Introduction
Bronchopulmonary dysplasia (BPD) is one of the most
common chronic lung diseases in preterm infants undergo
supplemental oxygen therapy in US (1). Oxygen toxicity or hyperoxia is one of
the major risk factors in the development of bronchopulmonary
dysplasia. Hyperoxia was reported to promote cell injury in
alveolar endothelial and epithelial cells in vivo and in
vitro (2,3), leading to impaired gas exchange and
increased epithelial apoptosis (4). Premature infants are more susceptible
to hyperoxia induced epithelial damage due to their respiratory
immaturity and deficiency of anti-oxidant enzyme activity (5,6). The
molecular mechanism in regulating epithelial apoptosis under
hyperoxia needs to be further elucidated.
The essential site responsible for protein synthesis
and maturation is endoplasmic reticulum (ER), into which newly
synthesized polypeptide chains enter through a peptide translocon,
and undergo maturation processes such as cleavage, glycosylation,
disulfide bond formation, folding and assembly. Much physiological
and pathological stimulation, such as ischemia, hyperoxia, and
poisons, cause ER stress, during which inhibition of protein
glycosylation or disulfide bond formation results in accumulation
of unfolded and misfolded proteins in the lumen of the ER. Gene
expression alteration occurs during ER stress (7). Glucose regulated protein 78 (GRP78),
a molecular chaperone, locates in the lumen of the ER that binds
newly synthesized proteins as they translocate into the ER, and
maintains them in a state competent for subsequent folding and
oligomerization. GRP78 protein is usually highly induced by the
microenvironment factors including hyperoxia, acidosis as well as
glucose deprivation (8).
Inositol-requiring enzyme-1 (IRE1), activating transcription
factor-6 (ATF6), and protein kinase regulated by RNA-like ER kinase
(PERK) play important role during ER stress (9). GRP78 recruitment to chaperone the
malfolded proteins results in GRP78 dissociation from its
conformational binding state of the above three trans-membrane
receptors (10,11), subsequently induces cell apoptosis.
C/EBP homologous protein (CHOP) is widely known as a participator
in the initiation of apoptosis. IRE1, ATF6 and PERK can trigger
CHOP (12). The Bcl2 family and
plays a crucial role in the apoptotic process of various cancers.
It has been documented that Bax (bcl-2-like protein 4) and Bcl-2
(B-cell lymphoma 2) are separately pro-apoptosis and anti-apoptosis
proteins of the Bcl2 family and that these two molecules can
finally regulate programmed cell death in ER (13). The pathway (via PERK) that induces
transcription of the pro-apoptotic factor CHOP can inhibit
anti-apoptotic protein Bcl-2, leading to activation of the
executioner caspase-3 and cell death (14). mRNA and protein levels of GRP78 and
CHOP were increased in lung tissue of preterm Sprague-Dawley rats
exposed to hyperoxia (15).
Exposure to 95–100% O2 induces CHOP mRNA expression in
the bronchiolar epithelium of adult mice and in isolated type II
cells in culture (16), as well as
postnatal day 2 and 7 murine developing lung tissue (17). Hyperoxia has also been shown to
enhance CHOP expression after 72 h exposure together with increased
ATF4 mRNA expression (18).
However, how GRP78 regulates lung epithelial cell apoptosis during
hyperoxia, especially the underlying mechanisms still remains
unknown.
In this study, we used siRNA targeted GPR78 to
transfect A549 cell under hyperoxia. GPR78 knockdown increased the
expression of CHOP at gene level or protein level, further induced
A549 cell apoptosis under hyperoxia. CHOP overexpression under
hyperoxia led to the robust apoptosis of A549 cells by inducing Bax
and inhibiting Bcl-2. CHOP knockdown (CHOP-siRNA) showed opposite
effect on Bax and Bcl-2. GRP78 silencing promoted lung epithelial
cells apoptosis during hyperoxia, probably through regulating CHOP
pathway.
Materials and methods
Cell culture and hyperoxia
exposure
The human airway epithelial cell line A549 was
purchased from American Type Culture Collection (ATCC; Manassas,
VA, USA) and cultured in DMEM/F12 with 10% FBS. Cells were grown
under humidified conditions consisting of 95% air and 5%
CO2 at 37°C (normoxia). For hyperoxia exposure, cells
were plated in an MIC-101 chamber (Modular Incubator;
Billups-Rothenberg, Inc., Del Mar, CA, USA) filled with 95%
O2 and 5% CO2 for up to 72 h at 37°C. The
gases were replaced every day.
Lipsome mediated cell transfection and
hyperoxia treatment of A549
siRNA sequences targeted GRP78, CHOP were designed
and called GRP78-siRNA and CHOP-siRNA, respectively. The sequences
of GRP78-siRNA or CHOP-siRNA were as follows:
5′-AAGAUCACAAUCACCAAUGACTT-3′, 5′-AAGAACCAGCAGAGGUCACAATT-3′. The
sequence of the corresponding negative control was
5′-AAAUCAUAGCGUAUGGUGCUGTT-3′. 3e5 A549 cells cultured in six-well
plate were transfected with 4 µg of siRNA or pEGFP-N1 plasmid with
CHOP by Lipofectamine® 2000 (Invitrogen Life Technologies,
Carlsbad, CA, USA). 24 h after transfection, fresh medium was
added. Next, A549 cells were treated with hyperoxia for 24, 48 and
72 h subsequently. A549 cells without transefection or hyperoxia
were designated as control (C). A549 cells treated with the
combination of negative control transfection and hyperoxia were
designated as negative control (N).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
RT-qPCR was carried out as previous described
(15) to detect the expression of
genes including ATF6, PERK, CHOP, Bcl-2 and Bax. Total RNA was
extracted with Trizol (~0.5e6 cells adding 0.5 ml TRIzol) reagent
(Applied Biosystems Life Technologies, Foster City, CA, USA) and
fractionated by electrophoresis on a 1.2% agarose/3-(N-morpholino)
propanesulfonic acid/formaldehyde gel to ensure RNA integrity.
Total RNA was reverse-transcribed to cDNA according to the
manufacturer's instructions (Multiscribe Reverse Transcriptase;
Applied Biosystems Life Technologies). Platinum Taq polymerase
(Invitrogen Life Technologies) and EvaGreen dye (Biotium, Inc.,
Hayward, CA, USA) were applied for RT-qPCR. In this system, the
increase in the concentration of EvaGreen dye fluorescent is
proportional to the increase in PCR products; the reaction
production can be accurately measured in the exponential phase of
amplification by the ABI prism 7700 Sequence Detection System. The
sequences of the primers used are listed in Table I. The signal of the housekeeping
gene GAPDH was used for normalization. The correct size of PCR
product was confirmed by electrophoresis on a 2% agarose gel
stained with ethidium bromide. Metling curve analysis was performed
to assess the specificity of the amplified PCR products.
 | Table I.Sequences of primers used in qPCR. |
Table I.
