Introduction
Allergic rhinitis is a type of nasal inflammation
which occurs when the immune system overreacts to inhaled
allergens, and is a frequent and serious public health concern.
Anti-allergic and antihistamine agents are commonly used in the
treatment of allergic rhinitis; however, their effects are
transient as they are able to suppress only the symptoms of the
inflammatory response (1).
Therefore, the development of novel therapeutic strategies that
target the pathogenesis of the disorder are required for the
treatment of allergic rhinitis.
During an allergic response, the activation of T
helper (Th)1 and Th2 cells appears to be mutually antagonistic.
Under physiological conditions, Th1 and Th2 populations exist in
equilibrium and cross-regulate each other, and the Th1/Th2 balance
has been suggested to be necessary for the maintenance of immune
homeostasis (2,3). GATA-3 is a transcription factor
revealed to be specifically expressed in Th2 cells, whereas T-box
protein expressed in T cells (T-bet) is a transcription factor
specifically expressed in Th1 cells. GATA-3 and T-bet have been
reported to modulate gene expression during T cell differentiation,
thus serving a critical role in the development of Th1 and Th2
lineages (4,5). However, the molecular mechanisms
underlying allergic rhinitis are complex and involve other pathways
apart from an aberrant Th2 response. Regulatory T (Treg) cells have
been suggested to serve critical roles in the maintenance of immune
homeostasis. Treg cells have been reported to inhibit the
proliferation and activation of conventional effector T cells in a
cell contact-dependent manner, either directly or via acting on
antigen-presenting cells (6). The
forkhead family transcription factor Foxp3 plays a key role on Treg
cells in inhibition of tissue inflammation reaction and regulate
the body's immune stability (7).
Th17 cells has the opposite effect of Treg cells, while RORγt is a
splice variant of ROR, which has been identified as an essential
factor during Th17 cellular differentiation (8,9).
However, the induction of RORγt has been reported to be dependent
on the activity of STAT-3. RORγt and STAT-3 may collaboratively
regulate the transcriptional profile of Th17 cells (10).
Astragalus membranaceus is a herb with a long
history of use in traditional Chinese medicine for the treatment of
allergic rhinitis (11).
3-O-β-D-xylopyranosyl-6-O-β-D-glucopyranosyl-cycloastragenol, or
astragaloside IV (AST), which belongs to the chemical class of
saponins, is one of the primary active compounds isolated from
Astragalus membranaceus roots with distinct pharmacological
effects, and has anti-inflammatory, immunoregulatory and
antifibrotic properties (12,13).
It has previously been demonstrated that AST markedly inhibits
airway inflammation and has profound immunoregulatory effects in
vivo (14). In addition, AST
has been reported to exert neuro- and cardioprotective effects
during cerebral and myocardial ischemic events, which have been
attributed to its anti-inflammatory, antioxidative, antithrombotic,
vasodilatory and immunoregulatory properties (15,16).
Therefore, the present study aimed to investigate whether AST
exhibited regulatory effects on allergic responses in vivo.
In addition, it aimed to examine the molecular mechanisms
underlying the putative protective effects of AST against allergic
rhinitis, potentially involving regulation of the of Th1/Th2 and
Treg cellular responses.
Materials and methods
Reagents
AST (purity>95%) was obtained from the National
Institutes for Food and Drug Control (Beijing, China) and was
suspended in a 1% carboxymethylcellulose solution. Dexamethasone
(DEX) was purchased from Shenyang Comeboard Technology Co., Ltd
(Shenyang, China). Ovalbumin (OVA, fraction V) was purchased from
Sigma-Aldrich; Merck KGaA (Darmstadt, Germany). Aluminium hydroxide
[Al (OH)3] was obtained from Pierce; Thermo Fisher
Scientific, Inc. (Waltham, MA, USA). Immunoglobulin (Ig)E (cat no.
SEA545Ra) and IgG (cat no. HEA544Ra) ELISA kits were purchased from
Cloud-Clone Corp. (Houston, TX, USA). Anti-GATA-3 (cat no. 5852T),
anti-T-box protein expressed in T cells (T-bet; cat no. 13232T),
anti-signal transducer and activator of transcription (STAT)-3 (cat
no. 12640S) and anti-β-actin antibody (cat no. 3700S) monoclonal
antibodies were obtained from Cell Signaling Technology, Inc.
(Danvers, MA, USA). An anti-forkhead box protein 3 (Foxp3; cat no.
ab20034) antibody was purchased from Abcam (Cambridge, MA,
USA).
Animals
A total of 25 male and 25 female BALB/c mice (age:
6–8 weeks; weight, 15–20 g) were purchased from the Laboratory
Animal Center of Chongqing Medical University (Chongqing, China),
and were maintained in horizontal laminar flow cabinets and
provided with free access to sterile food and water in a specific
pathogen-free facility (temperature, 18–29°C; 12/12 h light/dark
cycles). Mice were randomly assigned to the following five groups
(n=10 mice/group): Normal control (saline only), OVA-induced
allergic rhinitis (OVA), AST (25 mg/kg and 50 mg/kg; n=10
mice/sub-group) and DEX (3 mg/kg). The 25 and 50 mg/kg doses of AST
were selected based on previous studies (17).
The present study was conducted in accordance with
the internationally accepted principles for laboratory animal use
and care, as outlined in the European Commission guidelines (EEC
Directive of 1986; 86/609/EEC). The experimental procedures were
approved by the Animal Ethics Committee of Chongqing Medical
University.
Allergic rhinitis model and AST
administration
Mice in the OVA, AST and DEX groups were
administered intraperitoneally with 0.5 mg/ml OVA and 20 mg/ml
Al(OH)3 in saline, at a dose of 0.2 ml/mouse
(sensitization), as previously described (18). Sensitization was repeated three
times at weekly intervals (days 0, 7 and 14), and was followed by
daily administration of OVA solution (40 mg/ml in saline) into the
nostrils (0.02 ml/mouse), on days 21–42 (OVA challenge). Mice in
the AST and DEX treatment groups were administered intragastrically
with AST (25 or 50 mg/kg) or DEX (3 mg/kg) daily for 3 weeks, 30
min prior to the OVA challenge. Mice in the normal group were
administered only with the last intranasal OVA challenge on day 42.
Nasal symptoms were evaluated via counting the number of sneezes
for 10 min immediately following the last OVA intranasal
challenge.
Weight and spleen/hepatic index
measurements
The weight of each mouse was measured weekly using
an electronic scale. A total of 48 h following the last treatment,
all mice were weighed, anesthetized with ether and sacrificed. The
spleen and liver were harvested and used for further analysis. The
spleen and hepatic indices were calculated according to the
following formula: Spleen/hepatic index=spleen/liver weight
(µg)/body weight (g) × 10, as previously described (19).
ELISA
A total of 48 h following the last treatment, mice
were anesthetized with ether and sacrificed. Blood samples were
collected from the orbital sinus. Samples were kept standing for 30
min at room temperature, were centrifuged at 3,000 × g for 10 min
at room temperature and the serum was isolated. Concentrations of
OVA-specific IgE and total IgG were evaluated using the appropriate
ELISA kits.
