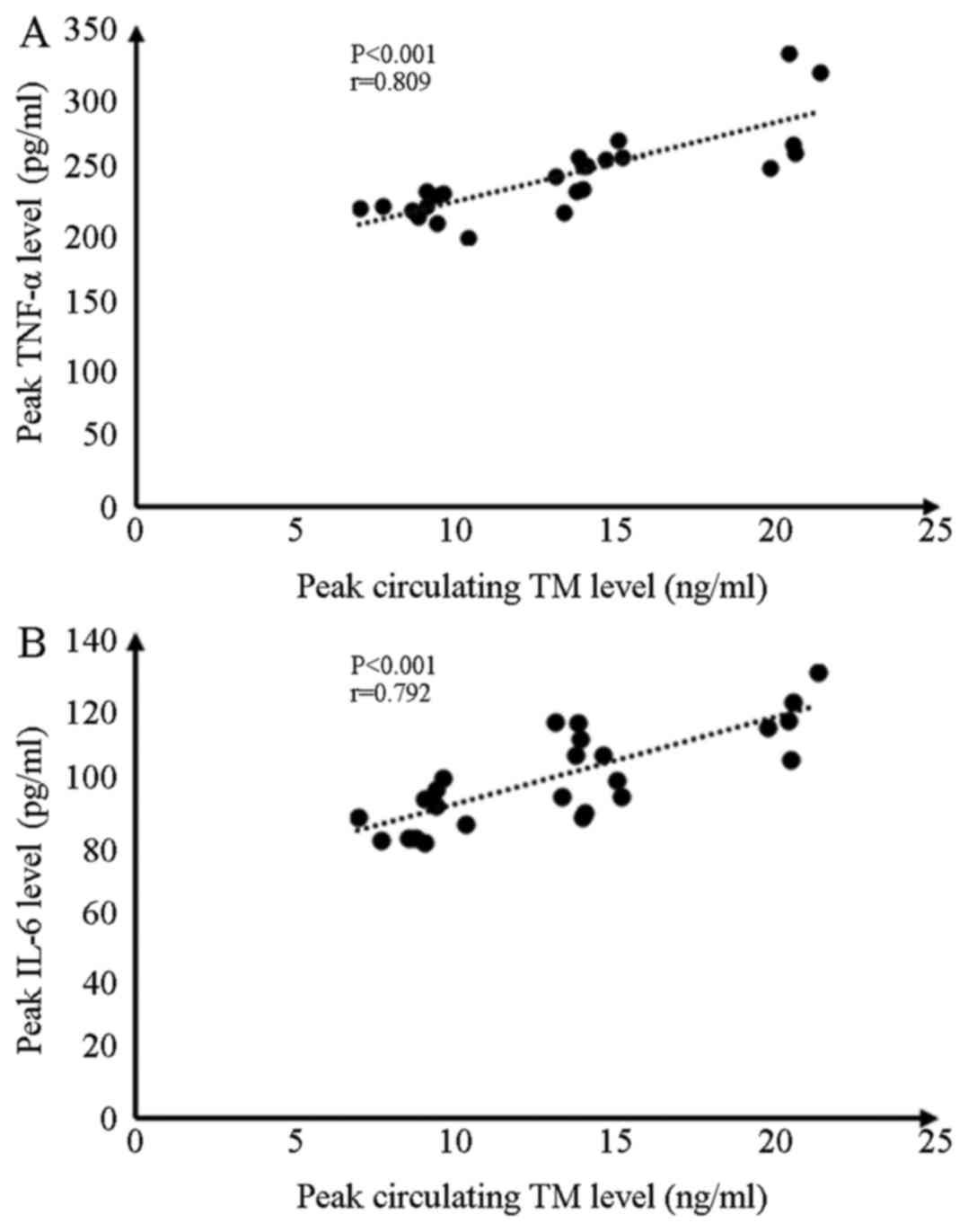
Statistical analysis demonstrated that, at 8 h, I/R
injury generated peak levels of circulating TM, TNF-α and IL-6
concentrations, and the interventions simultaneously induced the
most significant effects. Pearson's correlation analysis
demonstrated that peak TM levels were significantly associated with
peak levels of TNF-α and IL-6 concentrations (Fig. 7).