Sequences of primers used in qPCR.
| Gene | Sense | Antisense |
|---|
| GRP78 |
TCCTATGTCGCCTTCACT |
ACAGACGGGTCATTCCAC |
| PERK |
TTGTCGCCAATGGGATAG |
CAGTCAGCAACCGAAACC |
| IRE1 |
GACAGGCTCAATCAAATGG |
CGGTCAGGAGGTCAATAACA |
| ATF6 |
TCAATGGGCAGGACTACGA |
GGGAGCCAAAGAAGGTGT |
| CHOP |
CACTCTTGACCCTGCTTC |
AGTCGCCTCTACTTCCCT |
| Bcl-2 |
TCCAATCCTGTGCTGCTA |
ACTCTGTGAATCCCGTTT |
| Bax |
TTTTGCTTCAGGGTTTCATC |
GACACTCGCTCAGCTTCTTG |
| GAPDH |
GCACCGTCAAGGCTGAGAAC |
TGGTGAAGACGCCAGTGGA |
Western blotting
Total protein was isolated using RIPA lysis buffer
(Biyuntian Biotechnology Co., Ltd., Shanghai, China), and protein
concentrations were determined by the Bradford method. 80 µg
proteins were separated by SDS-PAGE and transferred onto
polyvinylidene fluoride membranes with the Bio-Rad Trans blot
system. Membranes were stained by Ponceau for 3 min, and cleaned by
double distilled H2O until the red blots were clear.
After blocking in 5% bovine serum albumin (BSA) and mixture of
Tris-Buffered Saline and Tween-20 (TBST) for 1 h, membranes were
incubated overnight in primary antibody diluted in 5% BSA at 4°C.
The following primary antibodies were used: anti-PERK (AF5304),
anti-IRE1 (DF7709), anti-ATF6 (DF6009), anti-CHOP (DF6025, 1:500,
Affinity biosciences, USA), anti-β-actin (A1978, 1:10,000; Sigma,
St. Louis, MO, USA). Membranes were incubated for 1 h with
HRP-conjugated goat anti-mouse (1:5,000) or rabbit (1:2,000)
immunoglobulin (Sigma) in 5% BSA. Chemiluminescence kit (Biyuntian
Biotechnology Co., Ltd.) was used to visualize the secondary
antibody. The bands were quantified using Image J software from
three independent experiments.
Flow cytometry
Transfected cells were harvested, washed by ice-cold
PBS, centrifuged at 300 × g for 5 min. 1e6 Cells were re-suspended
with 500 µl of 1xbinding buffer, then stained with FITC-conjugated
Annexin V and PI (Biyuntian Biotechnology Co., Ltd.) for 15 min at
4°C in the dark. The samples were analyzed using FACS (BD
Biosciences, San Diego, CA, USA).
Statistical analysis
The results are expressed as the mean ± standard
error of the mean (SEM) of at least three independent experiments.
All data were analyzed by SPSS 20.0 statistical software (SPSS,
