Introduction
Osteosarcoma is the most common primary malignant
bone tumor, which accounts for ~60% of all bone sarcomas (1,2). The
introduction of chemotherapy has led to a marked improvement in the
prognosis of patients with localized osteosarcoma. Long-term
survival rates, previously <20%, have improved to 65–70%
following the advent of multiagent chemotherapy regimens (3). However, the survival rate for
patients with osteosarcoma remains low. A significant proportion of
osteosarcomas cases are not sensitive to conventional chemotherapy,
with long-term survival rates of ~20% (4).
Adriamycin (ADM) is one of the most widely used
chemotherapeutic drugs for the treatment of human osteosarcoma.
However, the acquisition of ADM resistance is common in patients
with osteosarcoma, leading to local and distant failure. In
addition, high doses of ADM lead to toxic side effects in patients
with osteosarcoma (5,6). Therefore, an alternative strategy is
required, which enables a decrease in the dose of chemotherapeutics
required, but enhances the sensitivity of cancer cells to
chemotherapeutics.
Natural products are important in cancer
chemotherapy due to their beneficial pharmacological activities and
low toxicity. Tumor therapy with traditional Chinese herbs,
including curcumin (7–9), triptolide (10) and Danshen (11) is becoming increasingly attractive
(12). Shikonin (SK), an effective
constituent purified from the Chinese medicinal herb,
Lithospermum erythrorhixon, has been shown to exert
antitumor effects by inhibiting pyruvate kinase-M2 (PKM2). PKM2,
which catalyzes the final rate-limiting step in glycolysis, is
vital for cancer cell proliferation and is universally
overexpressed in cancer cells (13). SK was previously shown to be a
cytotoxic DNA-binding agent (14).
Furthermore, SK and its analogs have been shown to induce minimal
cancer drug resistance (15).
To decrease the side effects and enhance the
efficacy of traditional Chinese medicines, different combinations
of medicinal herbs have been used as an alternative strategy for
cancer therapy. The present study aimed to analyze the in
vitro effects of the combination treatment of SK and ADM on
human osteosarcoma cell lines.
Materials and methods
Cell lines and cell culture
The U2OS and MG63 human osteosarcoma cell lines were
obtained from American Type Culture Collection (Manassas, VA, USA).
All cells were cultured in high glucose Dulbecco's modified Eagle's
medium (DMEM-h) supplemented with 10% fetal bovine serum (FBS), 100
U/ml penicillin and 100 µg/ml streptomycin (all from Thermo Fisher
Scientific, Inc., Waltham, MA, USA) in a humidified incubator at
37°C in 5% CO2.
Drugs and antibodies
Purified SK (>98%) was purchased from Shanghai
Tauto Biotech Co., Ltd. (Shanghai, China). ADM (5 mmol/l) was
purchased from Selleck Chemicals (Houston, TX, USA). Stock
solutions were produced in dimethyl sulfoxide (Sigma-Aldrich; Merck
Millipore, Darmstadt, Germany) at a concentration of 50 mM and
stored in the dark at −20°C. SK and ADM were used at final
concentrations of 0.01 and 0.1 µΜ, respectively, by diluting the
stock solution in DMEM-h. The antibodies used for western blot
analysis were as follows: Rabbit anti-actin (Santa Cruz
Biotechnology, Inc., Dallas, TX, USA; cat no. sc130657),
anti-B-cell lymphoma 2-associated X protein (Bax; cat no. ab7977),
anti-caspase-3 (cat no. 9664), anti-caspase-8 (cat no. ca0689), and
anti-poly (ADP-ribose) polymerase (PARP; cat no. 9542) (all from
Cell Signaling Technology, Inc., Danvers, MA, USA).
CCK-8 assay
The U2OS and MG63 osteosarcoma cells (0.5×104/well)
were seeded into 96-well plates and cultured overnight for
adherence of cells. The cells were then treated with SK (0.01 µΜ),
ADM (0.1 µΜ) or a combination of SK (0.01 µΜ) and ADM (0.1 µΜ) for
48 h at 37°C. Cells incubated with DMEM-h were used as a control
group. Following incubation for 48 h, the supernatant was removed
and 2 ml CCK-8 (Beyotime Institute of Biotechnology, Shanghai,
China) was added into each well and incubated for 1 h to solubilize
the blue-purple crystals of formazan. The absorbance was then
measured at 490 nm using an ELX800 microplate reader (BioTek
Instruments, Inc., Winooski, VT, USA). The survival rate was
calculated according to the following formula: Survival rate =
absorbance of treatment/absorbance of control ×100%.