Discussion
The majority of studies on organ dysfunction
following resuscitation from CA have focused on the brain and
heart. The prevalence of extra-cerebral or cardiac organ injury and
its impact on outcomes has been less well-characterized. A previous
study highlighted acute kidney injury (AKI) in patients with CA;
the frequency of AKI development following CA ranged from 12 to 43%
and >75% of these episodes occurred within 3 days following CA
(19). Following more in-depth
research, a number of controversies arose regarding some aspects,
including whether AKI has an impact on hospital survival rates
(20,21) and whether AKI is associated with a
lower probability of favorable neurological outcomes (19,22).
In the present study, RMEC was chosen as the target as the kidney
is rich in micro-vessels and endothelial involvement in CPR as a
primary or secondary target has been described previously in animal
models and human patients (2).
Following ROSC, the systemic I/R response may lead
to PCAS, which is characterized by the activation of immunological
and coagulation pathways, and the release of inflammatory
mediators, all of which lead to tissue hypoperfusion and multiple
organ dysfunction (3).
Specifically, systemic inflammation promotes disorders in the
microcirculation due to metabolic imbalance, leukocyte activation,
endothelial toxicity, and impairment of mitochondrial respiratory
chain activity (23). Increases in
pro-inflammatory cytokines and soluble receptors have been reported
in patients h following resuscitation from CA (24). TNF-α and IL-6 are involved in
systemic inflammation and stimulate the acute phase reaction. They
are primarily involved in the regulation of immune cells and are
also implicated in the induction of fever, apoptosis and the
inhibition of cellular reproduction (25). Consistent with the present study,
the immediate reperfusion period after ROSC following CA has
previously been revealed to be characterized by an abrupt increase
in plasma TNF-α and IL-6 (26). A
previous study reported that early IL-6 levels are strongly
associated with mortality and appear to be excellent predictors of
the outcome of CA (12). However,
the use of inflammation biomarkers as an indicator following CA is
limited due to their poor specificity for I/R injury and their
exact mechanism and association with extensive endothelial damage
is still unclear. The present study used TM to associate the
inflammation markers with endothelial injury. TM is a transmembrane
glycoprotein expressed on the surface of all vascular endothelial
cells. Expression of TM is tightly regulated to maintain
homeostasis and to ensure a rapid and localized hemostatic and
inflammatory response to injury (27). The results of the correlation
analysis revealed that the peak TM levels and the peak levels of
the TNF-α and IL-6 concentrations are positively associated. This
may indicate that the inflammatory response is the pathway for
endothelial I/R injury in the early stages following ROSC.
Therefore, anti-inflammatory interventions may be effective
therapeutic strategies.
PGE1 is rarely used in PCAS patients, however, its
anti-inflammatory effect has been confirmed in other I/R injury
models, particularly in the kidney (28). Recent studies have indicated that
PGE1 may have a protective role as it blocks chemoattractant
factors and weakens leukocyte adhesion to the microvascular
endothelium, with lower tissue expression of some inflammatory
markers involved in this process, potentially leading to
cytoprotective activity (29,30).
The pathomorphological and ELISA results demonstrated that PGE1
reduced I/R injury to the RMEC following ROSC and markedly
inhibited the release of TM (at 3 time points) and TNF-α (at 4 and
8 h). In addition, the results from the VE-cadherin and VCAM-1 mRNA
expression analysis indicated that PGE1 was able to effectively
inhibit the rapid elevation of this mRNA expression, which may
imply the endogenous compensatory requirements for rapid and severe
I/R damage to the endothelial cell junction (31).
TTM is already a fundamental part of the treatment
for unconscious survivors of OHCA due to landmark studies that
concluded that mild hypothermia (32–34°C) improved survival and
neurological outcome (9,32,33).
As survivors of OHCA exhibit a ‘sepsis like syndrome’ (3), anti-inflammation effects are thought
to be a potential mechanism of TTM (34). In the present study, TTM alleviated
I/R injury to the RMEC, decreased VE-cadherin and VCAM-1 mRNA
expression, and suppressed the circulating TM concentration, which
is similar to PGE1. However, TTM did not exhibit an apparent
influence on inflammation biomarkers as there was no significant
difference between the ROSC group and TTM group during the majority
of the present study, except for the altered TNF-α levels at 4 h.