Inc., Chicago, IL, USA). Statistical analysis comparing the treated
and control groups was assessed using the Student's t-test, and
among multiple groups were tested by analysis of variance (ANOVA)
followed by Geisser-Greenhouse corrections post hoc test. P<0.05
was considered to indicate a statistically significant
difference.
Results
GRP78 silencing increase CHOP
expression in A549 cells under hyperoxia
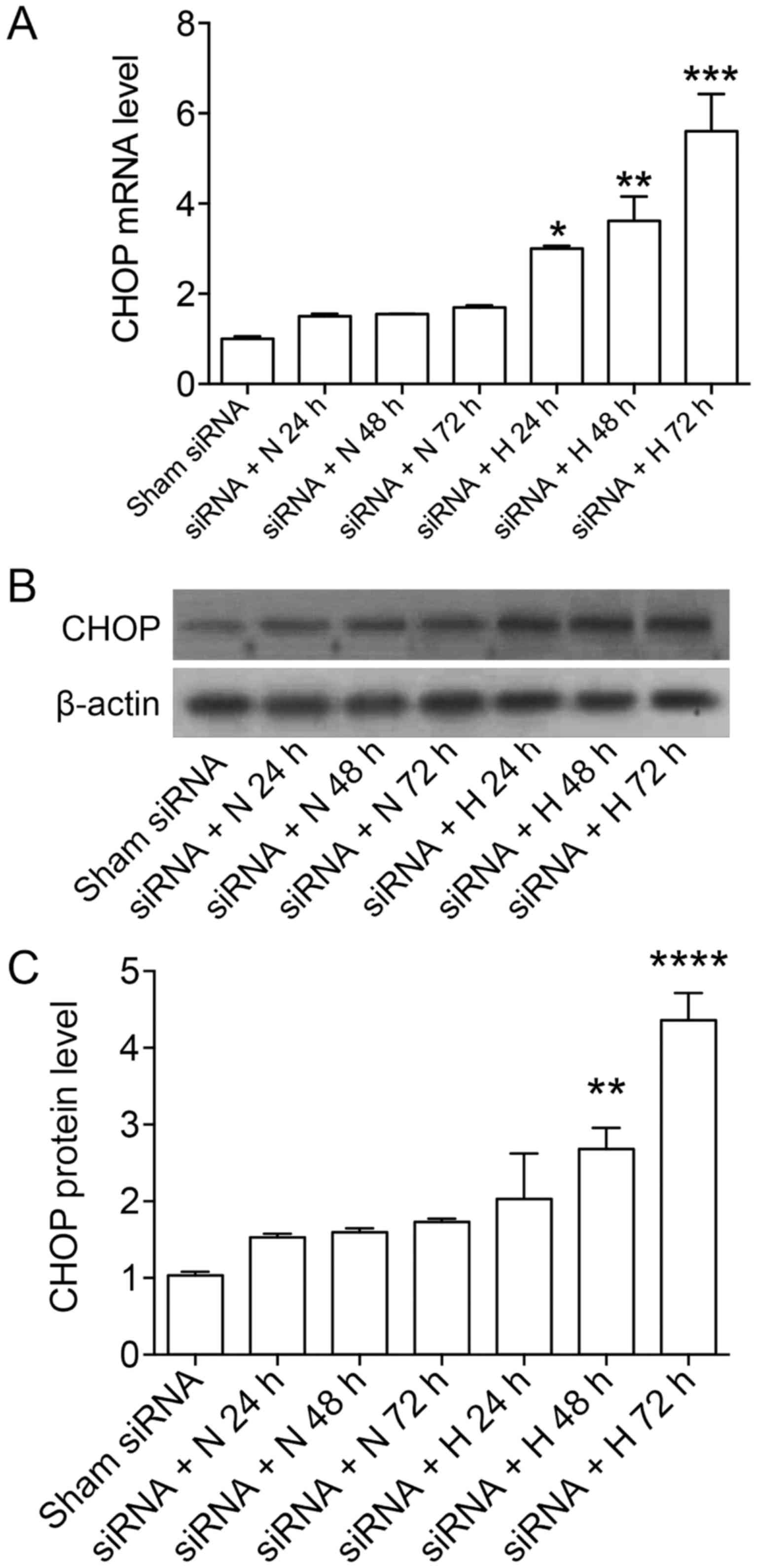
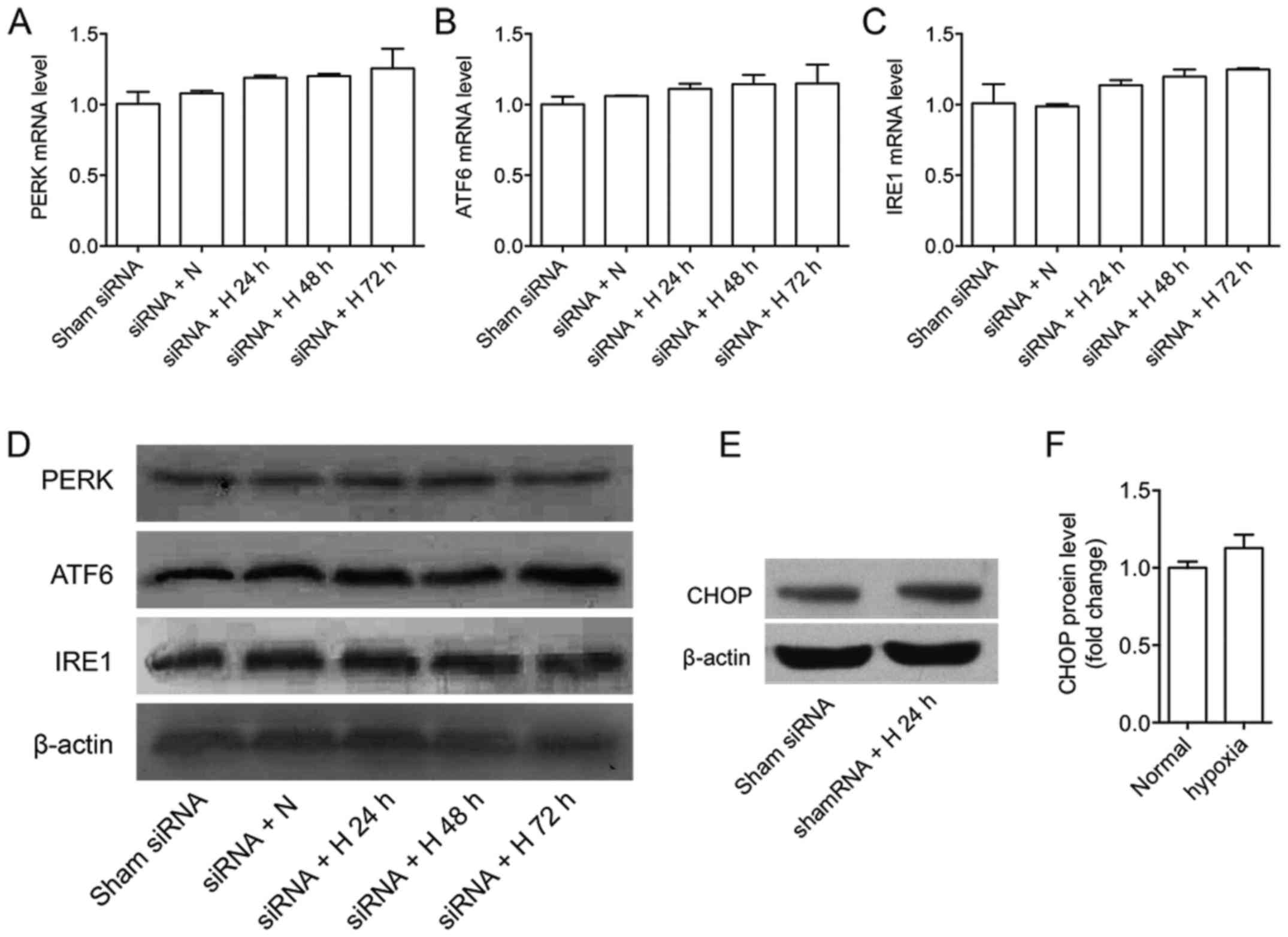
We used GPR78-siRNA to transfect A549 cells,
hyperoxia was established subsequently for 24, 48 and 72 h after
transfection, expressions of PERK, ATF6, IRE1 and CHOP at gene
level and protein level were detected. GRP78 mRNA level dropped
80–90% by RT-PCR and protein level dropped over 50% by western
blotting after knockdown (data not shown). The results showed that
the expressions of PERK, ATF6 and IRE1 were not affected at gene
level and protein level, while the expression of CHOP slightly
increased after GPR78 silencing at gene level and protein level
under normoxia, the increases were significantly enhanced under
hyperoxia (Figs. 1 and 2). Also, the expression of CHOP in A549
cells treated with GRP78-siRNA and hyperoxia increased gradually
with time. CHOP protein expression was slightly increased after 24
h under hypoxia after shamRNA treatment, not significantly
different from those under nomaxia (Fig. 2E and F). Our previous studies have
shown CHOP protein expression was increased for ~2 folds under
hypoxia for 72 h (15), GRP78
knockdown under hypoxia for 72 h further brought up CHOP protein
expression by ~4.5-folds.
The effect of GRP78-siRNA on the
apoptosis of A549 cells under hyperoxia
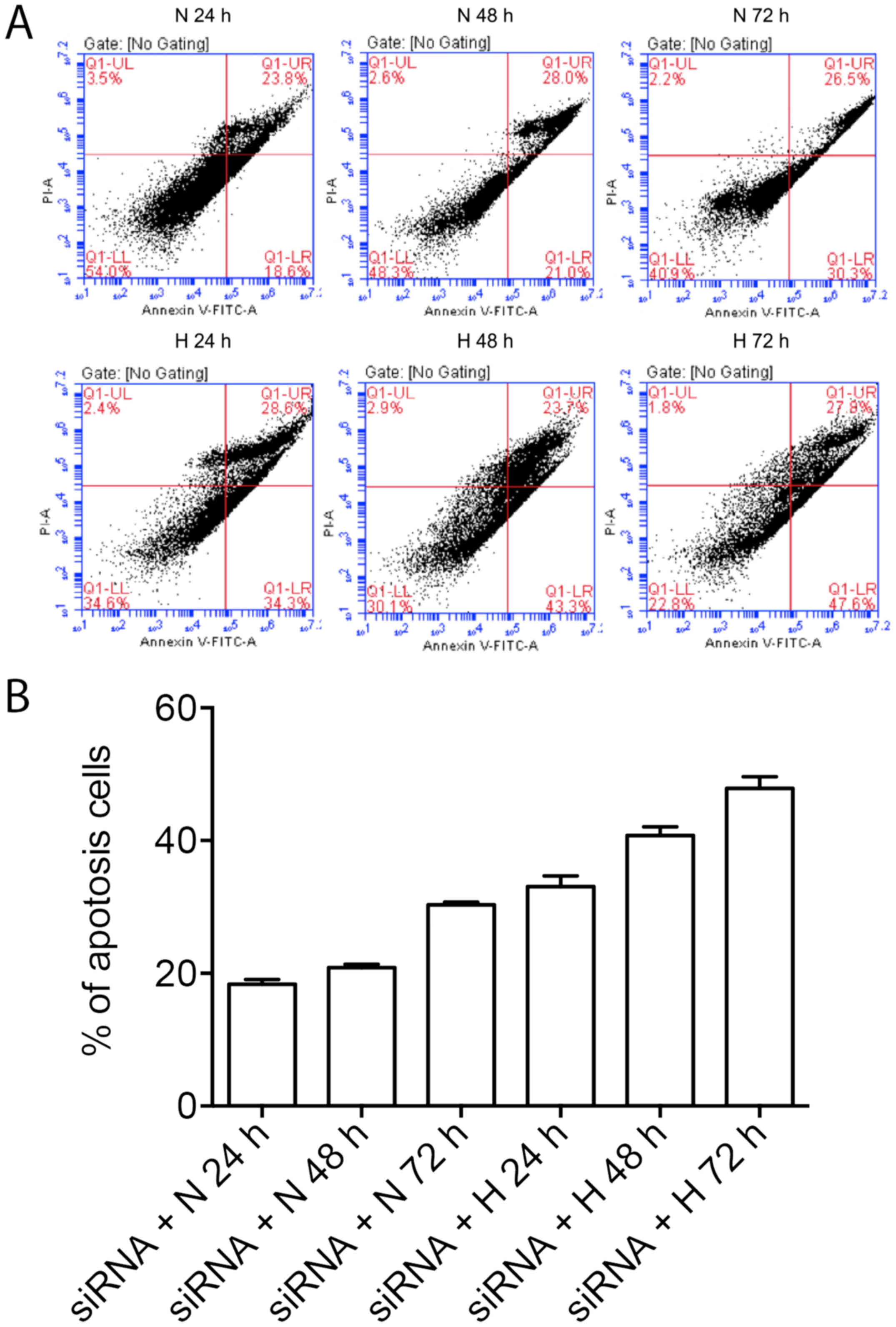
A549 cells were treated with GRP78 siRNA and
hyperoxia, induction of ER-mediated apoptosis was assessed. We
found that that the under normal oxygen levels, the percent of
apoptotic A549 cells was increased over time after GRP78 silencing,
in addition, the apoptosis of A549 cells were further enhanced at
presence of hyperoxia over time, which is consistent with the
increase of CHOP expression after GRP79 silencing (Fig. 3).
