Introduction
Essential hypertension (EH) is a major health burden
worldwide, and leads to poor mortality and morbidity from the
complications such as myocardial infarction, cerebrovascular
diseases, heart failure and renal dysfunctions (1). It is a multi-factorial condition
involving interactions among environmental, demographic and genetic
disorders (2), among which,
heritability accounts for 30–40% of blood pressure changes
(3). A previous study of the
authors identified 31 upregulated and 18 downregulated genes
through microarray analysis in the peripheral blood samples of EH
compared to normotensives, among which CD36 was upregulated
by 4.8-fold (4), and the
association between the +273A/G single nucleotide polymorphism
(SNP) of CD36 and EH has been identified (1). The complement component 4 binding
protein (C4BP)A gene is indicated to be significantly
upregulated by 2.4-fold in EH.
C4BPA, located in the 1q32 chromosome, with
12 exons and 11 introns, encodes the alpha chain of C4BP, a major
soluble inhibitor of both the lectin and the classical pathways of
complement (5). C4BP exists in
three different isoforms, α7β1,
α7β0, and α6β1
(6), with
α7β1 as the major form and
α6β1 as the minor, inhibiting complement
activation by binding to the activated complement component C4b
through the α chain, and works in the classical and lectin pathway
(7). Upon inflammation, the
α7β0 isoform is upregulated, so the level of
the α-chains of C4BP by and large reflects the total C4BP. The α
chain also contains the binding sites for C3b, serum amyloid
protein (8), heparin (9), low-density lipoprotein
receptor-related protein (LRP) (10) and the surface proteins of some
bacteria (11,12), which are key molecules involved in
inflammation, lipid metabolism and coagulation pathways (13,14).
It has been reported that the rs11120211-A allele in the 5′
upstream sequence is associated with increased C4BPA mRNA
levels corresponds to the rs3813948-C allele associated with
increased plasma levels of C4BPα and %α7β0.
In addition, the variants are associated to the venous thrombosis
susceptibility independent of the protein S regulation (15).
5′ upstream regions of certain genes contain
transcription factor binding sites, which are known as DNA response
elements (REs), and regulate the initiation of gene transcription
(16). Variations in the 5′
upstream regions may alter the RE sequence, regulate the gene
transcription and consequently alter the functions of the gene
(17). Therefore, the aim of the
present study was to identify candidate SNPs in the 5′ upstream
region of C4BPA and analyze the possible associations with
EH through a case-control study in a northeastern Han Chinese
population.
Materials and methods
Ethics statement
The present study complies with the 1975 Declaration
of Helsinki, and was approved by the local ethics committee of
China-Japan Union Hospital of Jilin University (Changchun, China).
Written informed consent was obtained from each participant.
Subjects
A total of 822 participants for genotyping in the
study aged from 25 to 65 were recruited from the Medical Physical
Examination Center in China-Japan Union hospital, Jilin University
(Changchun, China) during March 2014 to May 2014. Subjects with
secondary hypertension, primary renal disease, dyslipidemia,
diabetes mellitus, cancer, hepatic disorders and endocrine diseases
were excluded. Height, weight, systolic blood pressure (SBP),
diastolic blood pressure (DBP), total cholesterol (TC), high
density lipoprotein cholesterol (HDL-c), low density lipoprotein
cholesterol (LDL-c), triglyceride (TG), fasting blood glucose
(FBG), serum creatinine and blood urea nitrogen (BUN) were
measured.
A total of 371 EH subjects and 451 control subjects
were recruited. Blood pressure (BP) was measured in a seated
position using a mercury column sphygmomanometer twice with a 5 min
interval according to the common protocol recommended by European
Society of Hypertension (2). EH
was defined as follows: SBP ≥140 mmHg and/or the average DBP ≥90
mmHg and/or current antihypertensive medication treatment. The
controls with by SBP<140 mmHg, DBP<90 mmHg and had never been
treated for hypertension. Questionnaires were administered to
investigate the family history, smoking and drinking habits.
Participants who smoked 3100 cigarettes or drank ≥12
times a year were defined as smokers or drinkers (18,19).