This may support the idea that TTM has a protective effect with
respect to I/R injury to the endothelium, however, further research
is required to confirm whether anti-inflammatory effects are the
exact mechanism by which TTM improves the outcome during the early
stages of ROSC.
The present study focused on the combined
intervention of PGE1 and TTM. To the best of our knowledge, this
has not been studied previously. A number of medications are
normally given to patients with PCAS, in the same way they are for
any other critically ill patient. The influence of body temperature
on physicochemical properties of drug disposition, potentially
contributing to drug-therapy and drug-disease interaction, should
be considered when TTM is used for PCAS (35). The results demonstrated that the
PGE1/TTM combined intervention had more beneficial effects compared
with the single interventions in protecting the RMEC from I/R
injury in CA, with significant inhibition of VE-cadherin protein
loss, rapid promotion of VCAM-1 mRNA expression and TM release.
Only TTM/PGE1 combined interventions effectively reduced the
concentration of TNF-α and IL-6 at 8 h, producing similar results
to the sham operation group. TTM and PGE1 interventions alone did
not achieve this effect.
Due to limitations in experimental facilities, the
present study used an alternating current delivered through
transesophageal cardiac pacing instead of delivery to the classic
right ventricular endocardium to induce the VF. The present study
therefore differs from others to some extent with respect to
current stimulation parameters and VF duration time. Further
research is required to determine the response of proteins by using
quantitative analysis.
In conclusion, the present study considered RMEC to
be a useful indicator of general I/R injury following ROSC.
Anti-inflammatory interventions may be one of the most promising
options for future endothelial I/R injury treatment in the early
phase of PCAS. The PGE1 and TTM interventions had protective
effects with respect to I/R injury to the RMEC, while PGE1
exhibited a greater impact on inflammation markers than TTM. The
PGE1/TTM combined intervention may exhibit an improved synergistic
effect as an anti-inflammatory treatment compared with the single
interventions.
Acknowledgements
The present study was supported by funding from the
Transformation of Scientific and Technological Achievements of
Chengdu Grant (grant no. 11DXYB294SF-027). The authors of the
present study would like to thank the staff of the Transplantation
Engineering and Immunology Laboratory of the West China Hospital of
Sichuan University(Chengdu, Sichuan) for their technical help and
constructive criticism.
References
|
1
|
Roberts BW and Trzeciak S: Systemic
inflammatory response after cardiac arrest: Potential target for
therapy? Crit Care Med. 43:1336–1337. 2015. View Article : Google Scholar
|
|
2
|
Adams JA: Endothelium and cardiopulmonary
resuscitation. Crit Care Med. 34(12 Suppl): S458–S465. 2006.
View Article : Google Scholar
|
|
3
|
Adrie C, Laurent I, Monchi M, Cariou A,
Dhainaou JF and Spaulding C: Post resuscitation disease after
cardiac arrest: A sepsis-like syndrome? Curr Opin Crit Care.
10:208–212. 2004. View Article : Google Scholar
|
|
4
|
Soares BL, Freitas MA, Montero EF, Pitta
GB and Miranda F Jr: Alprostadil attenuates inflammatory aspects
and leucocytes adhesion on renal ischemia and reperfusion injury in
rats. Acta Cir Bras. 29 Suppl 2:S55–S60. 2014. View Article : Google Scholar
|
|
5
|
Vargas AV, Krishnamurthi V, Masih R,
Robinson AV and Schulak JA: Prostaglandin E1 attenuation of
ischemic renal reperfusion in the rat. J Am Coll Surg. 180:713–717.
1995.
|
|
6
|
Gupta PC, Matsushita M, Oda K, Nishikimi
N, Sakurai T and Nimura Y: Attenuation of renal
ischemia-reperfusion injury in rats by allopurinol and
prostaglandin E1. Eur Surg Res. 30:102–107. 1998. View Article : Google Scholar
|
|
7
|
Huk I, Brovkovych V, Nanobashvili J,
Neumayer C, Polterauer P, Prager M, Patton S and Malinski T:
Prostaglandin E1 reduces ischemia/reperfusion injury by normalizing
nitric oxide and superoxide release. Shock. 14:234–242. 2000.