Flow cytometric analysis
The U2OS and MG63 osteosarcoma cells (2×105/well)
were plated in 6-well plates and synchronized with DMEM-h
containing 10% FBS. Following incubation for 8 h, the SK-treated
cells (0.01 µΜ), ADM-treated cells (0.1 µΜ) and combination
treated-cells were treated with 0.01 µΜ SK and 0.1 µΜ ADM for 48 h
at 37°C. The cells were then collected and washed twice in cold
PBS. The cells were mixed in 100 µl of 1X binding buffer and
incubated at room temperature for 15 min with Annexin-V/7AAD
(eBioscience, Inc., San Diego, CA, USA) double staining solution.
The stained cells were analyzed using flow cytometry. The
percentages of necrotic cells and the proportions of cells in
different cell cycle stages were calculated using BD Accuri C6
software (C Flow Sampler Analysis version 1.0.208.2 Installer; BD
Biosciences, San Jose, CA, USA).
Western blot analysis
The U2OS and MG63 cells were treated with the
different solutions (DMEM-h, SK, ADM, or a combination of SK and
ADM) for 48 h. The cells were then washed twice with PBS solution
and lysed in RIPA lysis buffer (Beyotime Institute of
Biotechnology) containing protease inhibitors (Thermo Fisher
Scientific, Inc.). The tumor cells were retrieved from −80°C
storage and immersed rapidly in liquid nitrogen. The resulting
powder was lysed in RIPA lysis buffer containing protease
inhibitors. Protein concentrations were determined using a Pierce
BCA protein assay kit (Thermo Fisher Scientific, Inc.). Equivalent
quantities of total protein (50 µg) were boiled and
electrophoretically separated on a 10% polyacrylamide gel at 80