Body mass index (BMI)=weight/height2 (kg/m2).
According to the obesity guidelines of the World Health
Organization on Asian people (20), obesity was defined as a BMI ≥25
kg/m2.
C4BPA gene expression study
A total of 30 EH and 30 controls were randomly
selected to test the C4BPA expression in the peripheral
blood. C4BPA gene expression was evaluated by reverse
transcription-quantitative polymerase chain reaction (RT-qPCR), and
C4BPα expression level in the blood plasma was evaluated with a
human C4BPα ELISA test kit purchased from IBL International GmbH,
Hamburg, Germany (catalog no. IBATGP1664) according to the
manufacturer's protocol. To evaluate the mRNA expression level of
C4BPA, total RNA was extracted from the peripheral blood
using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) and purified using the RNeasy Mini cleanup kit
(Qiagen, Inc., Valencia, CA, USA) according to manufacturer's
protocol. cDNA was synthesized using the Superscript First Strand
Synthesis kit (Invitrogen; Thermo Fisher Scientific, Inc.)
following the manufacturer's protocol. C4BPA cDNA was
subjected to qPCR and amplifications were performed with BioEasy
SYBR®-Green I (Hangzhou Bori Technology Co., Ltd., Zhejiang, China)
and with the β-actin gene as a control. The primer sequences were
as follows: Forward, 5′-CTACGCATACGGCTTTTCTGT-3′ and reverse,
5′-CCCATGTGAAACATCTGGCTTG-3′ for C4BPA; forward,
5′-CCACGAAACTACCTTCAACTCC-3′ and reverse,
5′-TCATACTCCTGCTGCTTGCTGATCC-3′ for the gene encoding β-actin. qPCR
was conducted in a 25 µl reaction volume under the following
cycling conditions: An initial predenaturation step at 95°C for 5
min, followed by 40 cycles of denaturation at 95°C for 30 sec,
annealing at 60°C for 30 sec, extension at 72°C for 30 sec and a
final extension step at 72°C for 7 min. For data analysis, the
2−ΔΔCq quantification method was utilized (21).
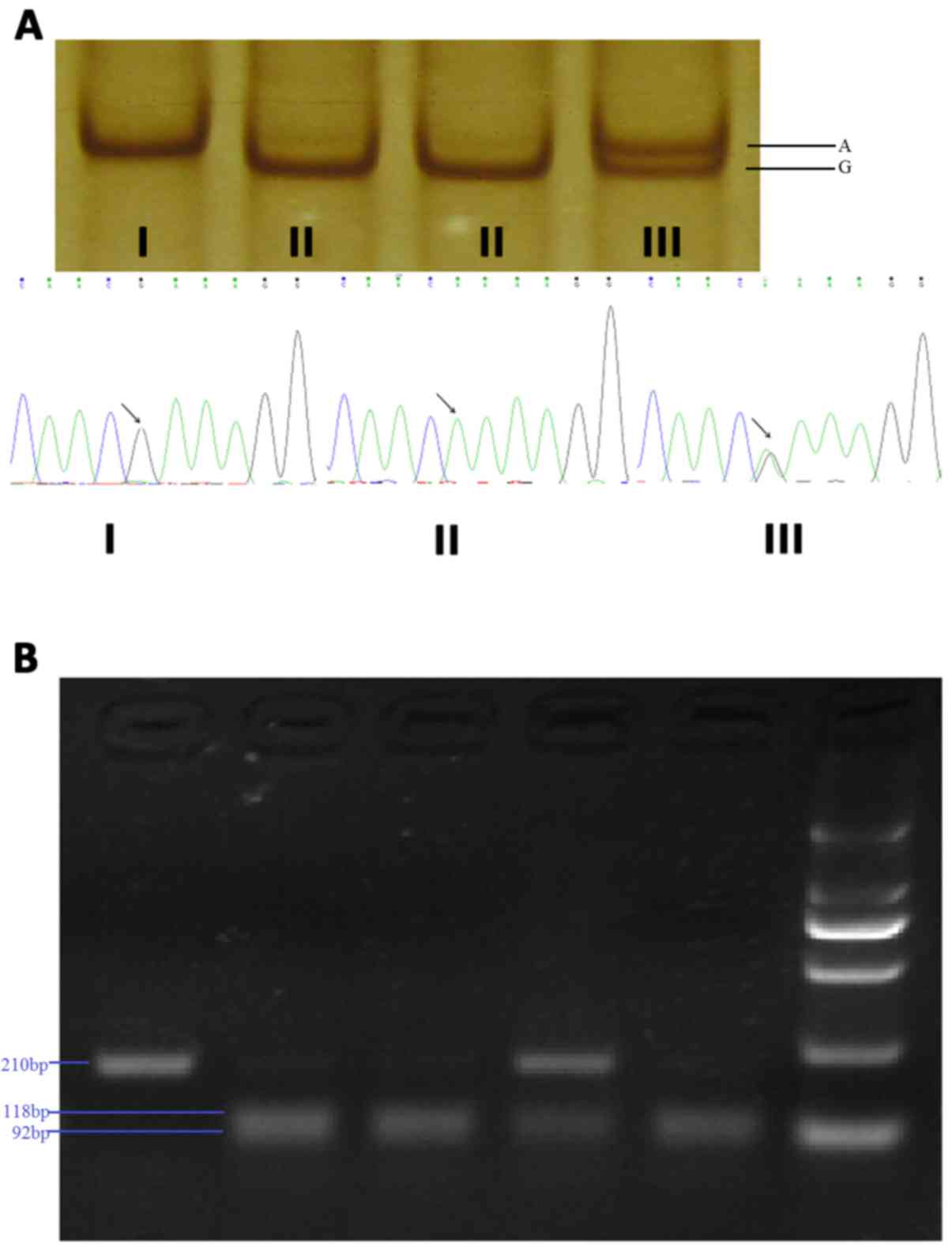
Genotyping
All blood samples were taken into EDTA-containing