Hematoxylin-eosin (HE) and
immunohistochemical staining
A total of 48 h following the last treatment, mice
were sacrificed and their heads and spleens were harvested and
immersed in 4% neutral-buffered formalin at room temperature for 7
days. Following fixation, heads were decalcified in 5% formic acid
at room temperature for 6 days. Subsequently, the heads and spleens
were trimmed, embedded in paraffin and sectioned coronally at a
thickness of 4 µm for HE and immunohistochemical staining. For the
immunohistochemical detection of GATA-3, T-bet and Foxp3, slides
were placed in 0.01 M citric buffer (pH 6.0) and autoclaved at
121°C for 20 min. Sections were blocked with mountain goat serum
(Hyclone; GE Healthcare Life Sciences, Logan, UT, USA) at room
temperature for 20 min and incubated with anti-GATA-3 (1:1,00),
anti-T-bet (1:1,00) and anti-Foxp3 (1:2,00) polyclonal antibodies
at 4°C for 12 h. Subsequently, sections were washed, incubated with
an anti-mouse horseradish peroxidase (HRP) -conjugated IgG
secondary antibody (1:1,000; Beyotime Institute of Biotechnology,
Haimen, China; cat no. A0216) at 37°C for 1 h and stained with
3,3′-diaminobenzidine. Numbers of eosinophils infiltrating the
nasal mucosa were counted in HE-stained sections in 3 randomly
selected fields under a ×400 magnification. Stained sections were
observed under a Leica DM2500 optical microscope (Leica
Microsystems, Inc., Buffalo Grove, IL, USA) and photomicrographs
were captured.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from spleen tissue samples
using TRIzol® reagent (Invitrogen; Thermo Fisher Scientific, Inc.)
and reverse transcribed into cDNA using the High Capacity cDNA
Reverse Transcription kit (Thermo Fisher Scientific, Inc.) and an
oligo (dT) primer (Thermo Fisher Scientific, Inc.), according to
the manufacturer's protocol. The reaction volume was 8 µl (5X
reaction buffer 4 µl, dNTP mix 1 µl, Rnase Inhibitor 0.5 µl, Rnase
free H2O 0.5 µl, M-MLV enzyme 2 µl). Amplification
conditions were as follows: at 30°C for 10 min, at 42°C for 60 min
and at 70°C for 15 min. Primer sequences for murine GATA-3, T-bet,
retinoic acid receptor-related orphan nuclear receptor (ROR) γt,
Foxp3 and β-actin, which served as the internal control, are
presented in Table I. The cDNA
products were used as templates for qPCR. The CFX96™ Real-Time PCR
detection system (Bio-Rad Laboratories, Inc., Hercules, CA, USA)
was used for analysis with SYBR® Premix Ex Taq™ (Takara
Biotechnology Co., Ltd., Dalian, China). The reaction was performed
in a total volume of 25 µl (SYBR® Premix Ex Taq™ 2X 12.5 µl,
forward primer (10 µmol/l) 1 µl, reverse primer (10 µmol/l) 1 µl,
cDNA 2 µl, ddH2O 8.5 µl) with the following
thermocycling conditions: Initial denaturation at 5°C for 2 min,
followed by 10 min at 95°C, 40 cycles of annealing at 95°C for 15
sec followed by extension at 60°C for 1 min. Gene expression was
quantified using the comparative 2−∆∆Cq method and
normalized to β-actin (20).
 | Table I.Primer sequences used for reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primer sequences used for reverse
transcription-quantitative polymerase chain reaction.
| Gene | Sequence
(5′-3′) |
|---|
| GATA-3 | F:
ACAGAAGGCAGGGAGTGTGT |
|
| R:
GGTAGAGTCCGCAGGCATT |
| T-bet | F:
TACAACAGCCAGCCAAACAG |
|
| R:
CACCCTTCAAACCCTTCCTC |
| RORγt | F:
TGCGACTGGAGGACCTTCTA |
|
| R:
TCACCTCCTCCCGTGAAAAG |
| Foxp3 | F:
GCCCATCCAATAAACTGTGG |
|
| R:
GTATCCGCTTTCTCCTGCTG |
| β-actin | F:
GAGACCTTCAACACCCCAGC |
|
| R:
ATGTCACGCACGATTTCCC |
Western blot assay
Spleen tissue was dissected and sonicated in T-PER™
Tissue Protein Extraction Reagent (Thermo Fisher Scientific, Inc.)
containing protease cocktail (Beyotime Institute of Biotechnology)
on ice. The lysates were centrifuged at 10,000 × g for 10 min at
4°C. Protein concentration was determined using a BCA assay kit
(Beyotime Institute of Biotechnology). Equal amounts of extracted
protein samples (50 µg) were separated by 10% SDS-PAGE and
transferred onto polyvinylidene difluoride membranes. The membranes
were blocked with 5% non-fat dry milk at 37°C for 1 h and incubated
overnight at 4°C with the following primary antibodies: Anti-GATA-3
(1:1,000), anti-T-bet (1:1,000), anti-STAT-3 (1:1,000), anti-Foxp3
(1:2,000) and anti-β-actin (1:5,000). Subsequently, membranes were
incubated with HRP-conjugated goat anti-rabbit IgG (cat no. A0208)
or goat anti-mouse IgG (cat no. A0258) secondary antibodies
(1:2,000; Beyotime Institute of Biotechnology) at 37°C for 2 h.
Protein bands were visualized using the Immun-Star™ HRP
Chemiluminescent kit (Bio-Rad Laboratories, Inc.) and the Molecular
Imager® VersaDoc™ MP imaging system (Bio-Rad Laboratories, Inc.).
β-actin served as the loading control.
Statistical analysis
The statistical significance of the difference
between groups was assessed by one-way analysis of variance
followed by a post hoc Dunnett's test for multiple comparisons.
Data are expressed as the mean ± standard deviation of three
independent experiments. P<0.05 was considered to indicate a
statistically significant difference. Statistical analysis was
performed using the SPSS software version 22.0 (IBM SPSS, Armonk,
NY, USA).
Results
AST administration alleviates allergic
rhinitis symptoms in mice
The putative protective effects of AST against
allergic rhinitis were examined in allergic rhinitis mice,
following intragastric administration of AST (25 or 50 mg/kg), DEX
(3 mg/kg) or saline (control). Mice in the OVA model group
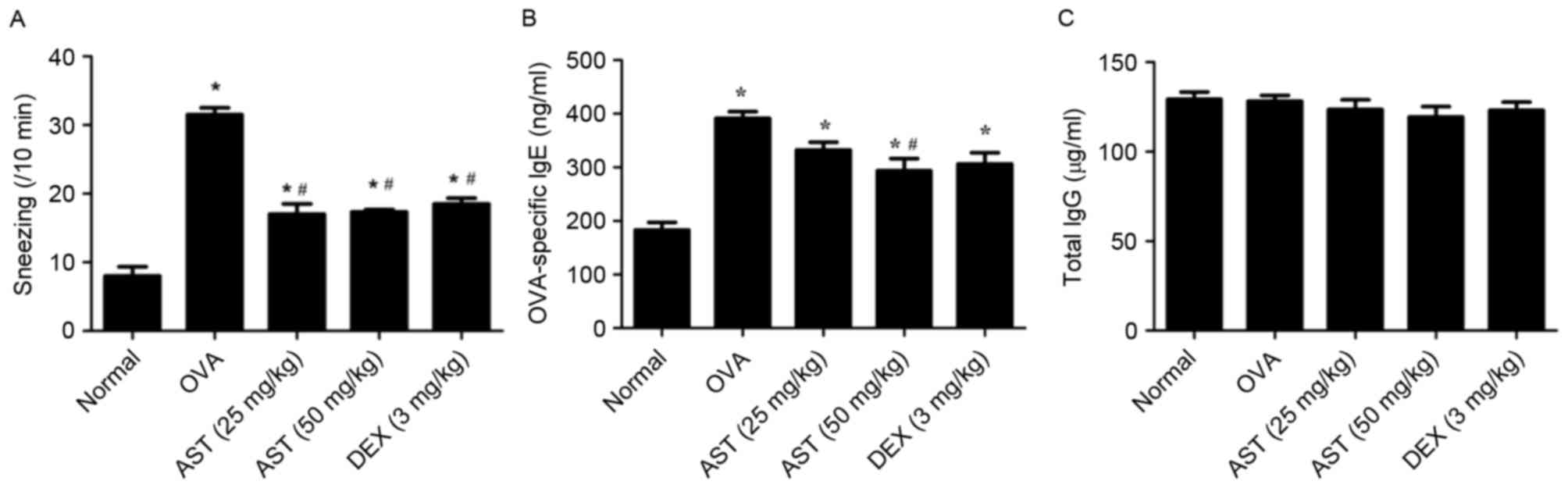
exhibited a marked increase in the number of sneezes (31.5±2.04
counts/10 min) compared with in the normal, non-sensitized group
that received only the last challenge immediately prior to
assessment (8.0±2.7 counts/10 min; P<0.05; Fig. 1A). Sneeze counts of AST-treated
allergic (17.0±2.6 counts/10 min, 25 mg/kg AST; 17.3±3.5 counts/10
min, 50 mg/kg AST) and DEX-treated mice (18.5±3.0 counts/10 min)
were significantly reduced compared with mice in the OVA model
group (P<0.05; Fig. 1A). These
results suggested that treatment with AST may ameliorate the
symptoms associated with allergic rhinitis.