volts. The proteins were then transferred onto a nitrocellulose
filter membrane. The membranes were blocked for 60 min with a 5%
milk solution prepared in PBS, and then incubated overnight at 4°C
with a 1:1,000 dilution of the primary antibodies (Bax, PARP,
caspase-3, caspase-8 and actin). The membranes were then washed
three times (10 min each) with Tween 20 (1:1,000 dilution)-PBS and
incubated for 1 h with the appropriate peroxidase-conjugated
secondary antibody (1:1,000 dilution; Odyssey Mouse IgG F(c)
Antibody IRDye® 800CW Conjugated; LI-COR Biotechnology, Lincoln,
NE, USA; cat no. 610-131-003) at 37°C. The membranes were washed
three times with Tween 20-PBS (10 min each) and developed using the
Odyssey two-color infrared laser imaging system (LI-COR
Biosciences, Lincoln, NE, USA). The signal generated by actin was
used as an internal control.
Statistical analysis
Statistical analysis was performed using GraphPad
Prism 5 (GraphPad Software, Inc., La Jolla, CA, USA). Data are
expressed as the mean ± standard deviation and comparisons between
two groups were performed using Student's t test. P<0.05 was
considered to indicate a statistically significant difference.
Results
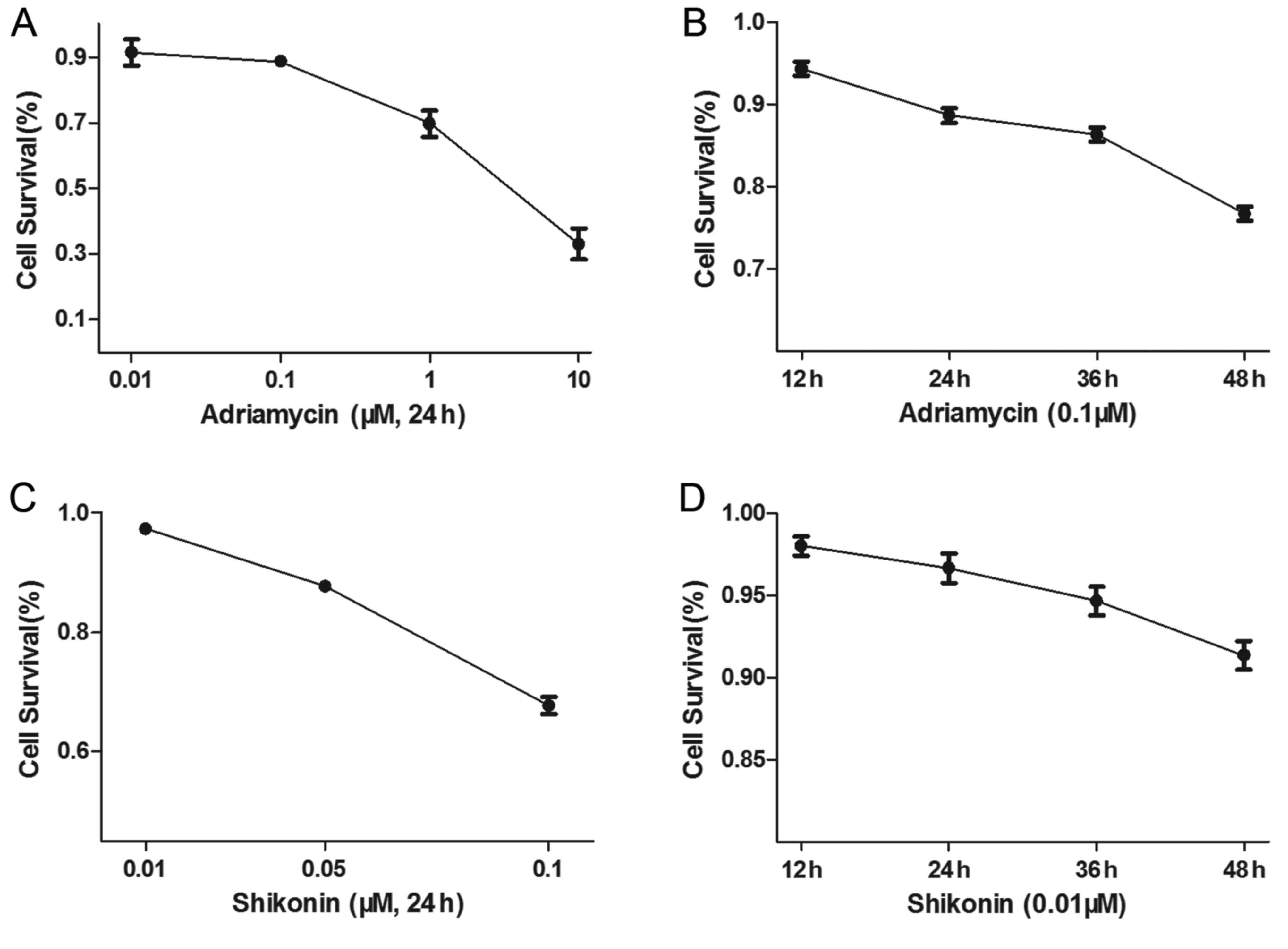
Treatment with SK and with ADM
decreases cell survival of osteosarcoma cells
The U2OS osteosarcoma cells were treated with
different concentrations of ADM, or with 0.1 µΜ ADM for different
durations. The U2OS cells were also treated with different
concentrations of SK for 48 h, or with 0.01 µΜ SK for different
durations. The results showed that ADM (Fig. 1A and B) and SK (Fig. 1C and D) increased apoptosis of the