receptacles and stored at −20°C until genomic DNA was extracted
using an AxyPrep DNA gel extraction kit (Axygen Scientific; Thermo
Fisher Scientific, Inc.). A total off 100 individuals were randomly
selected to scan and analyze the initial SNPs with 5 DNA pools of
20 samples in each pool. The 2kb 5′ upstream region of C4BPA
was PCR-amplified and sequence analysis was performed by Sangon
Biotech Co., Ltd. (Shanghai, China). A total of four SNPs were
identified and sequenced through PCR-sequencing among the 100
samples. Following preliminary analysis, genotype distribution of
two SNPs were different between EH and normotensives and were
further examined among all the participants through PCR-restriction
fragment length polymorphism (RFLP) and PCR-single strand
conformation polymorphism (SSCP). The primer sequences for PCR are
listed in Table I. Genotyping was
performed blindly to all other data.
 | Table I.The primer sequences of C4BPA
gene. |
Table I.
The primer sequences of C4BPA
gene.
| Fragment | Primer sequence (5′
to 3′) | Product size
(bp) |
|---|
| rs73079108 |
CATGAAGACATGGAAGCCTTGC | 288 |
|
|
TTGAACTCTTCCTCTCCCTCAC |
|
| rs74148971 |
TTCCCGAGAACCAGAGGTCAG | 210 |
|
|
CCAGTAAGAAGACTAGCCAGCACT |
|
| rs77660718 |
TGCTGGCTAGTCTTCTTACTGGT | 262 |
|
|
TGTCTGCAGCCTTTGTCACT |
|
| rs11120211 |
AAGCAACAGGTGGAGTGATGAATGAG | 924 |
|
|
CCACTATGTGCTGAGTTATCTAGAACGT |
|
Statistical analysis
SPSS software (version 19.0, IBM SPSS, Armonk, NY,
USA) was used for database management and statistical analyses.
Categorical variables were expressed as proportions (%) and
continuous variables as mean ± standard deviation. All comparisons
between two groups for allelic and genotypic frequencies were
performed by chi-squared test and continuous variables by
independent t-test. Genetic models (additive, dominant and
homozygote comparison) were analyzed by multivariate logistic
regression adjusted for covariates to calculate odds ratios (OR)
with 95% confidence intervals (CI) to predict the risk of EH.