The effect of CHOP overexpression on
the expressions of Bcl-2 or Bax under hyperoxia
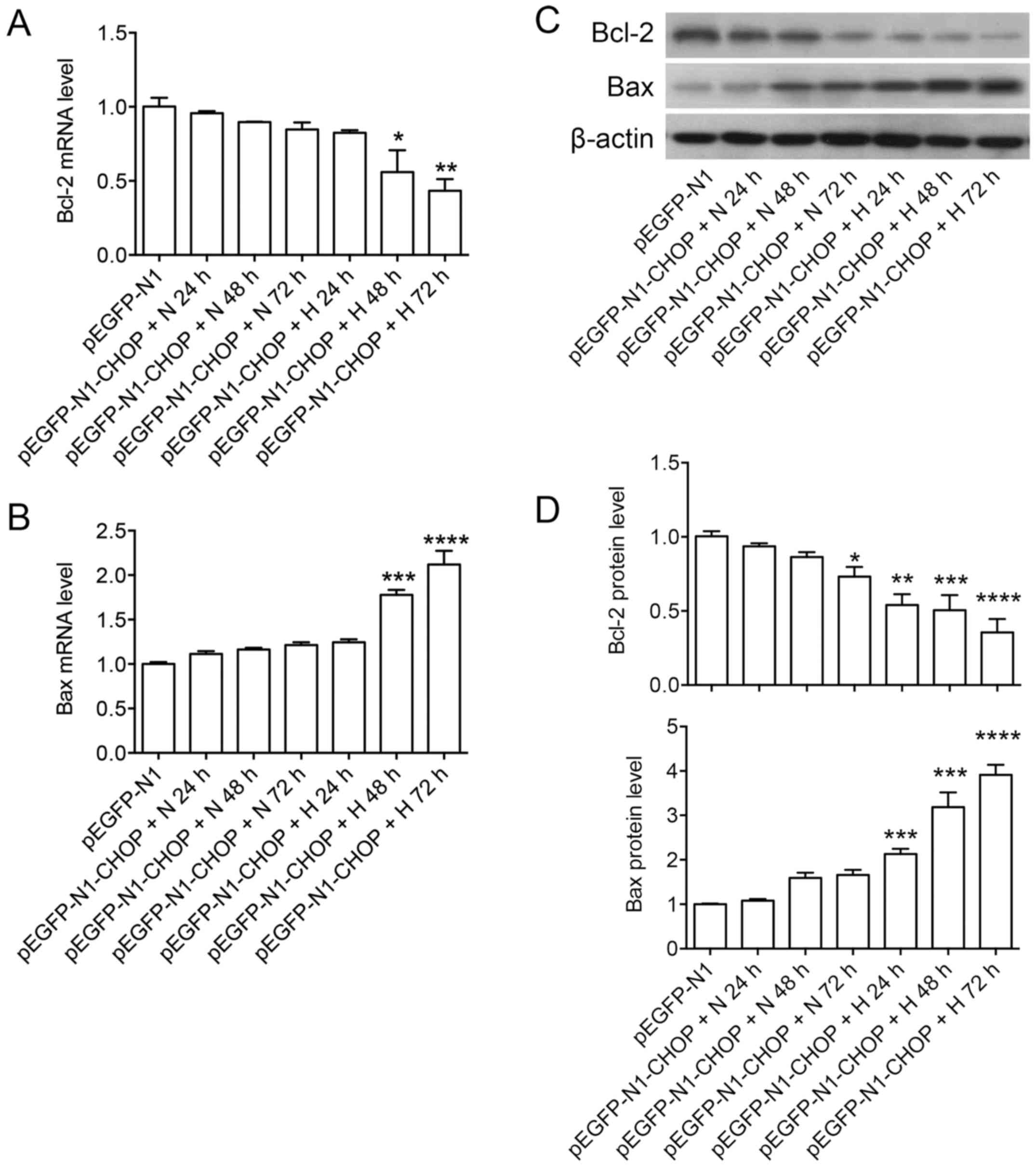
To test whether CHOP regulation by GRP78 was
correlated with the subsequent apoptotic events of A549 cells, we
overexpressed CHOP by using pEGFP-N1-CHOP (plasmid containing core
domain sequence of CHOP) on A549 cells, and establish hyperoxia
subsequently for 24, 48 and 72 h after transfection, and monitored
the expressions of anti-apoptotic protein Bcl-2 and pro-apoptotic
protein Bax at gene level and protein level. CHOP overexpression
induced 2–3-folds mRNA (24 h) by RP-PCR and protein expression
levels (48 h) by western blot (data not shown). We found that the
expression of Bcl-2 and Bax at RNA level were not affected by CHOP
overexpression under normal oxygen level for up to 72 h after
transfection. Decreased expression of Bcl-2 and increased
expression of Bax at protein level were found in later time points
after CHOP overexpression (48 and 72 h). Importantly, CHOP