osteosarcoma cells in a dose- and time-dependent manner.
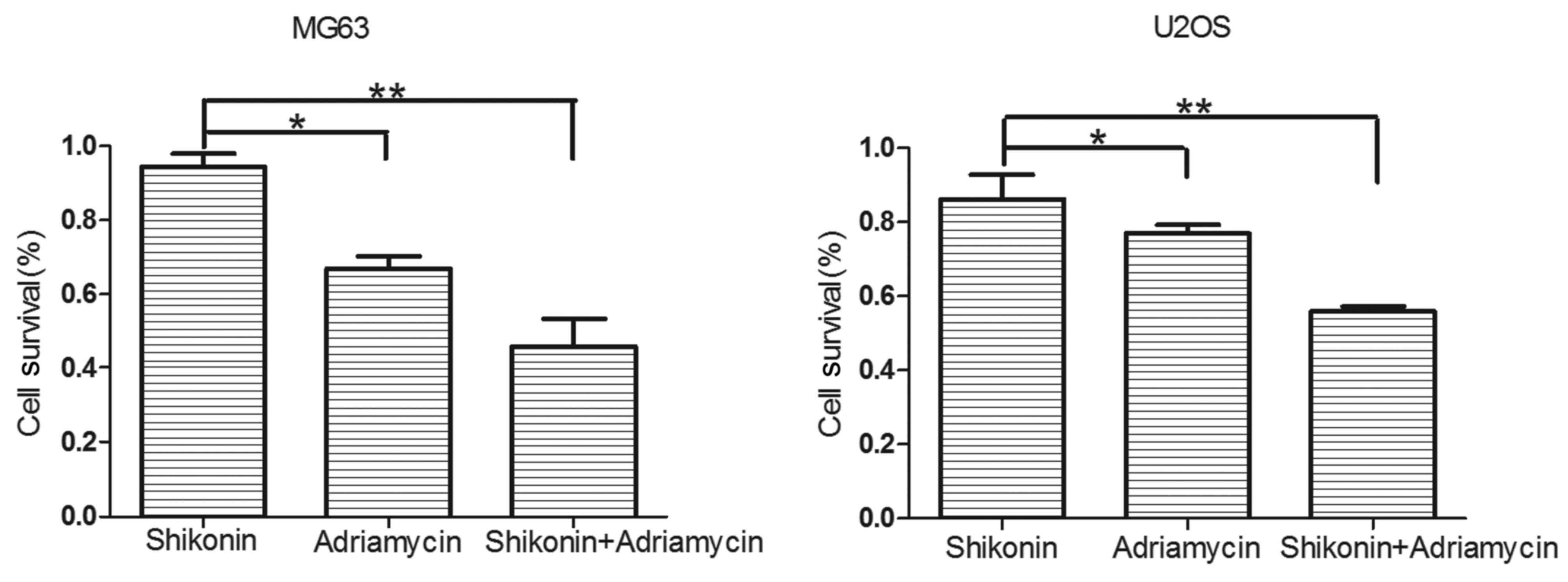
SK and ADM act synergistically to
decrease osteosarcoma cell survival
A CCK-8 assay was used to measure the cell survival
rates of the U2OS and MG63 osteosarcoma cells, which were treated
with DMEM-h, SK (0.01 µΜ), ADM (0.1 µΜ), or SK (0.01 µΜ) and ADM
(0.1 µΜ) for 48 h (Fig. 2). The
results revealed that SK and ADM acted synergistically to decrease
the survival rates of the osteosarcoma cells.
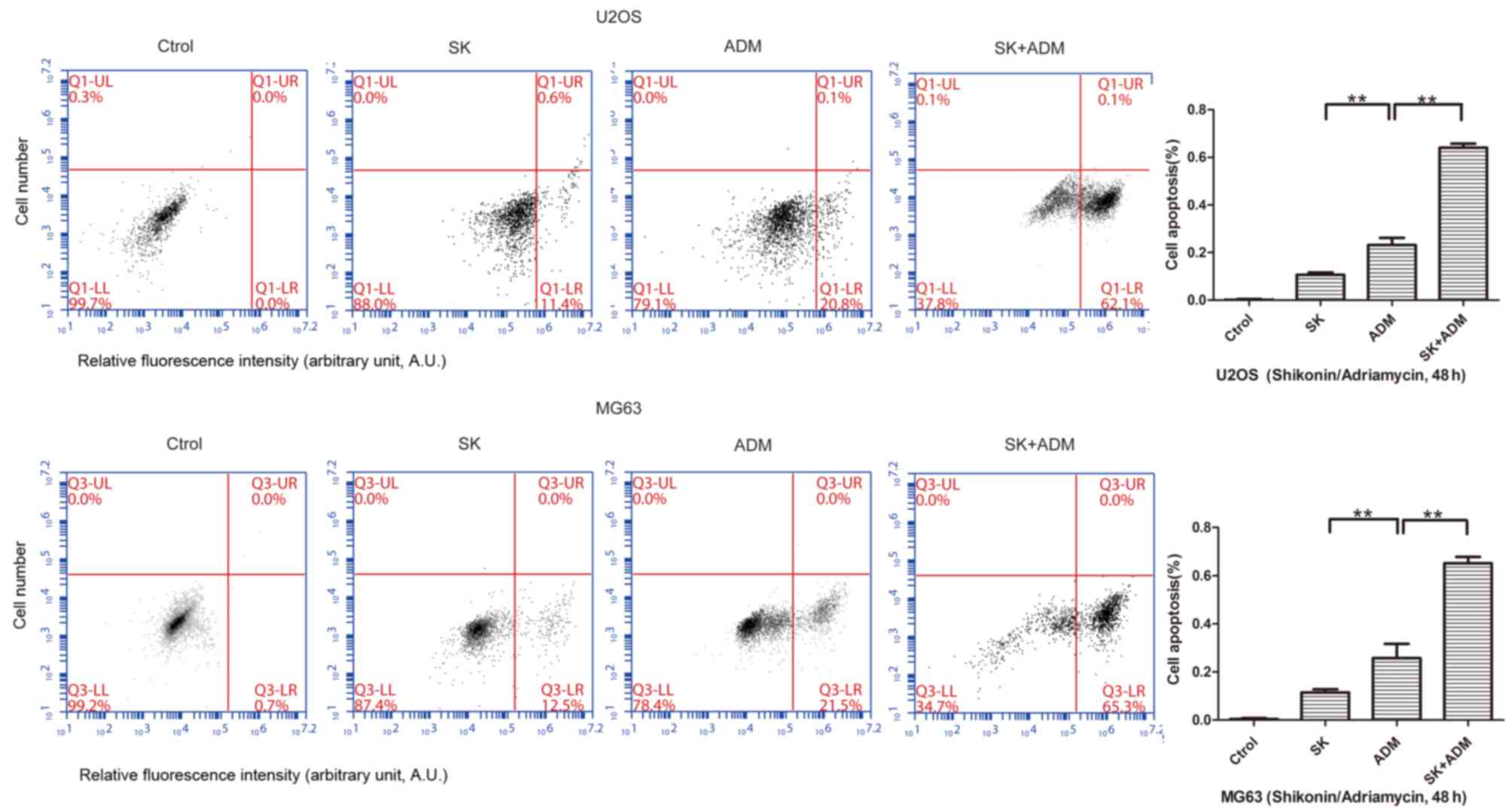
SK enhances the apoptotic effect of
ADM in osteosarcoma
The present study further examined differences in
cell apoptosis following treatment of osteosarcoma cells (U2OS and
MG63) with SK and/or ADM. The results of the flow cytometric
analysis revealed significant increases in cell apoptosis following
treatment with SK and ADM for 48 h (Fig. 3). These data suggested that SK
induced the apoptotic effects of ADM on the osteosarcoma cells.
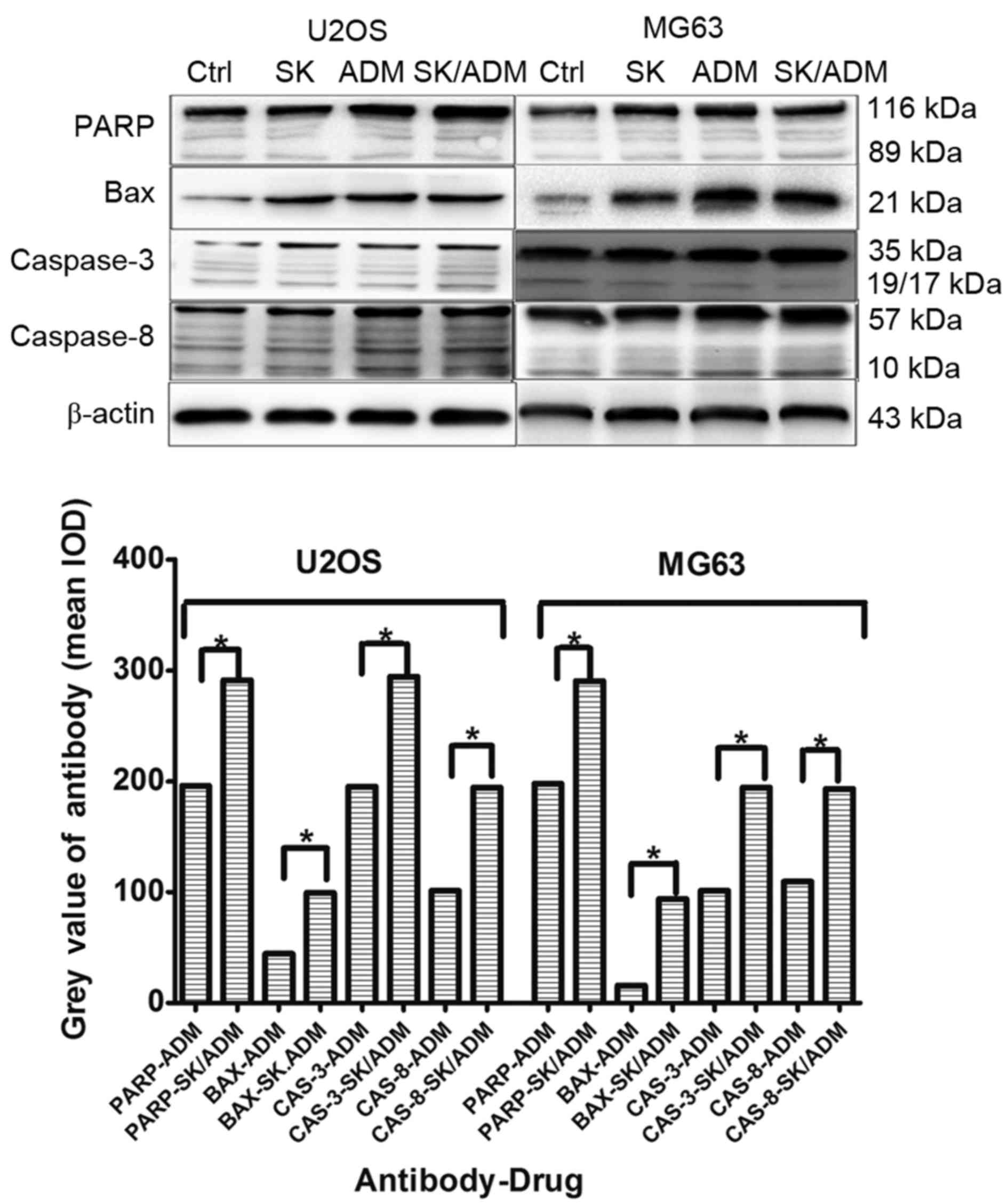
SK and ADM promote expression levels
of caspase-3 and −8 in osteosarcoma
Caspase-3 and caspase-8 are considered crucial
modulators of apoptosis. As shown in Fig. 4, the protein levels of caspase-3
and caspase-8 increased significantly in the U2OS and MG63 cells
following treatment with SK and ADM for 48 h at the lowest drug
concentrations (P<0.05). The data indicated that SK promoted
ADM-induced cell death of the osteosarcoma cells by activating
caspase-3- and caspase-8-dependent apoptosis.
Discussion
Osteosarcoma is the most common type of primary