AST administration decreases serum IgE
levels
OVA-specific IgE and IgG levels in mouse serum
samples were examined by ELISA. OVA-specific IgE serum levels were
significantly increased in mice with OVA-induced allergic rhinitis
compared with control mice (Fig.
1B). However, treatment with 50 mg/kg AST reversed this effect.
Conversely, IgG levels in mouse serum samples remained unaffected,
regardless of the treatment administered (Fig. 1C).
Histopathological alterations in nasal
mucosa and spleen
Morphological alterations in nasal mucosal and
spleen tissue samples of allergic mice were investigated using HE
staining. Nasal mucosal tissue isolated from allergic mice
exhibited prominent alterations, including eosinophil infiltration,
blood vessel sprouting, gland hyperplasia and enlargement, and
mucosal remodeling. In addition, the nasal epithelium became
multilayered (Fig. 2A). Spleen
tissue isolated from allergic mice was characterized by splenic
corpuscle proliferation (Fig. 2B).
Notably, the administration of AST or DEX appeared to prevent the
infiltration of eosinophils in the submucosa, the acidophilic
alterations and the development of fibrosis. Furthermore, AST and
DEX appeared to attenuate splenic corpuscle proliferation (Fig. 2B). These findings suggested that
AST administration may alleviate allergic responses in
OVA-challenged mice.
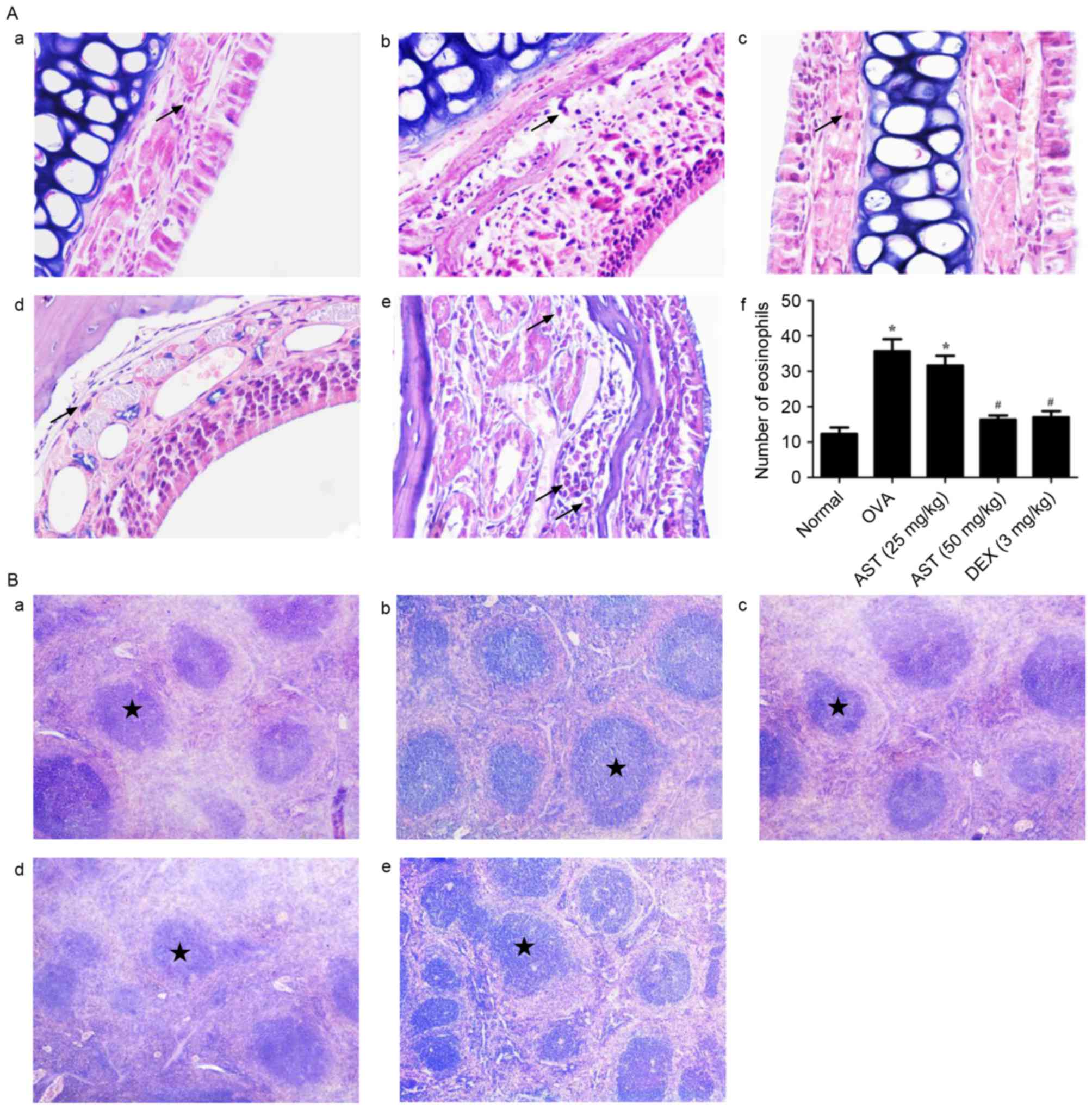 | Figure 2.Morphological alterations following
OVA sensitization and AST administration in mouse nasal mucosa and
spleens. (A) Representative photomicrographs of the numbers of
eosinophils infiltrating the nasal mucosa in the (a) normal, (b) 25
mg/kg AST, (c) 50 mg/kg AST, (d) DEX and (e) OVA groups, and (f)
quantification. Data are expressed as the mean ± standard
deviation. Arrows indicate the presence of eosinophils. *P<0.05
vs. the normal group; #P<0.05 vs. the OVA model
group. (B) Representative photomicrographs of mouse spleen tissue
in the (a) normal, (b) 25 mg/kg AST, (c) 50 mg/kg AST, (d) DEX and
(e) OVA groups. Stars indicate the location of the splenic
corpuscle. Magnification, ×400. OVA, ovalbumin; AST, Astragaloside
IV; DEX, dexamethasone. |
AST downregulates GATA-3 and
upregulates T-bet mRNA and protein expression levels in allergic
mice
It has previously been reported that Th2-type
cytokines, as well as the GATA-3/T-bet expression ratio in lung
tissue isolated from allergic mice were affected following
Astragalus membranaceus administration (21) Therefore, it may be hypothesized
that the mechanisms underlying the antiallergic effects of AST
involve the regulation of transcription factors, including T-bet
and GATA-3, during Th1 and Th2 cellular differentiation. The
present study used immunohistochemistry, RT-qPCR and western blot
analysis to investigate the expression of GATA-3 and T-bet in nasal
mucosal and spleen tissue of allergic mice. Immunohistochemical
results demonstrated that GATA-3 expression levels were
downregulated in nasal mucosal (Fig.