overexpression under hyperoxia significantly decreased the
expression of Bcl-2 and increased expression of Bax at both RNA and
protein level (Fig. 4). Bcl-2 and
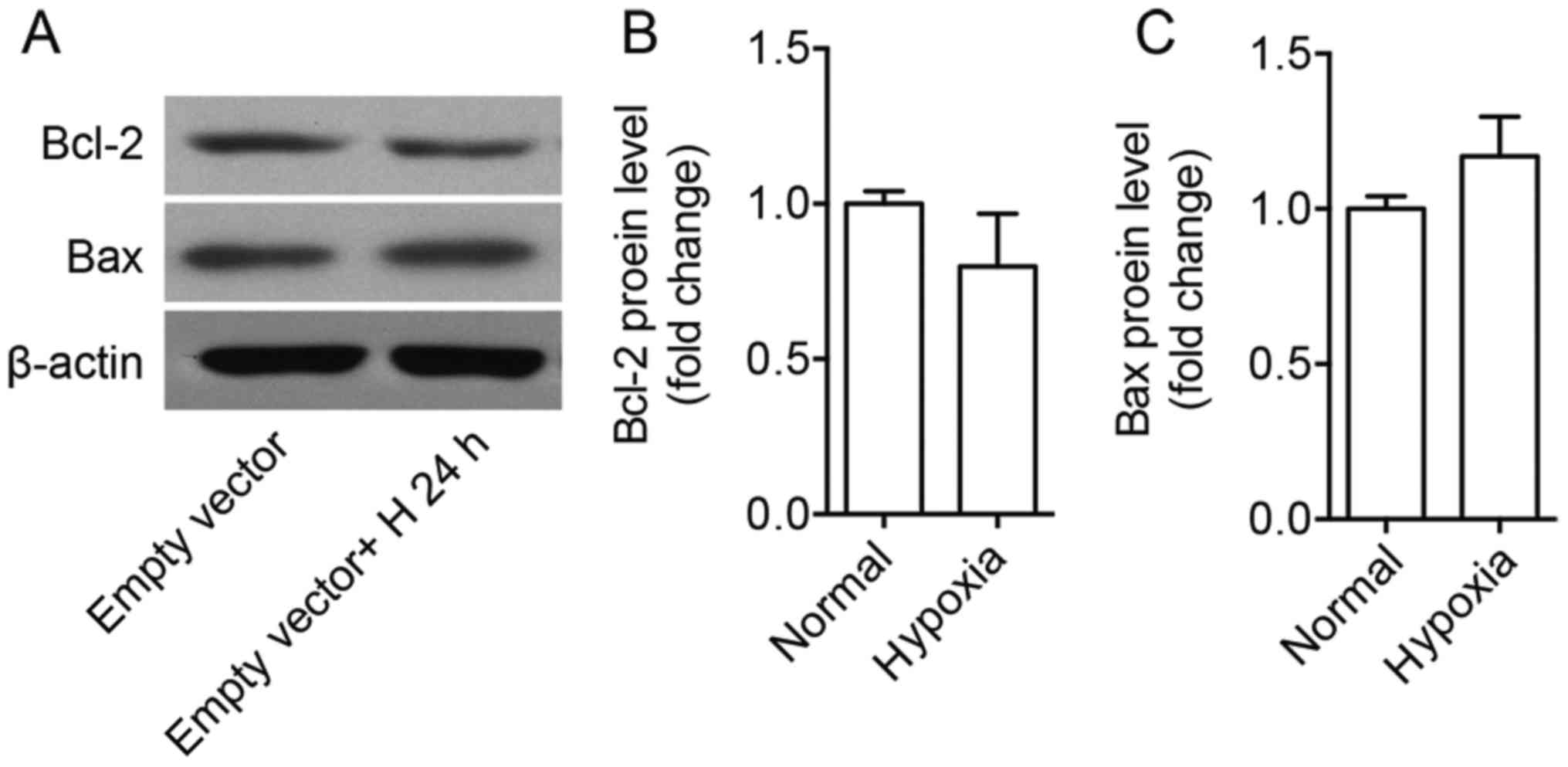
Bax protein levels were not changed after 24 h under hyperoxia
compared with normoxia on empty vector treated cells (Fig. 5).
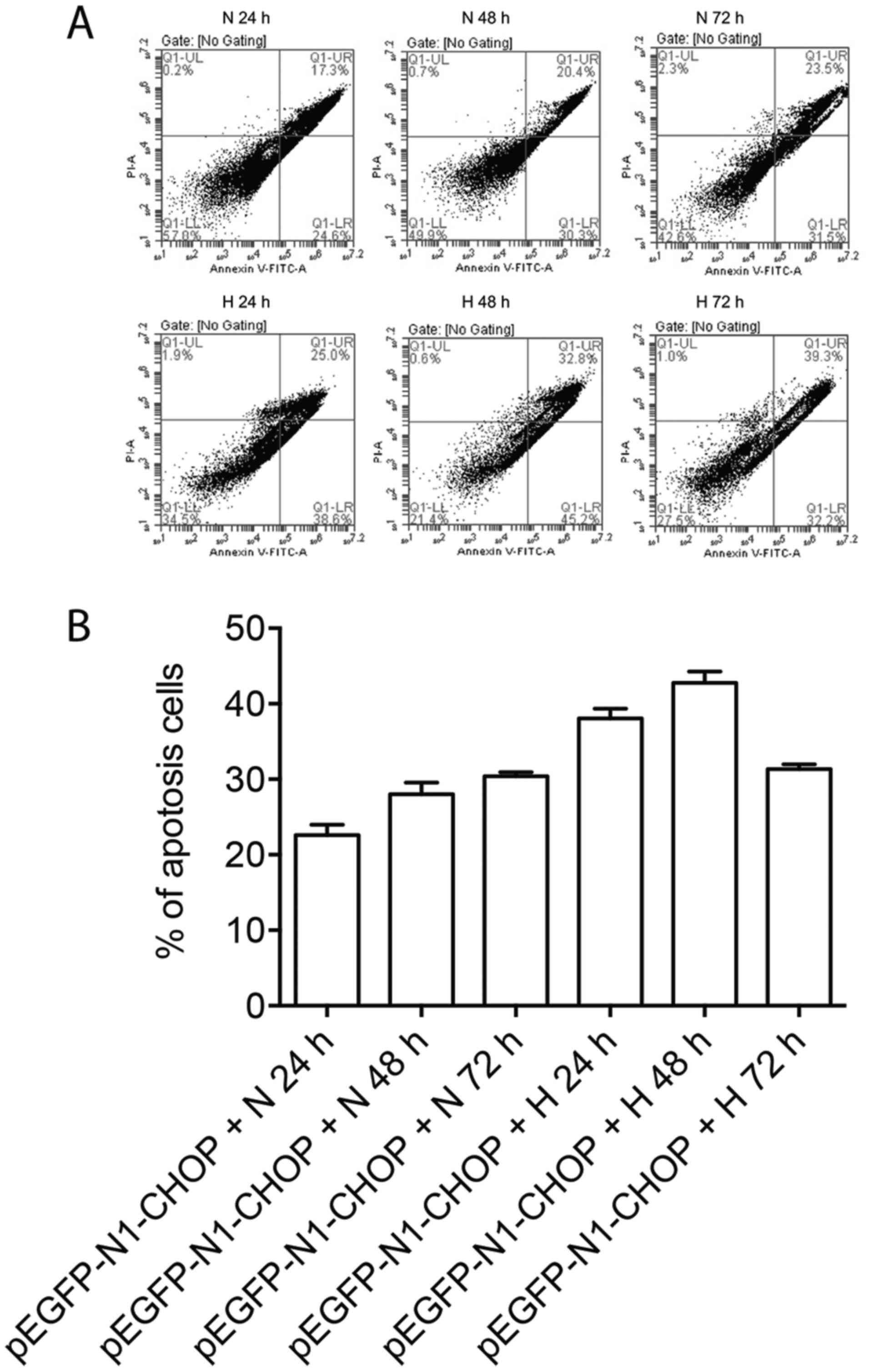
CHOP overexpression promoted apoptosis
of A549 cells under hyperoxia
To examine whether regulation of Bcl-2 and Bax by
CHOP could impact the apoptosis of A549 cells, we stained A549
cells with PI and Annexin V-FITC under normoxia or hyperoxia after
CHOP overexpression. The percent of apoptotic A549 cells (defined
by Annexin-V+PI-) increased over time after CHOP overexpression
under normoxia using BD FACSCanto, furthermore, the apoptosis of
A549 cells was significantly enhanced under hyperoxia, with the
peak apoptotic phase at 48 h after hyperoxia treatment, suggesting
the early apoptosis of A549 cells treated with pEGFP-N1-CHOP
transfection under hyperoxia (Fig.
6).