Analyses used two-tailed estimation of significance. Presence of
Hardy-Weinberg equilibrium was tested by the chi-squared test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Characteristics of the
participants
Samples comprising 822 unrelated participants
comprising 371 hypertensive patients (214 men and 157 women; mean
age 50.66±8.64) and 451 normotensive controls (237 men and 214
women; mean age 48.43±8.60) were genotyped for the 5′ upstream
region. The participants were further divided into two subgroups
according to sex. The clinical and laboratory parameters were
summarized in Table II. For total
subjects, female and male, when compared with the normotensives,
the following variables were significant higher in EH: BMI, SBP,
DBP, MBP, HR, TG and FBG. Age and TC were higher in EH compared
with the controls in total and in female. Significant differences
were identified in weight and family history incidence in total and
in male.
 | Table II.Clinical characteristics of EH
participants and normotensives. |
Table II.
Clinical characteristics of EH
participants and normotensives.
|
| Total (n) | Male (n) | Female (n) |
|---|
|
|
|
|
|
|---|
|
| EH (371) | Control (451) | EH (276) | Control (313) | EH (276) | Control (313) |
|---|
| Sex, M/F | 214/157 | 237/214 | / | / | / | / |
| Age (years) |
50.66±8.64b | 48.43±8.60 | 49.47±7.94 | 48.64±8.51 | 52.28±9.29d | 48.19±8.72 |
| BMI
(kg/m2) |
25.49±0.68b | 23.75±0.36 |
24.63±6.05c | 22.84±4.15 |
24.63±6.05d | 22.84±4.15 |
| SBP (mmHg) |
154.63±14.01b | 118.93±10.48 |
152.34±13.65c | 120.74±9.51 |
157.75±13.94d | 116.92±11.13 |