3A) and spleen (Fig. 3B)
tissue samples following AST or DEX administration, whereas T-bet
expression levels were upregulated (Fig. 4). Similar expression alterations
for GATA-3 and T-bet were observed at the protein (Fig. 5A) and mRNA (Fig. 5B) level, as determined by western
blotting and RT-qPCR, respectively. These findings suggested that
treatment with AST may downregulate the expression of GATA-3 and
upregulate the expression of T-bet during allergic responses in
vivo.
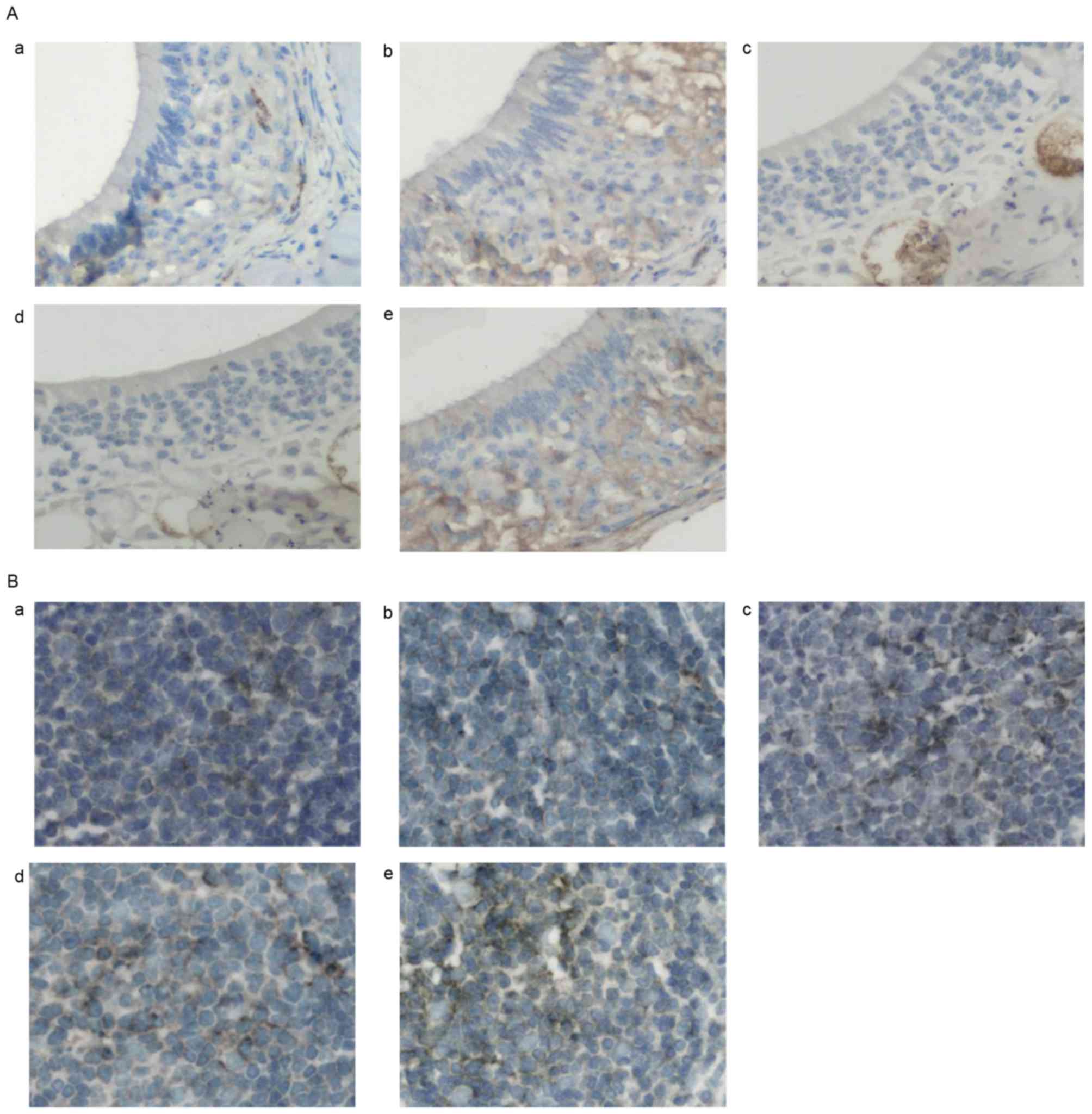 | Figure 3.Effects of AST administration on
GATA-3 protein expression. (A) Representative photomicrographs of
GATA-3 protein expression in nasal mucosa tissue samples in the (a)
normal, (b) 25 mg/kg AST, (c) 50 mg/kg AST, (d) DEX and (e) OVA
groups. (B) Representative photomicrographs of mouse spleen tissue
in the (a) normal, (b) 25 mg/kg AST, (c) 50 mg/kg AST, (d) DEX and
(e) OVA groups. Magnification, ×400. AST, Astragaloside IV; OVA,
ovalbumin; DEX, dexamethasone. |
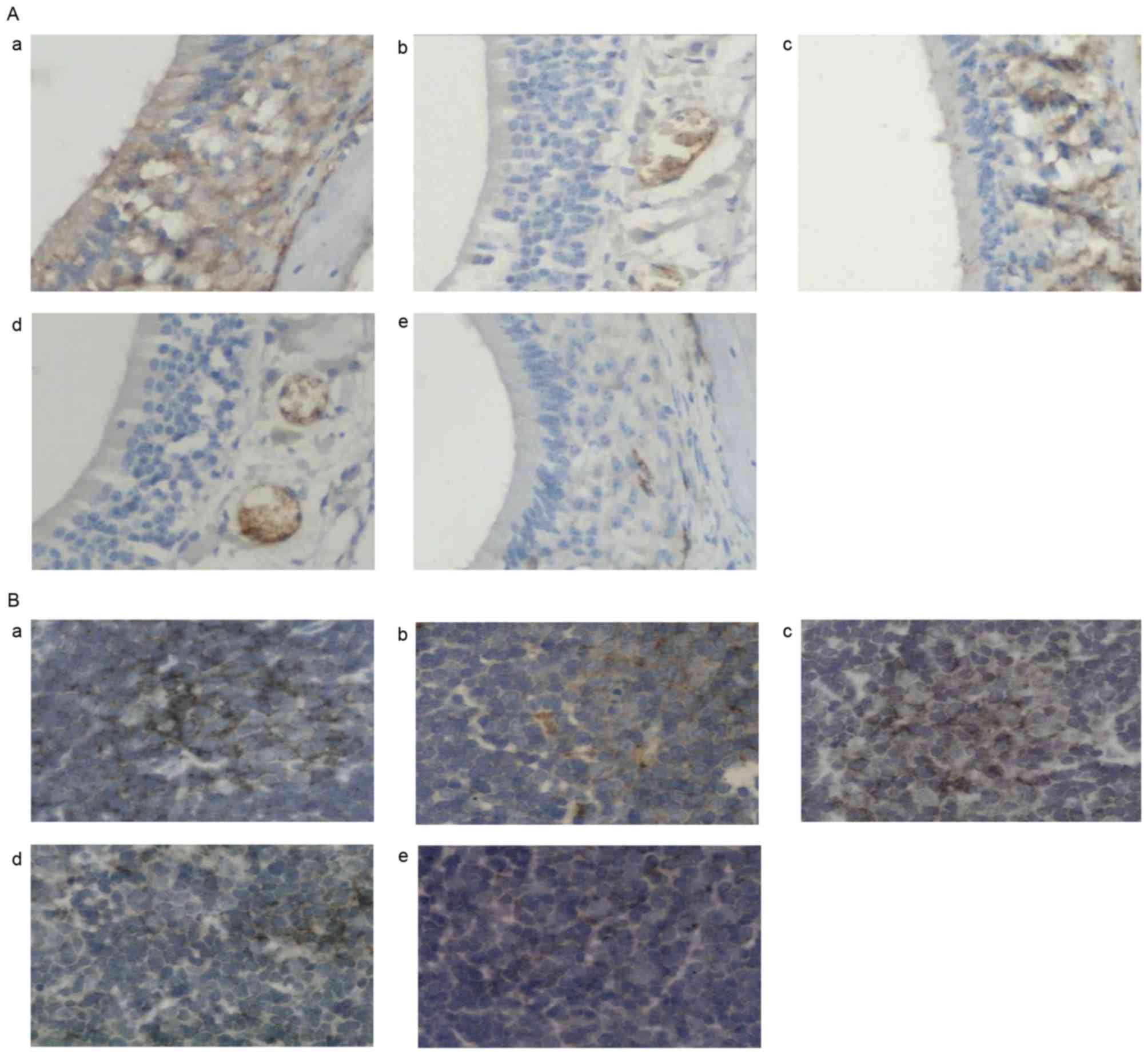 | Figure 4.Effects of AST administration on
T-bet protein expression. (A) Representative photomicrographs of
T-bet protein expression in nasal mucosa tissue samples in the (a)
normal, (b) 25 mg/kg AST, (c) 50 mg/kg AST, (d) DEX and (e) OVA
groups. (B) Representative photomicrographs of mouse spleen tissue
in the (a) normal, (b) 25 mg/kg AST, (c) 50 mg/kg AST, (d) DEX and
(e) OVA groups. Magnification, ×400. AST, Astragaloside IV; OVA,
ovalbumin; DEX, dexamethasone; T-bet, T-box protein expressed in T