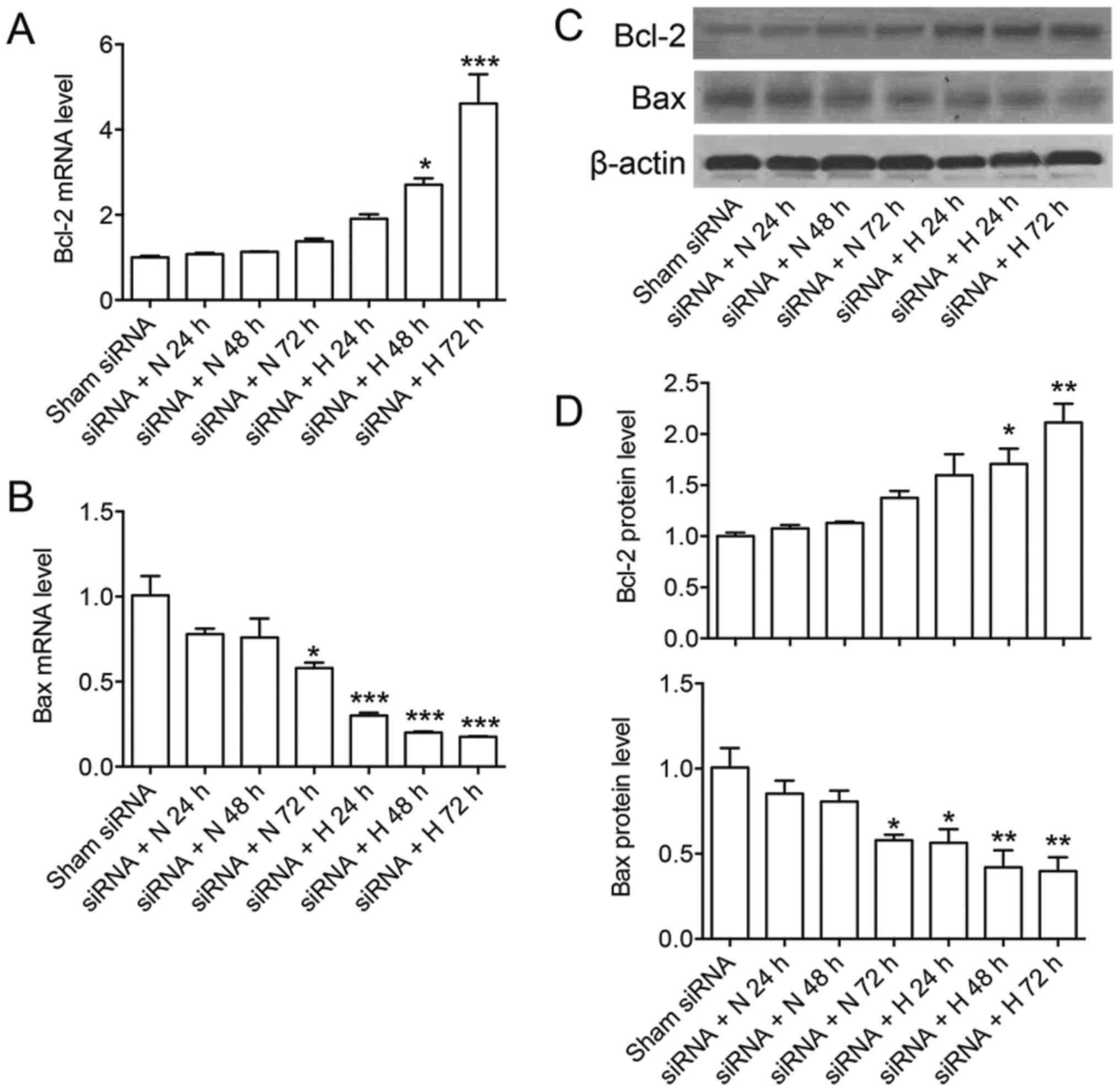
The effect of CHOP-siRNA on the
expression of Bcl-2 and Bax on A549 cells under hyperoxia
To further confirm the role of CHOP expression on
regulation of apoptotic related genes, we used CHOP-siRNA to
transfect A549 cell, established hyperoxia subsequently for 24, 48
and 72 h after transfection, and monitored the expressions of Bcl-2
and Bax at gene level and protein level. CHOP siRNA induced over
90% mRNA by RT-PCR and around 50–60% protein downregulation by
western blot compared with shamRNA (data not shown). We found the
relative mRNA expression of Bcl-2 was increased and Bax was
decreased at later time points after CHOP siRNA at gene level and
protein level, while significant increase of Bcl-2 and decrease of
Bax were shown when A549 cells were treated with CHOP siRNA under
hyperoxia. These results further confirmed the important role of
CHOP and hyperoxia in promoting apoptosis of A549 cells, probably
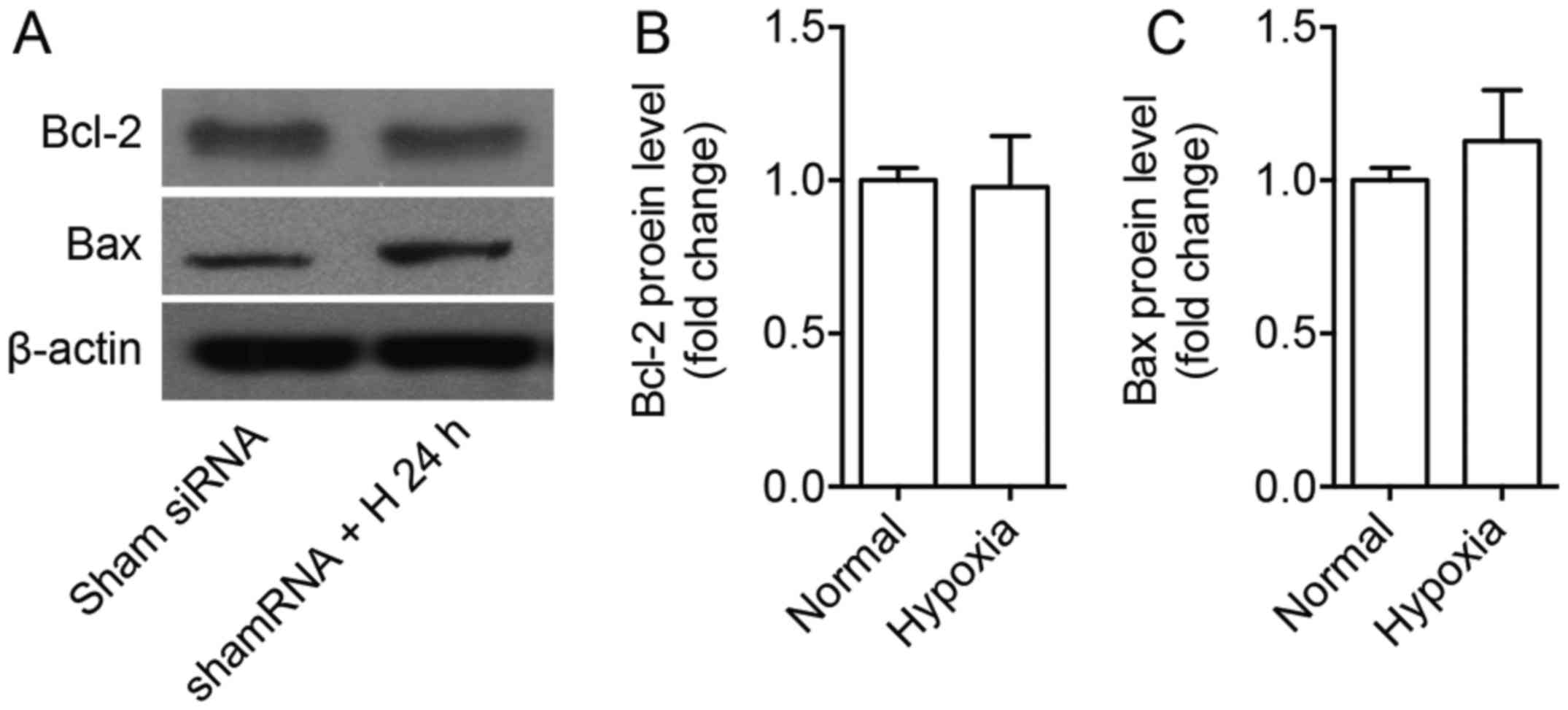
through regulation of Bcl-2 and Bax (Fig. 7). Bcl-2 and Bax protein levels were
not significantly changed after 24 h under hyperoxia compared to
normoxia on Sham siRNA treated cells (Fig. 8).