View Article : Google Scholar
|
|
8
|
Hong JP, Chung YK and Chung SH: The effect
of prostaglandin E1 versus ischemia-reperfusion injury of
musculocutaneous flaps. Ann Plast Surg. 47:316–321. 2001.
View Article : Google Scholar
|
|
9
|
Callaway CW, Donnino MW, Fink EL, Geocadin
RG, Golan E, Kern KB, Leary M, Meurer WJ, Peberdy MA, Thompson TM
and Zimmerman JL: Part 8: Post-cardiac arrest care: 2015 American
Heart Association Guidelines Update for Cardiopulmonary
Resuscitation and Emergency Cardiovascular Care. Circulation.
132(18 Suppl 2): S465–S482. 2015. View Article : Google Scholar :
|
|
10
|
Fries M, Stoppe C, Brücken D, Rossaint R
and Kuhlen R: Influence of mild therapeutic hypothermia on the
inflammatory response after successful resuscitation from cardiac
arrest. J Crit Care. 24:453–457. 2009. View Article : Google Scholar
|
|
11
|
Bisschops LL, van der Hoeven JG, Mollnes
TE and Hoedemaekers CW: Seventy-two h of mild hypothermia after
cardiac arrest is associated with a lowered inflammatory response
during rewarming in a prospective observational study. Crit Care.
18:5462014. View Article : Google Scholar :
|
|
12
|
Bro-Jeppesen J, Kjaergaard J, Wanscher M,
Nielsen N, Friberg H, Bjerre M and Hassager C: Systemic
inflammatory response and potential prognostic implications after
out-of-hospital cardiac arrest: A sub-study of the target
temperature management trial. Crit Care Med. 43:1223–1232. 2015.
View Article : Google Scholar
|
|
13
|
Chen MH, Liu TW, Xie L, Song FQ, He T,
Zeng ZY and Mo SR: Ventricular fibrillation induced by
transoesophageal cardiac pacing: A new model of cardiac arrest in
rats. Resuscitation. 74:546–551. 2007. View Article : Google Scholar
|
|
14
|
Zhou J, Huang GQ, Li XM and Li XG: Effects
of different stimulating parameters and their various combinations
on transoesophageal electrical stululation induced cardiac arrest
in rats. China J Emergency Resuscitaion and Disaster Med.
8:596–598. 2013.
|
|
15
|
Idris AH, Becker LB, Ornato JP, Hedges JR,
Bircher NG, Chandra NC, Cummins RO, Dick W, Ebmeyer U, Halperin HR,
et al: Utstein-style guidelines for uniform reporting of laboratory
CPR research. Resuscitation. 33:69–84. 1996. View Article : Google Scholar
|
|
16
|
Cardiff RD, Miller CH and Munn RJ: Manual
hematoxylin and eosin staining of mouse tissue sections. Cold
Spring Harb Protoc. 2:665–668. 2014.
|
|
17
|
Pallor MS, Hoidal JR and Ferris TF: Oxygen
free radicals in ischemic acute renal failure in the rat. J Clin
Inves. 74:1156–1164. 1984. View Article : Google Scholar
|
|
18
|
Garvard J and Gutkind JS: VEGF controls
endothelial permeability by promoting the beta-arrestin-dependent
endocytosis of VE-cadherin. Nat Cell Biol. 8:1223–1234. 2006.
View Article : Google Scholar
|
|
19
|
Tujjar O, Mineo G, Dell'Anna A,
Poyatos-Robles B, Donadello K, Scolletta S, Vincent JL and Taccone
FS: Acute kidney injury after cardiac arrest. Critical Care.