| DBP (mmHg) |
94.89±10.03b | 74.57±8.60 |
96.60±9.64c | 77.19±7.48 |
92.56±10.12d | 71.65±8.83 |
| HR (bpm) |
79.92±13.70a | 76.05±14.12 | 79.32±11.48 | 77.38±15.11 |
80.74±16.25d | 74.58±9.72 |
| HDL-C (mmol/l) | 1.16±0.31 | 1.16±0.27 | 1.11±0.34 | 1.07±0.27 | 1.24±0.25 | 1.28±0.28 |
| LDL-C (mmol/l) | 3.00±0.95 | 2.92±0.77 | 2.86±0.95 | 2.94±0.78 |
3.19±0.92d | 2.89±0.76 |
| TG (mmol/l) |
2.18±1.48b | 1.66±1.23 |
2.35±1.53c | 1.97±1.37 |
1.93±1.37d | 1.32±0.94 |
| TC (mmol/l) |
5.08±0.92b | 4.81±0.89 | 4.96±0.86 | 4.84±0.86 |
5.23±0.97d | 4.76±0.92 |
| FBG (mmol/l) |
5.30±0.78b | 5.02±0.69 |
5.34±0.74c | 5.12±0.73 |
5.24±0.82d | 4.93±0.63 |
| Height (cm) | 168.03±7.32 | 168.12±7.26 | 172.81±4.85 | 172.79±5.35 | 161.43±4.44 | 162.95±5.34 |
| Weight (kg) |
72.03±14.05b | 67.67±13.11 |
76.96±9.90c | 73.97±10.68 | 65.22±15.98 | 60.70±11.99 |
| Cr (µmol/l) | 78.87±29.91 | 72.59±20.82 | 85.21±16.12 | 88.30±17.20 | 75.15±30.11 | 72.94±20.12 |
| BUN | 4.96±1.34 | 4.94±2.53 | 5.03±1.29 | 5.51±1.64 | 4.93±1.37 | 4.59±1.32 |
| FH (yes/no) |
125/111b | 107/186 | 66/67c | 46/111 | 59/44 | 61/75 |
| SH (yes/no) | 56/180 | 78/215 | 55/78 | 75/82 | 1/102 | 3/133 |
| DH (yes/no) | 70/166 | 74/219 | 70/63 | 71/86 | 0/103 | 3/133 |
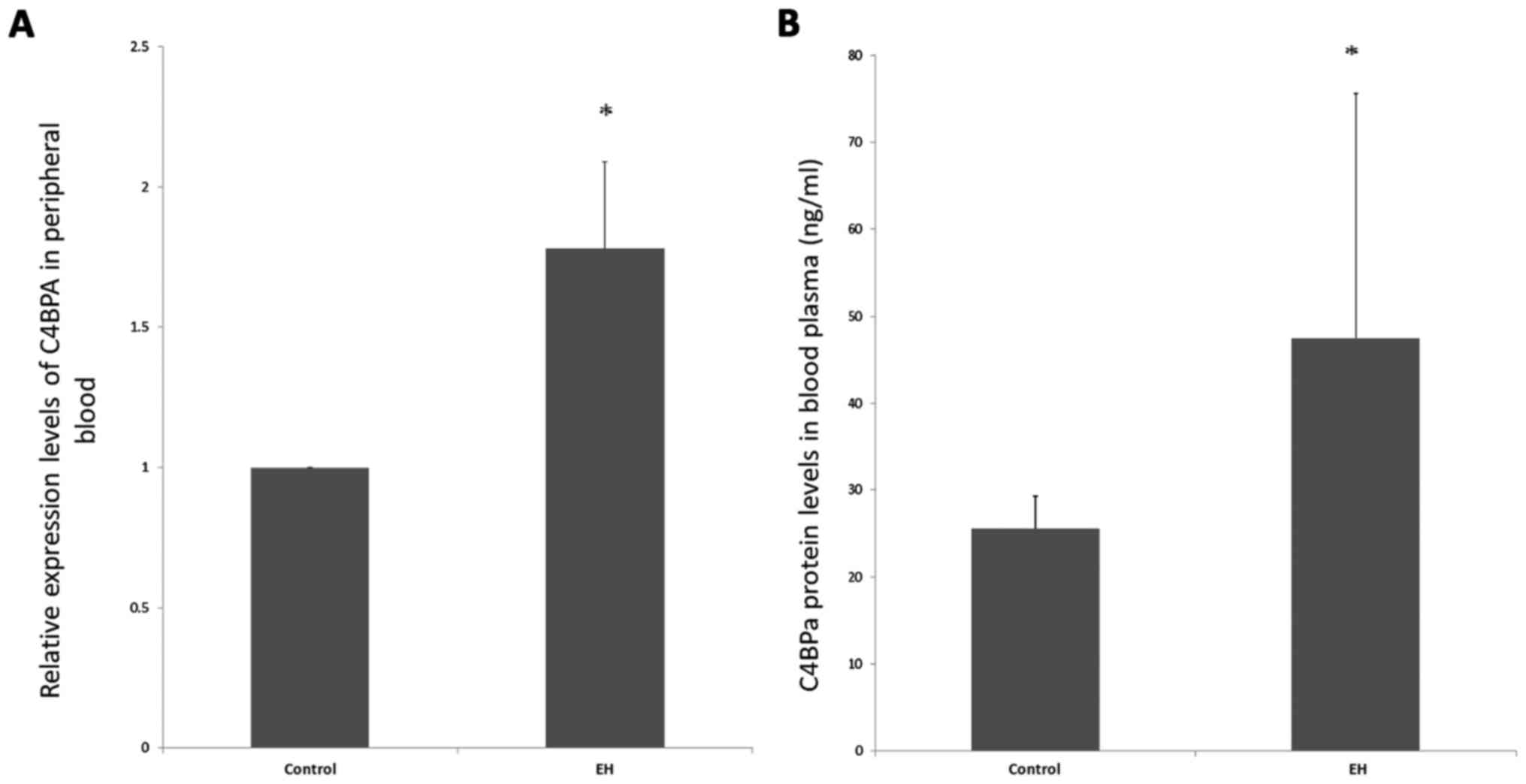
Expression valuation of C4BPA in
peripheral blood
The expression levels of C4BPA mRNA and C4BPα
protein in peripheral blood in the EH and normotensives were tested
(Fig. 1). C4BPA expression was
significantly higher in EH than in controls both in the
transcriptional level and translational level (P<0.05).