cells. |
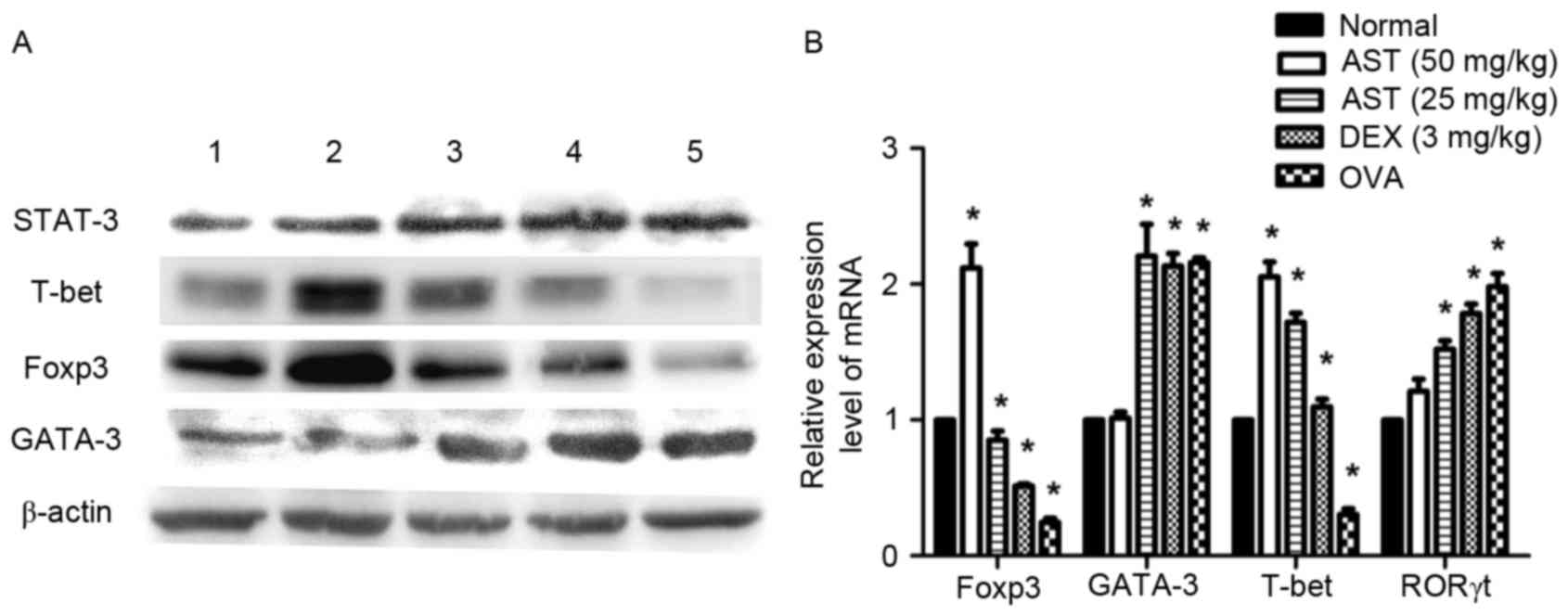 | Figure 5.Effects of AST administration on
factors implicated in T helper cell differentiation. (A)
Representative western blot images and (B) reverse
transcription-quantitative polymerase chain reaction analysis of
GATA-3, T-bet, Foxp3 and STAT-3 protein and mRNA expression levels,
respectively, in spleen tissue samples. β-actin served as an
internal control. Data are expressed as the mean ± standard
deviation. *P<0.05 vs. normal; #P<0.05 vs. OVA
model. AST, Astragaloside IV; T-bet, T-box protein expressed in T
cells; Foxp, forkhead box protein; STAT, signal transducer and
activator of transcription; OVA, ovalbumin; DEX, dexamethasone;
ROR, retinoic acid receptor-related orphan nuclear receptor. |
Effects of AST on Foxp3, RORγt and
STAT-3
The expression of the inflammation-associated
proteins Foxp3, RORγt and STAT-3 in mouse spleen tissue was altered
following AST and DEX administration. Mice in the OVA model group
demonstrated decreased Foxp3 protein (Fig. 5A) and mRNA (Fig. 5B) expression levels compared with
the normal group. Notably, AST and DEX administration upregulated
Foxp3 protein and mRNA expression levels compared with the OVA
model group (Fig. 5A and B,
respectively). Similar effects were observed via
immunohistochemical staining (Fig.
6). Conversely, protein expression levels of STAT-3 and mRNA
expression levels of RORγt appeared to be upregulated in allergic
mice; however, treatment with AST and DEX ameliorated this effect
(Fig. 5A and B, respectively).