19:1692015. View Article : Google Scholar :
|
|
20
|
Yanta J, Guyette FX, Doshi AA, Callaway CW
and Rittenberger JC: Post Cardiac Arrest Service: Renal dysfunction
is common following resuscitation from out-of-hospital cardiac
arrest. Resuscitation. 84:1371–1374. 2013. View Article : Google Scholar
|
|
21
|
Geri G, Guillemet L, Dumas F, Charpentier
J, Antona M, Lemiale V, Bougouin W, Lamhaut L, Mira JP, Vinsonneau
C and Cariou A: Acute kidney injury after out-of-hospital cardiac
arrest: Risk factors and prognosis in a large cohort. Intensive
Care Med. 41:1273–1280. 2015. View Article : Google Scholar
|
|
22
|
Hasper D, von Haehling S, Storm C, Jörres
A and Schefold JC: Changes in serum creatinine in the first 24 h
after cardiac arrest indicate prognosis: An observational cohort
study. Crit Care. 13:R1682009. View
Article : Google Scholar :
|
|
23
|
Huet O, Dupic L, Batteux F, Matar C, Conti
M, Chereau C, Lemiale V, Harrois A, Mira JP, Vicaut E, et al:
Postresuscitation syndrome: Potential role of hydroxyl
radical-induced endothelial cell damage. Crit Care Med.
39:1712–1720. 2011. View Article : Google Scholar
|
|
24
|
Adrie C, Adib-Conquy M, Laurent I, Monchi
M, Vinsonneau C, Fitting C, Fraisse F, Dinh-Xuan AT, Carli P,
Spaulding C, et al: Successful cardiopulmonary resuscitation after
cardiac arrest as a ‘sepsis-like’ syndrome. Circulation.
106:562–568. 2002. View Article : Google Scholar
|
|
25
|
Scolletta S, Donadello K, Santonocito C,
Franchi F and Taccone FS: Biomarkers as predictors of outcome after
cardiac arrest. Expert Rev Clin Pharmacol. 5:687–699. 2012.
View Article : Google Scholar
|
|
26
|
Samborska-Sablik A, Sablik Z and Gaszynski
W: The role of the immuno-inflammatory response in patients after
cardiac arrest. Arch Med Sci. 7:619–626. 2011. View Article : Google Scholar :
|
|
27
|
Conway EM: Thrombomodulin and its role in
inflammation. Semin Immunopathol. 34:107–125. 2012. View Article : Google Scholar
|
|
28
|
Vargas AV, Krishnamurthi V, Masih R,
Robinson AV and Schulak JA: Prostaglandin E1 attenuation of
ischemic renal reperfusion injury in the rat. J Am Coll Surg.
180:713–717. 1995.
|
|
29
|
Kawamura T, Nara N, Kadosaki M, Inada K
and Endo S: Prostaglandin E1 reduces myocardial reperfusion injury
by inhibiting proinflammatory cytokines production during cardiac
surgery. Crit Care Med. 28:2201–2208. 2000. View Article : Google Scholar
|
|
30
|
Soares BL, Freitas MA, Montero EF, Pitta
GB and Miranda F Jr: Alprostadil attenuates inflammatory aspects
and leucocytes adhesion on renal ischemia and reperfusion injury in
rats. Acta Cir Bras. 29 Suppl 2:S55–S60. 2014. View Article : Google Scholar
|
|
31
|
Chen F, Kondo N, Sonobe M, Fujinaga T,
Wada H and Bando T: Expression of endothelial cell-specific
adhesion molecules in lungs after cardiac arrest. Interact
Cardiovasc Thorac Surg. 7:437–440. 2008. View Article : Google Scholar
|
|
32
|
Hypothermia after Cardiac Arrest Study
Group, . Mild therapeutic hypothermia to improve the neurologic
outcome after cardiac arrest. N Engl J Med. 346:549–556. 2002.
View Article : Google Scholar
|
|
33
|
Bernard SA, Gray TW, Buist MD, Jones BM,
Silvester W, Gutteridge G and Smith K: Treatment of comatose
survivors of out-of-hospital cardiac arrest with induced
hypothermia. N Engl J Med. 346:557–563. 2002. View Article : Google Scholar
|
|
34
|
Bro-Jeppesen J, Kjaergaard J, Wanscher M,
Nielsen N, Friberg H, Bjerre M and Hassager C: The inflammatory
response after out-of-hospital cardiac arrest is not modified by
targeted temperature management at 33°C or 36°C. Resuscitation.
85:1480–1487. 2014. View Article : Google Scholar
|
|
35
|
Zhou J and Poloyac SM: The effect of
therapeutic hypothermia on drug metabolism and drug response:
Cellular mechanisms to organ function. Expert Opin Drug Metab
Toxicol. 7:803–816. 2011. View Article : Google Scholar :
|