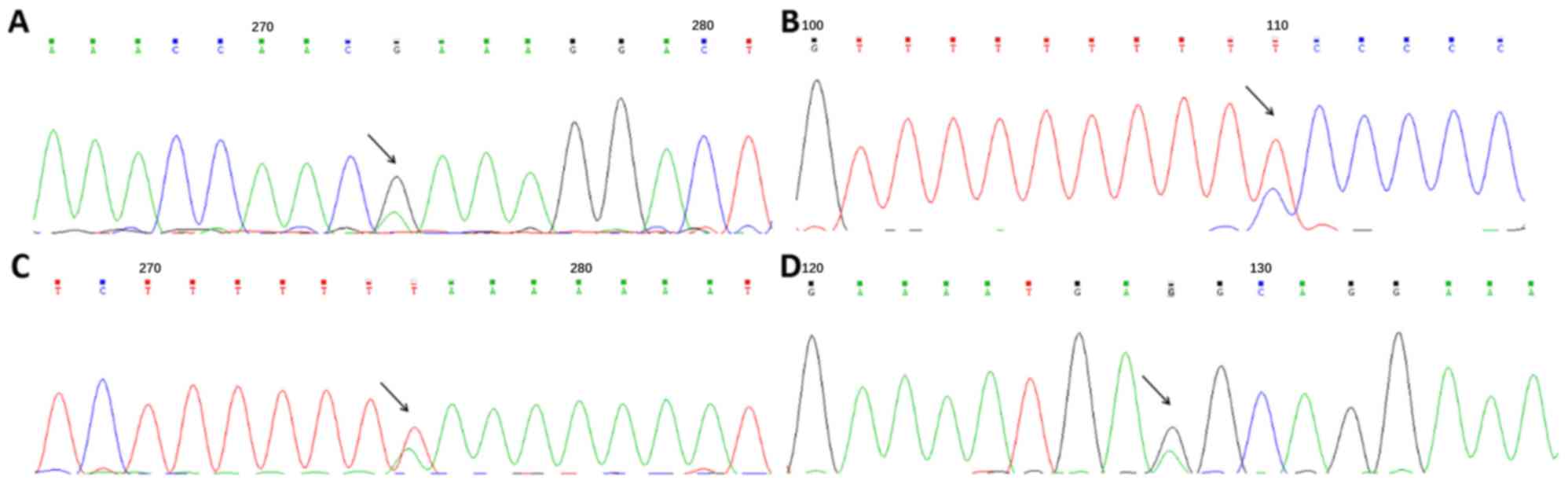
Detection and distribution of the
SNPs
Through direct sequencing of the 5′ upstream region
of C4BPA, four SNPs were identified (Fig. 2), and they were consistent with the
SNPs labeled rs73079108, rs74148971, rs77660718 and rs11120211 in
the National Center for Biotechnology Information database.
Following preliminary analysis of genotype distributions in 100
samples, rs73079108 and rs74148971 presented a different trend in
two groups and were further tested through PCR-SSCP and PCR-RFLP,
respectively (Fig. 3). No
deviation from the Hardy-Weinberg equilibrium expectation was
observed for rs73079108 and rs74148971 in either normotensives or
hypertensives (P>0.05).
Univariate analysis indicated that the genotype and
allele distribution of the rs73079108 polymorphism differed
significantly between EH and normotensive subjects (P<0.05). The
AA and GA genotypes were significantly more prevalent in the
control cases, and the A allele frequency was significantly higher
in the normotensives. When subdivided by sex, the difference of
genotype distribution was also observed in males and females. The
prevalence of G allelic frequencies was significantly higher in the
hypertensives than in the normotensives both in total and in female
subgroup, but not in male subgroup. For rs74148971, no significant
differences in the proportion of genotypes and alleles were found
between the two groups whether in total case, in male or in female
(Table III).
 | Table III.The frequencies of the C4BPA gene
rs73079108 and rs74148971 genotypes. |
Table III.