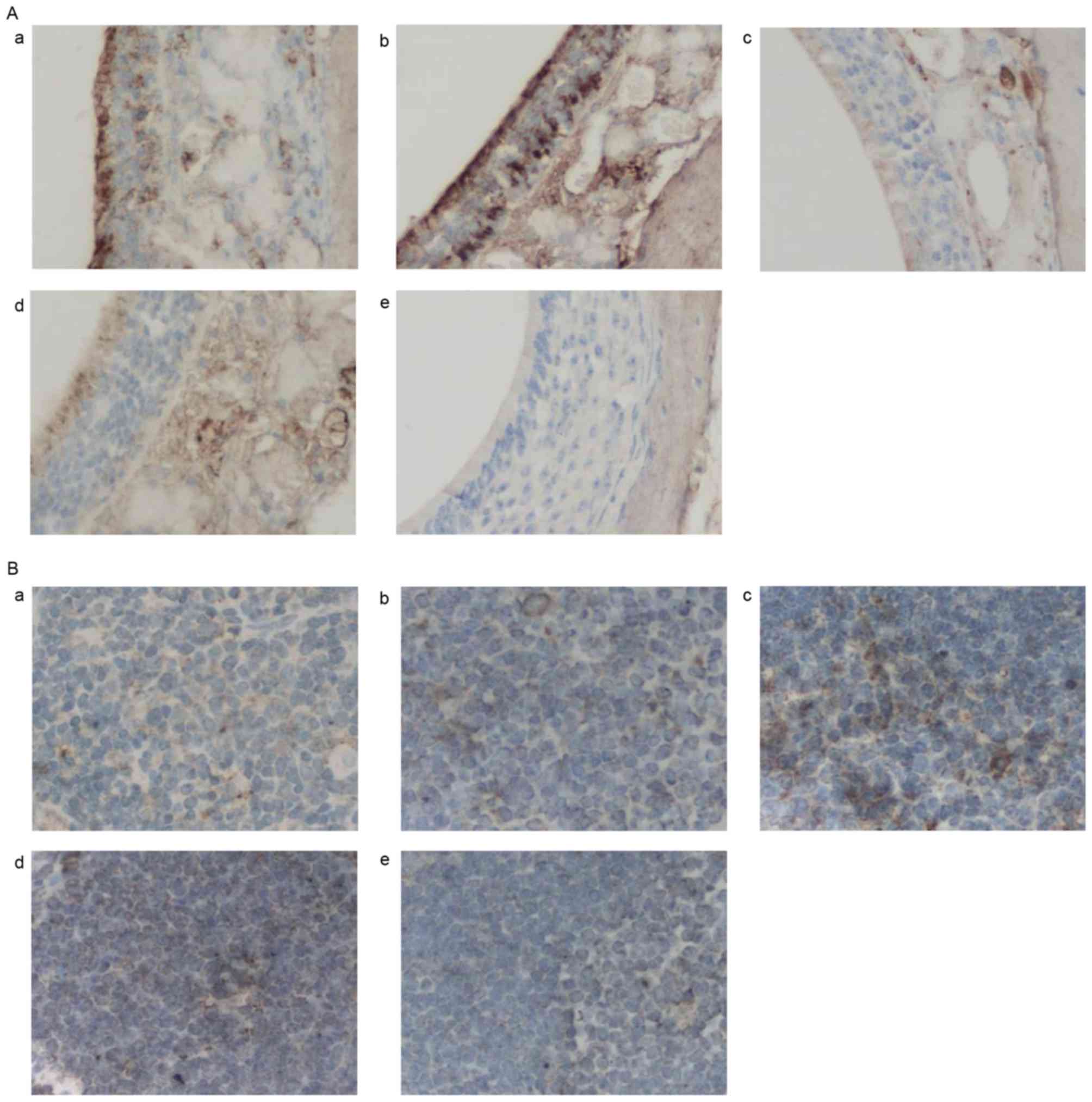 | Figure 6.Effects of AST administration on
Foxp3 protein expression. (A) Representative photomicrographs of
Foxp3 protein expression in nasal mucosa tissue samples in the (a)
normal, (b) 25 mg/kg AST, (c) 50 mg/kg AST, (d) DEX and (e) OVA
groups. (B) Representative photomicrographs of mouse spleen tissue
in the (a) normal, (b) 25 mg/kg AST, (c) 50 mg/kg AST, (d) DEX and
(e) OVA groups. Magnification, ×400. AST, Astragaloside IV; OVA,
ovalbumin; DEX, dexamethasone; T-bet, Foxp 3, forkhead box protein
3. |
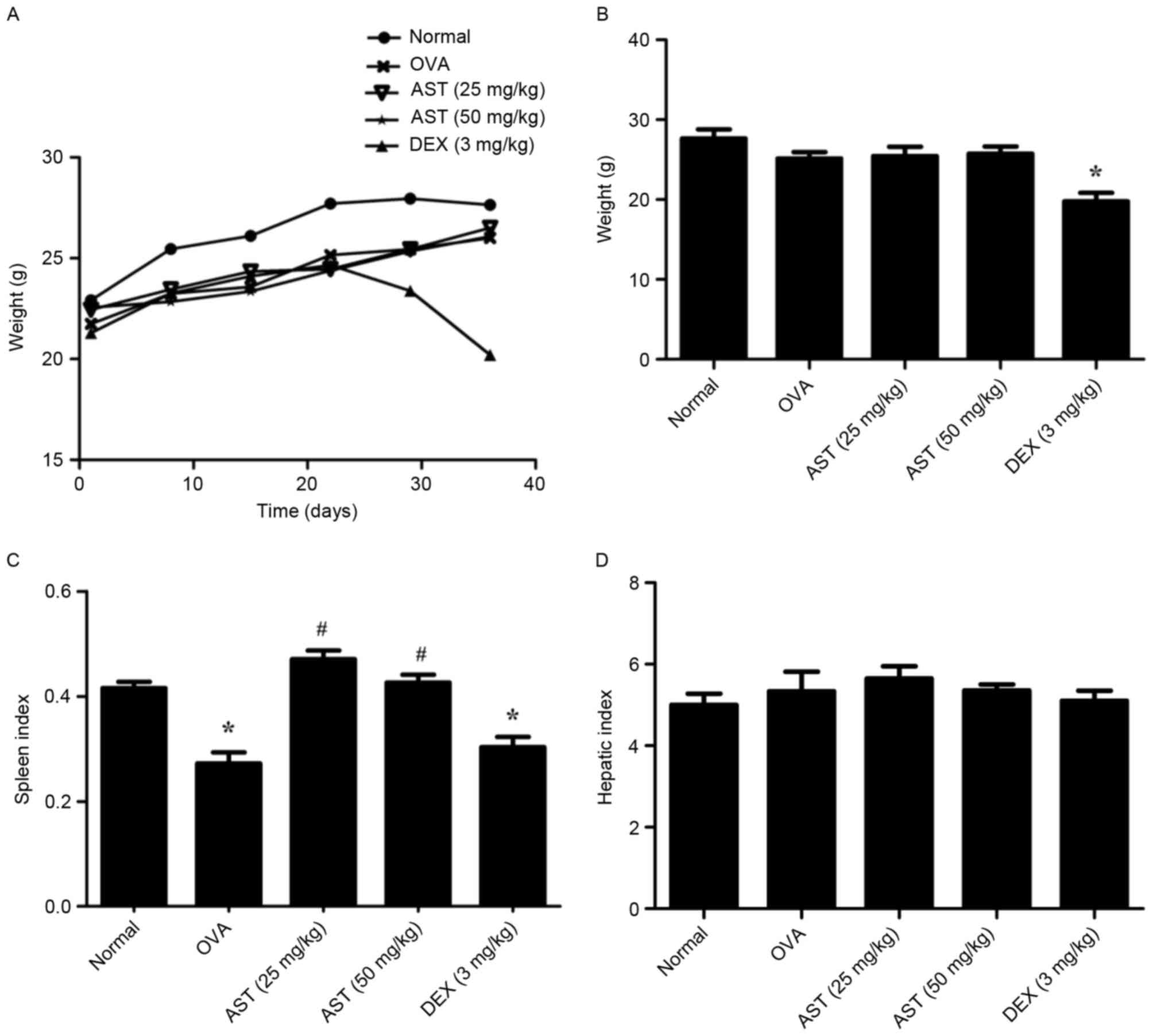
Adverse events associated with DEX
administration
The body weight of mice from all groups was measured
from days 1–42. The results demonstrated that mice in the DEX group
started to lose weight from day 29 (Fig. 7A). Following long-term therapy with
DEX (42 days), the weight and spleen index of mice in the DEX group
were significantly reduced (P<0.05 vs. the normal group;
Fig. 7B and C, respectively).