Discussion
Hyperoxia-induced lung injury after oxygen
supplementation is one of the major risk factors in the
pathogenesis of BPD (1).
Observations from prenatal and postnatal lung studies revealed that
apoptosis plays an important role in lung development in animals as
well as in humans. Apoptosis is more prominent in mesenchymal cells
and less frequent in epithelium in developing lungs, which are
normal processes in alveolar wall thinning and alveolar formation
(19,20). Preterm infants underwent
supplemental oxygen therapy showed disrupted lung development
featured by larger and simplified alveoli, increased alveolar
macrophages, and thickened alveolar walls due to interstitial
fibrosis and smooth muscle hyperplasia (21,22).
Hyperoxia causes apoptosis in peripheral airways (23,24).
It has demonstrated that apoptosis is significantly increased in
alveolar epithelial cells in preterm infants with BPD and
respiratory distress syndrome (25,26).
All these results suggest that adaptive apoptosis is a critical
process in lung development, neonatal lung injury and the
pathogenesis of BPD. In this study, the A549 cell line was selected
for this study due to its human alveolar type II epithelial cell
origin, we cultured A549 cells under 95% O2 to mimics
hyperoxia in vitro, apoptosis were induced and cellular
events associated with apoptosis were evaluated. There are
potential limitations of using A549 for this study, hTERT
immortalized cell line, primary cultured cells, or a panel of
cancer cell lines will be included in future study to confirm the
findings we discovered on A549 cells.
Prolonged oxygen exposure regulates the expression
of a variety of genes involved in cellular oxidative stress, cell
cycle, growth, and death (27).
GRP78 is a HSP70 molecular chaperone located in the lumen of the ER
that binds newly synthesized proteins as they are translocated into
the ER, and maintain them in a state competent for subsequent
folding and oligomerization. Inhibition or downregulation of GRP78
has been demonstrated to increase ER stress-induced cell death in
melanoma and cancer cells (28,29).
GRP78 siRNA lipoplex inhibited the growth of the renal carcinoma
cell line, which highly expresses GRP78 basally (11). Previous studies showed that
2-deoxyglucose (2-dG), tunicamycin (TM), and cigarette smoke
extract (CSE) treatments induced apoptosis of alveolar epithelial
cells, downregulation of GRP78 expression by GRP78 siRNA led to the
increased expression of caspase-3 and sensitivity to apoptosis
(30,31). ER protein ERp57 knockdown protected
hyperoxia- or tunicamycin-induced apoptosis of A549 cells by
induction of BiP/GRP78 (8). In
current study, we observed significant increase of apoptosis of
A549 cells treated with the combination of GRP78-siRNA under
hyperoxia, suggesting that GRP78 signaling pathway plays an
important role for lung epithelial injuries in preterm infants
undergo supplemental oxygen therapy.
ER stress induced cell death signaling occur via
three ER-resident transmembrane proteins, IRE-1α, ATF6α, and PERK
(14,32): i) Activated IRE-1α can recruit
c-Jun N-terminal inhibitory kinase (JIK) and tumor necrosis factor
receptor-associated factor-2 (TRAF2) to activate apoptosis-signal
regulating kinase-1 and c-Jun N-terminal kinase (JNK), leading to
the activation of a mitochondria-dependent cell-death pathway
(activation of caspase-3, 8, 9, Bcl-2-associated X protein or Bax,
the release of cytochrome c); ii) The release of JIK from
procaspase-12 allows for activation to caspase-12, which activates
procaspase-9, which in turn activates procaspase-3, the executioner
of cell death. iii) Activated PERK phosphorylates the eukaryotic
initiation factor-2α that enhances the translation of ATF4 mRNA,
which in turn induces CHOP (33,34).
CHOP can inhibit antiapoptotic Bcl-2, leading to the activation of
the executioner caspase-3. Newborn murine lung exposed to hyperoxia
and IFN-γ showed marked increase in cyclooxygenase-2 (Cox2) and the
upregulation of the endoplasmic reticulum (ER) stress pathway
mediator CHOP, which resulted in increased alveolar epithelial cell
death in as well as murine BPD (17). We investigate the effects of GRP78
siRNA on the gene expression profile of ER stress signaling pathway
molecules, and found that both gene and protein expression of CHOP
increased compared with those treated with sham siRNA.
Overexpression of CHOP under hyperoxia caused significant
downregulation of Bcl-2 and upregulation of Bax, enhanced apoptosis
of A549 cells, which suggested that imbalance of CHOP expression by
GRP78 knockdown under hyperoxia might be the cause of increased
apoptosis of A549 cells. Mouse embryo fibroblasts from
Bax−/−Bak−/− mice were resistant to apoptosis
induced by ER stress, suggesting the role of Bax and Bak as
executioner in ER-stress mediated apoptosis (35). Also, Matsumoto et al
reported that overexpression of Bcl-2 blocked CHOP-induced
apoptosis (36). On the other
hand, we treated A549 cells with the CHOP siRNA under hyperoxia,
the results showed the expression of Bcl-2 increased, while the
expression of Bax decreased, which further supported our hypothesis
on the role of GRP78-CHOP-Bcl-2/Bax pathway in ER related apoptosis
of lung epithelial cells under hyperoxia.
In this study, we determined the effect of GRP78
siRNA and CHOP overexpression under hyperoxia on human lung
epithelial cell line A549 cells, and found that the GRP78 siRNA or
CHOP overexpression could lead the enhanced apoptosis of A549 cells
under hyperoxia, suggesting that the important role of GRP78 in
promoting lung epithelial apoptosis under hyperoxia, probably
through regulating CHOP-Bcl-2/Bax pathway, targeting GRP78 might
help to reduce lung epithelial injury for preterm infants undergo
oxygen supplementary treatment and lower the incidence of BPD.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (NNSFC) (nos. 81370746 and no.
81300521), the Natural Science Foundation of Jiangsu Province,
China (no. BK20161356), and the Social Development Foundation of
Zhenjiang, China (no. SH2015071).
References
|
1
|
Eichenwald EC and Stark AR: Management and
outcomes of very low birth weight. N Engl J Med. 358:1700–1711.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
McGrath-Morrow SA and Stahl J: Apoptosis
in neonatal murine lung exposed to hyperoxia. Am J Respir Cell Mol
Biol. 25:150–155. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
de Paepe ME, Mao Q, Chao Y, Powell JL,
Rubin LP and Sharma S: Hyperoxia-induced apoptosis and Fas/FasL
expression in lung epithelial cells. Am J Physiol Lung Cell Mol
Physiol. 289:L647–L659. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bourbon J, Boucherat O, Chailley-Heu B and
Delacourt C: Control mechanisms of lung alveolar development and
their disorders in bronchopulmonary dysplasia. Pediatr Res.