malignant tumor of bone and is treated predominantly by surgical
resection. However, chemotherapy is important in preoperative and
postoperative treatment. Clinical studies have shown that the most
commonly used osteosarcoma chemotherapeutic drug, ADM, exhibits an
effective rate of only ~40% when used as a single agent (16). The limited efficacy of cytotoxic
chemotherapy remains a key obstacle in the treatment of advanced
osteosarcoma.
The combined effect of two or more drugs can be
superior to that of a single drug in chemotherapy. Combination
therapy can increase the rate of tumor cell death and decrease the
growth of tumor cells during treatment. As a result, toxic side
effects of chemotherapeutic drugs are commonly found in patients
with osteosarcoma. In addition, long-term exposure of the tumor to
certain drugs can lead to drug resistance. However, the addition of
a second drug markedly reduces the incidence of drug resistance,
particularly when the mechanism of drug-induced cell death varies
significantly between the two drugs. Drug resistance in cancer is
tightly associated with the apoptotic pathway, including the
overexpression of anti-apoptotic proteins, mutations in
pro-apoptotic proteins and reduction of caspases (17–19).
For these reasons, ~40% patients with osteosarcoma do not respond
to commonly used anticancer drugs.
ADM is the most commonly used chemotherapeutic drug
for the clinical treatment of bone tumors. It exerts antitumor
effects by targeting the activity of topoisomerase II (20,21).
In addition, ADM is a not a cell cycle-specific drug, being
involved in various stages of the cell cycle and inducing cellular