However, DEX administration exerted no effects on the liver index
(Fig. 7D).
Discussion
Allergic rhinitis is one of the most common
allergies in children and young individuals, and poses a serious
public health concern (22). A
2011 epidemiological investigation reported that in western China,
the prevalence of allergic rhinitis was 30.3% (Nanning), 37.9%
(Urumqi), 34.3% (Chengdu) and 32.30% (Chongqing) (23). Current treatment strategies aimed
at allergic disorders include leukotriene inhibitors, mast cell
stabilizers and β2 adrenergic agonists, which have been
associated with serious adverse events (24–26).
Therefore, the development of novel complementary and alternative
therapeutic strategies for the treatment of allergic disorders is
required. Treatments used in traditional Chinese medicine include
various natural products, including AST (11), and may have potential as
alternative therapeutic approaches for the management of allergic
rhinitis.
AST has previously been reported to suppress
interleukin (IL)-13 and tumor necrosis factor-α levels in the
supernatants of D10.G4.1 Th2 lymphocyte cultures in vitro,
and inhibited GATA-3 expression (27). The present study used an
OVA-induced mouse model of allergic rhinitis to assess the putative
anti-inflammatory effects of AST and investigate the molecular
mechanisms underlying its actions on allergic rhinitis. The present
results suggested that AST may alleviate allergic symptoms, as it
was demonstrated to inhibit eosinophilic infiltration, to suppress
the production of OVA-specific IgE, and to attenuate splenic
corpuscle proliferation. The recruitment of eosinophils in allergic
rhinitis has been suggested to be initiated by Th2-type cytokines
(28). The present results
demonstrated that eosinophil counts were decreased in AST-treated
allergic mice compared with untreated controls. The effects of AST
in preventing eosinophilic infiltration in the nasal mucosa may be
attributed to the inhibition of Th2-type cytokines. Class-switch
recombination has been reported to occur in restricted areas,
including the germinal center of lymph nodes, and B cells have been
demonstrated to undergo class switching to IgE production in nasal
mucosa following allergen exposure (29). However, it is possible that IgE
production in the spleen and lymphatic system may differ during
Th2-based immune responses induced by OVA immunization in mice.
OVA-specific IgE is predominantly produced in lymphoid tissue, but
not in the spleen (30). The
present results revealed that AST suppressed the proliferation of
splenic corpuscles and the production of OVA-specific IgE, thus
suggesting that AST may regulate the production of OVA-specific
IgEs in the spleen.
Potentiated Th2-mediated responses and impaired Th1
activation have been reported to mediate allergic inflammatory
responses (31,32). GATA-3 belongs to the GATA family of
transcription factors, and naive cluster of differentiation
(CD)4+ T cells express low levels of GATA-3 mRNA;
however, the expression of GATA-3 has been demonstrated to be
markedly upregulated in cells differentiating along the Th2
lineage, and downregulated in cells differentiating along the Th1
pathway (33,34). The present study revealed that in
mice with OVA-induced allergic rhinitis, GATA-3 expression was
markedly upregulated in nasal mucosal and spleen tissue compared
with normal mice. Notably, GATA-3 expression levels were markedly
decreased following treatment with AST. Conversely, T-bet levels
were upregulated following AST administration in OVA-induced
allergic mice. T-bet is a member of the T-box family of
transcription factors that has been revealed to serve a critical
role in the regulation of Th1-lineage commitment (35). The present findings suggested that
the expression of GATA-3 and T-bet may be altered during allergic
immune responses and may be implicated in the regulation of Th1/Th2
differentiation.
Treg and Th17 cells have been identified as two Th
cell subtypes independent from Th1 and Th2, that serve opposing
roles in vivo (36). Tregs
have been reported to inhibit inflammatory and allergic responses,
and exert crucial functions in autoimmunity and immunological
tolerance. Tregs expressing the transcription factor Foxp3 have
been demonstrated to possess anti-inflammatory properties and to be
involved in the maintenance of immunological tolerance under
physiological conditions (7). In
addition, previous studies have reported that CD25hi
Foxp3+ Tregs were able to effectively suppress
Th2-mediated responses to allergens in health, whereas this effect
was abolished in atopic allergic diseases (37,38).
The present study revealed that treatment with AST markedly
upregulated Foxp3 levels in nasal mucosal and spleen tissue
compared with allergic untreated mice.
RORγt is a splice variant of ROR, which has been
identified as an essential factor during Th17 cellular
differentiation. Following the retroviral vector-mediated
transduction of RORγt into naive T cells, Th17 cell development was
enhanced, therefore suggesting that RORγt may be essential for Th17
cellular proliferation (9). The
induction of RORγt has been reported to be dependent on the
activity of STAT-3. Chromatin immunoprecipitation analysis
demonstrated that STAT-3 was able to directly bind the IL-17A
promoter, thus suggesting that RORγt and STAT-3 may collaboratively
regulate the transcriptional profile of Th17 cells (10). The present study revealed that the
protein expression of STAT-3 and the mRNA expression of RORγt were
downregulated following AST administration in vivo.
DEX and AST were demonstrated to exert similar
effects on OVA-induced allergic rhinitis; however, the weight and
the spleen index of mice receiving long-term treatment with DEX was
significantly reduced, suggesting that AST treatment may have fewer
side effects compared with the traditional anti-allergic agent
DEX.
In conclusion, the results of the present study
suggested that treatment with AST may alleviate the symptoms of
OVA-induced allergic rhinitis, potentially by mediating the Th1/Th2
cell balance, via regulating the expression levels of T-bet,
GATA-3, Foxp3 and RORγt. Therefore, AST may represent an
alternative therapeutic approach for the treatment of patients with
allergic rhinitis.
Acknowledgements
The present study was supported by the Health Bureau
Cooperation Project of Chongqing (grant no. 20142042) and the
Science and Technology Project of Yuzhong District, Chongqing
(grant no. 20140123).
The authors of the present study would like to
acknowledge the College of Life Science (Chongqing Medical
University, Chongqing, China) for their technical support.
References
|
1
|
Adamia N, Jorjoliani L, Khachapuridze D,
Katamadze N and Chkuaseli N: Allergic diseases and asthma in
adolescents. Georgian Med News. 58–62. 2015.PubMed/NCBI
|
|
2
|
Gotoh M: Allergen immunotherapy for
allergic rhinitis. Arerugi. 64:693–699. 2015.(In Japanese).
PubMed/NCBI
|
|
3
|
Ma C, Ma Z, Liao XL, Liu J, Fu Q and Ma S:
Immunoregulatory effects of glycyrrhizic acid exerts anti-asthmatic
effects via modulation of Th1/Th2 cytokines and enhancement of
CD4(+)CD25(+)Foxp3+ regulatory T cells in ovalbumin-sensitized
mice. J Ethnopharmacol. 148:755–762. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ku CJ, Lim KC, Kalantry S, Maillard I,
Engel JD and Hosoya T: A monoallelic-to-biallelic T-cell
transcriptional switch regulates GATA3 abundance. Genes Dev.