57:38R–46R. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Frank L and Groseclose EE: Preparation for
birth into an O2-rich environment: The antioxidant enzymes in the
developing rabbit lung. Pediatr Res. 18:240–244. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tanswell AK and Freeman BA: Pulmonary
antioxidant enzyme maturation in the fetal and neonatal rat. I.
Developmental profiles. Pediatr Res. 18:584–587. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xu C, Bailly-Maitre B and Reed JC:
Endoplasmic reticulum stress: Cell life and death decisions. J Clin
Invest. 115:2656–2664. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xu D, Perez RE, Rezaiekhaligh MH, Bourdi M
and Truog WE: Knockdown of ERp57 increases BiP/GRP78 induction and
protects against hyperoxia and tunicamycin-induced apoptosis. Am J
Physiol Lung Cell Mol Physiol. 297:L44–L51. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Maurel M and Chevet E: Endoplasmic
reticulum stress signaling: The microRNA connection. Am J Physiol
Cell Physiol. 304:C1117–C1126. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi
NN, Ozdelen E, Tuncman G, Görgün C, Glimcher LH and Hotamisligil
GS: Endoplasmic reticulum stress links obesity, insulin action, and
type 2 diabetes. Science. 306:457–461. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wu J, Ruas JL, Estall JL, Rasbach KA, Choi
JH, Ye L, Boström P, Tyra HM, Crawford RW, Campbell KP, et al: The
unfolded protein response mediates adaptation to exercise in
skeletal muscle through a PGC-1α/ATF6α complex. Cell Metab.
13:160–169. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Puthalakath H, O'Reilly LA, Gunn P, Lee L,
Kelly PN, Huntington ND, Hughes PD, Michalak EM, McKimm-Breschkin
J, Motoyama N, et al: ER stress triggers apoptosis by activating
BH3-only protein Bim. Cell. 129:1337–1349. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zinkel S, Gross A and Yang E: BCL2 family
in DNA damage and cell cycle control. Cell Death Differ.
13:1351–1359. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rutkowski DT and Kaufman RJ: That which
does not kill me makes me stronger: Adapting to chronic ER stress.
Trends Biochem Sci. 32:469–476. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lu HY, Zhang J, Wang QX, Tang W and Zhang
LJ: Activation of the endoplasmic reticulum stress pathway
involving CHOP in the lungs of rats with hyperoxia-induced
bronchopulmonary dysplasia. Mol Med Rep. 12:4494–4500.
2015.PubMed/NCBI
|
|
16
|
O'Reilly MA, Staversky RJ, Watkins RH,
Maniscalco WM and Keng PC: p53-independent induction of GADD45 and
GADD153 in mouse lungs exposed to hyperoxia. Am J Physiol Lung Cell
Mol Physiol. 278:L552–L559. 2000.PubMed/NCBI
|
|
17
|
Choo-Wing R, Syed MA, Harijith A, Bowen B,
Pryhuber G, Janér C, Andersson S, Homer RJ and Bhandari V:
Hyperoxia and interferon-γ-induced injury in developing lungs occur
via cyclooxygenase-2 and the endoplasmic reticulum stress-dependent
pathway. Am J Respir Cell Mol Biol. 48:749–757. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lozon TI, Eastman AJ, Matute-Bello G, Chen
P, Hallstrand TS and Altemeier WA: PKR-dependent CHOP induction
limits hyperoxia-induced lung injury. Am J Physiol Lung Cell Mol
Physiol. 300:L422–L429. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dieperink HI, Blackwell TS and Prince LS:
Hyperoxia and apoptosis in developing mouse lung mesenchyme.
Pediatr Res. 59:185–190. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bruce MC, Honaker CE and Cross RJ: Lung
fibroblasts undergo apoptosis following alveolarization. Am J
Respir Cell Mol Biol. 20:228–236. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Stenmark KR and Abman SH: Lung vascular
development: Implications for the pathogenesis of bronchopulmonary
dysplasia. Annu Rev Physiol. 67:623–661. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Thibeault DW, Mabry S and Rezaiekhaligh M:
Neonatal pulmonary oxygen toxicity in the rat and lung changes with
aging. Pediatr Pulmonol. 9:96–108. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Scavo LM, Ertsey R, Chapin CJ, Allen L and
Kitterman JA: Apoptosis in the development of rat and human fetal
lungs. Am J Respir Cell Mol Biol. 18:21–31. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kresch MJ, Christian C, Wu F and Hussain
N: Ontogeny of apoptosis during lung development. Pediatr Res.
43:426–431. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hargitai B, Szabó V, Hajdú J, Harmath A,
Pataki M, Farid P, Papp Z and Szende B: Apoptosis in various organs
of preterm infants: Histopathologic study of lung, kidney, liver,
and brain of ventilated infants. Pediatr Res. 50:110–114. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
May M, Strobel P, Preisshofen T,
Seidenspinner S, Marx A and Speer CP: Apoptosis and proliferation
in lungs of ventilated and oxygen-treated preterm infants. Eur
Respir J. 23:113–121. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
O'Reilly MA: DNA damage and cell cycle
checkpoints in hyperoxic lung injury: Braking to facilitate repair.
Am J Physiol Lung Cell Mol Physiol. 281:L291–L305. 2001.PubMed/NCBI
|
|
28
|
Stuhr LE, Raa A, Oyan AM, Kalland KH,
Sakariassen PO, Petersen K, Bjerkvig R and Reed RK: Hyperoxia
retards growth and induces apoptosis, changes in vascular density
and gene expression in transplanted gliomas in nude rats. J
Neurooncol. 85:191–202. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Martin S, Hill DS, Paton JC, Paton AW,
Birch-Machin MA, Lovat PE and Redfern CP: Targeting GRP78 to
enhance melanoma cell death. Pigment Cell Melanoma Res. 23:675–682.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ahmad M, Hahn IF and Chatterjee S: GRP78
up-regulation leads to hypersensitization to cisplatin in A549 lung
cancer cells. Anticancer Res. 34:3493–3500. 2014.PubMed/NCBI
|
|
31
|
He B, Luo B, Chen Q and Zhang L: Cigarette
smoke extract induces the expression of GRP78 in A549 cells via the
p38/MAPK pathway. Mol Med Rep. 8:1683–1688. 2013.PubMed/NCBI
|
|
32
|
Zhang K and Kaufman RJ: The unfolded
protein response: A stress signaling pathway critical for health
and disease. Neurology. 66 2 Suppl 1:S102–S109. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu G, Su L, Hao X, Zhong N, Zhong D,
Singhal S and Liu X: Salermide up-regulates death receptor 5
expression through the ATF4-ATF3-CHOP axis and leads to apoptosis
in human cancer cells. J Cell Mol Med. 16:1618–1628. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Konsavage WM, Zhang L, Wu Y and Shenberger
JS: Hyperoxia-induced activation of the integrated stress response
in the newborn rat lung. Am J Physiol Lung Cell Mol Physiol.
302:L27–L35. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wei MC, Zong WX, Cheng EH, Lindsten T,
Panoutsakopoulou V, Ross AJ, Roth KA, MacGregor GR, Thompson CB and
Korsmeyer SJ: Proapoptotic BAX and BAK: A requisite gateway to
mitochondrial dysfunction and death. Science. 292:727–730. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Matsumura K, Sakai C, Kawakami S,
Yamashita F and Hashida M: Inhibition of cancer cell growth by
GRP78 siRNA lipoplex via activation of unfolded protein response.
Biol Pharm Bull. 37:648–653. 2014. View Article : Google Scholar : PubMed/NCBI
|