apoptosis (22,23). The use of PEGylated liposomal
doxorubicin has become a novel treatment strategy for patients with
cancer (24). As a traditional
chemotherapeutic drug, ADM has inevitable side effects and drug
resistance. The treatment of cancer patients with PEGylated
liposomal ADM (24) increases drug
sensitivity to a certain extent and improves side effects. However,
the potential limitations, including the complicated formulation
process, potential toxic effects to humans and high medical costs
of ADM, have limited the use of liposomal ADM.
As a natural product, SK is important in cancer
chemotherapy owing to its beneficial pharmacological activities and
low toxicity. SK has also been identified as a cytotoxic
DNA-binding agent (14).
Furthermore, SK and its analogs have been shown to induce minimal
cancer drug resistance (15).
Compared with ADM and its different forms (24), SK has inherent advantages,
including being a pure and natural product, showing low toxicity,
causing few side effects, exhibiting low drug resistance, being low
cost. It also shows high cooperative activity with other anticancer
drugs (14,15).
ADM, a classic bone tumor chemotherapeutic drug, and
SK, a novel Chinese herb, cooperate in human tumor cell lines and
interact to produce apoptotic effects.
In the present study, U2OS and MG63 human tumor cell
lines were examined to examine the efficacy of SK in combination
with ADM on osteosarcoma. It was found that SK promoted ADM-induced
apoptosis at the lowest drug concentrations in osteosarcoma. In
addition, SK enhanced the sensitivity of ADM at a low toxicity.