29:1930–1941. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Han LN, Guo SL, Li TL, Ding GL, Zhang YJ
and Ma JL: Effect of immune modulation therapy on cardiac function
and T-bet/GATA-3 gene expression in aging male patients with
chronic cardiac insufficiency. Immunotherapy. 5:143–153. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sanchez AM, Zhu J, Huang X and Yang Y: The
development and function of memory regulatory T cells after acute
viral infections. J Immunol. 189:2805–2814. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lloyd CM and Hawrylowicz CM: Regulatory T
cells in asthma. Immunity. 31:438–449. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen Z, Lin F, Gao Y, Li Z, Zhang J, Xing
Y, Deng Z, Yao Z, Tsun A and Li B: FOXP3 and RORγt: Transcriptional
regulation of Treg and Th17. Int Immunopharmacol. 11:536–542. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Korn T, Bettelli E, Oukka M and Kuchroo
VK: IL-17 and Th17 cells. Annu Rev Immunol. 27:485–517. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang XO, Panopoulos AD, Nurieva R, Chang
SH, Wang D, Watowich SS and Dong C: STAT3 regulates
cytokine-mediated generation of inflammatory helper T cells. J Biol
Chem. 282:9358–9363. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Guo H and Liu MP: Mechanism of traditional
Chinese medicine in the treatment of allergic rhinitis. Chin Med J
(Engl). 126:756–760. 2013.PubMed/NCBI
|
|
12
|
Wang B and Chen MZ: Astragaloside IV
possesses antiarthritic effect by preventing interleukin 1β-induced
joint inflammation and cartilage damage. Arch Pharm Res.
37:793–802. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huang LF, Yao YM, Li JF, Zhang SW, Li WX,
Dong N, Yu Y and Sheng ZY: The effect of Astragaloside IV on immune
function of regulatory T cell mediated by high mobility group box 1
protein in vitro. Fitoterapia. 83:1514–1522. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Qiu YY, Zhu JX, Bian T, Gao F, Qian XF, Du
Q, Yuan MY, Sun H, Shi LZ and Yu MH: Protective effects of
astragaloside IV against ovalbumin-induced lung inflammation are
regulated/mediated by T-bet/GATA-3. Pharmacology. 94:51–59. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhao J, Yang P, Li F, Tao L, Ding H, Rui
Y, Cao Z and Zhang W: Therapeutic effects of astragaloside IV on
myocardial injuries: Multi-target identification and network
analysis. PLoS One. 7:e449382012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li M, Qu YZ, Zhao ZW, Wu SX, Liu YY, Wei
XY, Gao L and Gao GD: Astragaloside IV protects against focal
cerebral ischemia/reperfusion injury correlating to suppression of
neutrophils adhesion-related molecules. Neurochem Int. 60:458–465.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen SM, Tsai YS, Lee SW, Liu YH, Liao SK,
Chang WW and Tsai PJ: Astragalus membranaceus modulates Th1/2
immune balance and activates PPARγ in a murine asthma model.
Biochem Cell Biol. 92:397–405. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hiromura Y, Kishida T, Nakano H, Hama T,
Imanishi J, Hisa Y and Mazda O: IL-21 administration into the
nostril alleviates murine allergic rhinitis. J Immunol.
179:7157–7165. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dao-jun X: Studies on the effects of
Freud's adjuvant and aluminum hydroxide adjuvant on Tregs and
CD4+T cell relative genes in spleen of mice. PhD
thesisHunan Agricultural University 2010, (In Chinese).
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method: Methods. 25:402–408. 2001.
|
|
21
|
Chen SM, Tsai YS, Lee SW, Liu YH, Liao SK,
Chang WW and Tsai PJ: Astragalus membranaceus modulates Th1/2
immune balance and activates PPARγ in a murine asthma model.
Biochem Cell Biol. 92:397–405. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mandhane SN, Shah JH and Thennati R:
Allergic rhinitis: An update on disease, present treatments and
future prospects. Int Immunopharmacol. 11:1646–1662. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shen J, Ke X, Hong S, Zeng Q, Liang C, Li
T and Tang A: Epidemiological features of allergic rhinitis in four
major cities in western China. J Huazhong Univ Sci Technolog Med
Sci. 31:433–440. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Eid N, Morton R, Olds B, Clark P, Sheikh S
and Looney S: Decreased morning serum cortisol levels in children
with asthma treated with inhaled fluticasone propionate.
Pediatrics. 109:217–221. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Klimek L, Mullol J, Hellings P, Gevaert P,
Mösges R and Fokkens W: Recent pharmacological developments in the
treatment of perennial and persistent allergic rhinitis. Expert
Opin Pharmacother. 17:657–669. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Keith PK, Koch C, Djandji M, Bouchard J,
Psaradellis E, Sampalis JS, Schellenberg RR and McIvor RA:
Montelukast as add-on therapy with inhaled corticosteroids alone or
inhaled corticosteroids and long-acting beta-2-agonists in the
management of patients diagnosed with asthma and concurrent
allergic rhinitis (the RADAR trial). Can Respir J. 16 Suppl
A:17A–31A. 2009.(In English, French). View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jin HL, W LM, Luo QL, Li B, Du YJ, Lv YB
and Dong JC: Effects of astragaloside IV on Th2 lymphocyte D 10. G
4.1 in vitro. Chinese Pharmacological Bulletin. 30:1508–1513.
2014.(In Chinese).
|
|
28
|
Juneja L and Parmar HS: Ovalbumin induced
allergic rhinitis and development of prediabetes to rats: Possible
role of Th2 cytokines. Inflamm Allergy Drug Targets. 12:199–205.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Takhar P, Smurthwaite L, Coker HA, Fear
DJ, Banfield GK, Carr VA, Durham SR and Gould HJ: Allergen drives
class switching to IgE in the nasal mucosa in allergic rhinitis. J
Immunol. 174:5024–5032. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shang XZ, Armstrong J, Yang GY, Volk A, Li
J, Griswold DE, Emmell E and Li L: Regulation of antigen-specific
versus by-stander IgE production after antigen sensitization. Cell
Immunol. 229:106–116. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Melli LC, do Carmo-Rodrigues MS,
Araújo-Filho HB, Solé D and de Morais MB: Intestinal microbiota and
allergic diseases: A systematic review. Allergol Immunopathol
(Madr). 44:177–188. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nogueira JC and Goncalves Mda C:
Probiotics in allergic rhinitis. Braz J Otorhinolaryngol.
77:129–134. 2011.(In Portuguese). View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ouyang W, Ranganath SH, Weindel K,
Bhattacharya D, Murphy TL, Sha WC and Murphy KM: Inhibition of Th1
development mediated by GATA-3 through an IL-4-independent
mechanism. Immunity. 9:745–755. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang DH, Cohn L, Ray P, Bottomly K and
Ray A: Transcription factor GATA-3 is differentially expressed in
murine Th1 and Th2 cells and controls Th2-specific expression of
the interleukin-5 gene. J Biol Chem. 272:21597–21603. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Szabo SJ, Kim ST, Costa GL, Zhang X,
Fathman CG and Glimcher LH: Pillars article: A novel transcription
factor, T-bet, directs Th1 lineage commitment. Cell. 2000. 100:
655–669. J Immunol. 194:2961–2975. 2015.PubMed/NCBI
|
|
36
|
Chen Z, Lin F, Gao Y, Li Z, Zhang J, Xing
Y, Deng Z, Yao Z, Tsun A and Li B: FOXP3 and RORγt: Transcriptional
regulation of treg and Th17. Int Immunopharmacol. 11:536–542. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kündig TM and Bachmann MF:
Allergen-specific immunotherapy: Regulatory T cells or
allergen-specific IgG? Hum Vaccin. 6:673–675. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Nouri-Aria KT and Durham SR: Regulatory T
cells and allergic disease. Inflamm Allergy Drug Targets.
7:237–252. 2008. View Article : Google Scholar : PubMed/NCBI
|