Decreases in the required dosage of ADM and expected clinical side
effects were observed.
Previous studies (14,15)
have shown that SK induces antitumor effects via two major
mechanisms: Inducing osteosarcoma necrosis and promoting apoptosis
of osteosarcoma cells. Few studies have reported on the use of this
drug as a synergistic antitumor agent. Thus, investigating the
antitumor mechanism of SK is of important clinical
significance.
Caspase-3 and caspase-8 are considered to be crucial
modulators of apoptosis (25). In
the present study, it was found that the protein levels of
caspase-3 and caspase-8 were significantly increased following
treatment with SK and ADM at low concentrations in the U2OS and
MG63 osteosarcoma cell lines. The levels of Bax and PARP were also
significantly increased (26),
further validating the apoptotic pathway. These results indicated
that SK induced cell death in U2OS and MG63 osteosarcoma cell lines
via the caspase-3- and caspase-8-dependent apoptotic pathway.
In the present study, a potential therapeutic
pathway was identified, targeted by SK and ADM to induce apoptosis
in osteosarcoma cells. The cooperation of SK with ADM resulted in
enhanced apoptosis in osteosarcoma cells via the induction of
apoptotic cell death, accompanied by upregulation in the expression
levels of caspase-3, caspase-8, Bax and PARP. SK, as a
sensitization agent and chemotherapeutic drug, is of clinical value
and may serve as a standard for the development of other Chinese
herbal medicines. Limitations of the present study include the lack
of in vivo experiments; therefore, additional investigations
using animal models are required in the future.
In conclusion, data obtained in the present study
showed that the suppression of growth in osteosarcoma by SK in
combination with ADM resulted from the induction of apoptosis in
vitro. These findings may lead to further investigations on
Chinese herbal medicines with unexpected antitumor effects.
References
|
1
|
Cormier JN and Pollock RE: Soft tissue
sarcomas. CA Cancer J Clin. 54:94–109. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liao YX, Zhou CH, Zeng H, Zuo DQ, Wang ZY,
Yin F, Hua YQ and Cai ZD: The role of the CXCL12-CXCR4/CXCR7 axis
in the progression and metastasis of bone sarcomas (Review). Int J
Mol Med. 32:1239–1246. 2013.PubMed/NCBI
|
|
3
|
Isakoff MS, Bielack SS, Meltzer P and
Gorlick R: Osteosarcoma: Current treatment and a collaborative
pathway to success. J Clin Oncol. 33:3029–3035. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jiang Y, Ludwig J and Janku F: Targeted
therapies for advanced Ewing sarcoma family of tumors. Cancer Treat
Rev. 41:391–400. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
McTiernan A, Jinks RC, Sydes MR, Uscinska
B, Hook JM, van Glabbeke M, Bramwell V, Lewis IJ, Taminiau AH,
Nooij MA, et al: Presence of chemotherapy-induced toxicity predicts
improved survival in patients with localised extremity osteosarcoma
treated with doxorubicin and cisplatin: A report from the European
Osteosarcoma Intergroup. Eur J Cancer. 48:703–712. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ferrari S, Ruggieri P, Cefalo G, Tamburini
A, Capanna R, Fagioli F, Comandone A, Bertulli R, Bisogno G,
Palmerini E, et al: Neoadjuvant chemotherapy with methotrexate,
cisplatin, and doxorubicin with or without ifosfamide in
nonmetastatic osteosarcoma of the extremity: An Italian sarcoma
group trial ISG/OS-1. J Clin Oncol. 30:2112–2118. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Khaw AK and Hande MP, Kalthur G and Hande
MP: Curcumin inhibits telomerase and induces telomere shortening
and apoptosis in brain tumour cells. J Cell Biochem. 114:1257–1270.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
James MI, Iwuji C, Irving G, Karmokar A,
Higgins JA, Griffin-Teal N, Thomas A, Greaves P, Cai H, Patel SR,
et al: Curcumin inhibits cancer stem cell phenotypes in ex vivo
models of colorectal liver metastases, and is clinically safe and
tolerable in combination with FOLFOX chemotherapy. Cancer Lett.
364:135–141. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Blakemore LM, Boes C, Cordell R and Manson
MM: Curcumin-induced mitotic arrest is characterized by spindle
abnormalities, defects in chromosomal congression and DNA damage.
Carcinogenesis. 34:351–360. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Patil S, Lis LG, Schumacher RJ, Norris BJ,
Morgan ML, Cuellar RA, Blazar BR, Suryanarayanan R, Gurvich VJ and
Georg GI: Phosphonooxymethyl prodrug of triptolide: Synthesis,
physicochemical characterization, and efficacy in human colon
adenocarcinoma and ovarian cancer xenografts. J Med Chem.
58:9334–9344. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen X, Guo J, Bao J, Lu J and Wang Y: The
anticancer properties of Salvia miltiorrhiza Bunge (Danshen): A
systematic review. Med Res Rev. 34:768–794. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhuang SR, Chiu HF, Chen SL, Tsai JH, Lee
MY, Lee HS, Shen YC, Yan YY, Shane GT and Wang CK: Effects of a
Chinese medical herbs complex on cellular immunity and
toxicity-related conditions of breast cancer patients. Br J Nutr.
107:712–718. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen J, Xie J, Jiang Z, Wang B, Wang Y and
Hu X: Shikonin and its analogs inhibit cancer cell glycolysis by
targeting tumor pyruvate kinase-M2. Oncogene. 30:4297–4306. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Andújar I, Recio MC, Giner RM and Ríos JL:
Traditional Chinese medicine remedy to jury: The pharmacological
basis for the use of shikonin as an anticancer therapy. Curr Med
Chem. 20:2892–2898. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Andújar I, Ríos JL, Giner RM and Recio MC:
Pharmacological properties of shikonin-a review of literature since
2002. Planta Med. 79:1685–1697. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Anninga JK, Gelderblom H, Fiocco M, Kroep
JR, Taminiau AH, Hogendoorn PC and Egeler RM: Chemotherapeutic
adjuvant treatment for osteosarcoma: Where do we stand? Eur J
Cancer. 47:2431–2445. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li S, Sun W, Wang H, Zuo D, Hua Y and Cai
Z: Research progress on the multidrug resistance mechanisms of
osteosarcoma chemotherapy and reversal. Tumour Biol. 36:1329–1338.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
de C, arné Trécesson S, Guillemin Y,
Bélanger A, Bernard AC, Preisser L, Ravon E, Gamelin E, Juin P,
Barré B and Coqueret O: Escape from p21-mediated oncogene-induced
senescence leads to cell dedifferentiation and dependence on
anti-apoptotic Bcl-xl and MCL1 proteins. J Biol Chem.
286:12825–12838. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
PosthumaDeBoer J, Witlox MA, Kaspers GJ
and van Royen BJ: Molecular alterations as target for therapy in
metastatic osteosarcoma: A review of literature. Clin Exp
Metastasis. 28:493–503. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Momparler RL, Karon M, Siegel SE and Avila
F: Effect of adriamycin on DNA, RNA, and protein synthesis in
cell-free systems and intact cells. Cancer Res. 36:2891–2895.
1976.PubMed/NCBI
|
|
21
|
Tewey KM, Rowe TC, Yang L, Halligan BD and
Liu LF: Adriamycin-induced DNA damage mediated by mammalian DNA
topoisomerase II. Science. 226:466–468. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bilim VN, Tomita Y, Kawasaki T, Takeda M
and Takahashi K: Adriamycin (ADM) induced apoptosis in transitional
cell cancer (TCC) cell lines accompanied by p21 WAF1/CIP1
induction. Apoptosis. 2:207–213. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bilim V, Kawasaki T, Takahashi K and
Tomita Y: Adriamycin induced G2/M cell cycle arrest in transitional
cell cancer cells with wt p53 and p21(WAF1/CIP1) genes. J Exp Clin
Cancer Res. 19:483–488. 2000.PubMed/NCBI
|
|
24
|
Voorhees PM, Orlowski RZ, Mulkey F, Watson
P, Geyer S, Sanford BL, Bennett E, Chanan-Khan AA, Bloomfield CD
and Larson RA: Long-term outcomes for newly-diagnosed multiple
myeloma patients treated with pegylated liposomal doxorubicin and
bortezomib: Final results of CALGB (Alliance) 10301, a multicentre
phase II study. Br J Haematol. 171:373–377. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Taylor RC, Cullen SP and Martin SJ:
Apoptosis: Controlled demolition at the cellular level. Nat Rev Mol
Cell Biol. 9:231–241. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Oliver FJ, de la Rubia G, Rolli V,
Ruiz-Ruiz MC, de Murcia G and Murcia JM: Importance of poly
(ADP-ribose) polymerase and its cleavage in apoptosis. Lesson from
an uncleavable mutant. J Biol Chem. 273:33533–33539. 1998.
View Article : Google Scholar : PubMed/NCBI
|