The frequencies of the C4BPA gene
rs73079108 and rs74148971 genotypes.
|
| Genotype
(frequency, %) |
| Allele (frequency,
%) |
|
|---|
|
|
|
|
|
|
|---|
| Group | GG | GA | AA |
P-valuea | G allele | A allele |
P-valueb |
|---|
| rs73079108 |
| EH
(Total) | 324 (87.33) | 44 (11.86) | 3 (0.81) | 0.011 | 692 (93.26) | 50 (6.74) | <0.001 |
| Control
(Total) | 350 (77.61) | 92 (20.40) | 9 (2.00) |
| 792 (77.80) | 110 (12.20) |
|
| EH
(Male) | 187 (87.38) | 27 (12.62) | 0 (0) | 0.038 | 401 (93.69) | 27 (6.31) | 0.184 |
| Control
(Male) | 201 (84.81) | 31 (13.08) | 5 (2.11) |
| 433 (91.35) | 41 (8.65) |
|
| EH
(Female) | 137 (87.26) | 17 (10.83) | 3 (1.91) | <0.001 | 291 (92.68) | 23 (7.32) | <0.001 |
| Control
(Female) | 149 (69.63) | 61 (28.50) | 4 (1.87) |
| 359 (83.88) | 69 (16.12) |
|
| rs74148971 |
| EH
(Total) | 156 (65.27) | 71 (29.71) | 12 (5.02) | 0.709 | 383 (80.13) | 95 (19.87) | 0.517 |
| Control
(Total) | 128 (61.54) | 70 (33.65) | 10 (4.81) |
| 326 (78.37) | 90 (21.63) |
|
| EH
(Male) | 72 (65.45) | 32 (29.09) | 6 (5.45) | 0.806 | 176 (80.00) | 44 (20.00) | 0.640 |
| Control
(Male) | 59 (61.46) | 32 (33.33) | 5 (5.21) |
| 150 (78.13) | 42 (21.87) |
|
| EH
(Female) | 84 (65.12) | 39 (30.23) | 6 (4.65) | 0.828 | 207 (80.23) | 51 (19.77) | 0.479 |
| Control
(Female) | 69 (61.61) | 38 (33.93) | 5 (4.46) |
| 166 (77.57) | 48 (22.43) |
|
Age, SBP, DBP, FBG, TC, TG and BMI levels were
compared among genotypes of the rs73079108 polymorphism (Table IV). Lipid profiles, age and BMI
did not differ significantly among the genotypes, but there was a
significantly higher level of SBP, DBP and FBG in the GG
genotype.
 | Table IV.The association between rs73079108
and clinical features. |
Table IV.
The association between rs73079108
and clinical features.
|
| Age | SBP (mmHg) | DBP (mmHg) | FBG (mmol/l) | TG (mmol/l) | TC (mmol/l) | BMI
(kg/m2) |
|---|
| AA | 50.00±9.24 | 121.08±19.95 | 78.33±1.00 | 4.69±0.24 | 1.52±1.00 | 4.48±0.54 | 23.34±2.96 |
| AG | 50.14±8.22 | 129.42±22.30 | 80.07±14.99 | 5.02±0.59 | 1.61±1.06 | 4.76±0.78 | 24.15±4.65 |
| GG | 49.28±8.77 | 136.42±21.18 | 84.56±13.30 | 5.18±0.77 | 1.95±1.42 | 4.97±0.94 | 24.47±3.52 |
| P-value | 0.562 | <0.001 | 0.001 | 0.021 | 0.276 | 0.066 | 0.423 |
Association analysis
Logistic regression analysis was performed under
different genetic models (additive, dominant and recessive) after
adjusting for confounding risk factors, including age, sex, BMI,
HDL-c, LDL-c, TG, TC, FBG, smoking and drinking history. As
presented in Table V, for the
rs73079108 polymorphism, significant association could be
identified in the additive genetic model (AA vs. GA vs. GG,
OR=0.604, 95% CI: 0.418–0.873, P=0.007) and dominant genetic model
(AA+GA vs. GG, OR=0.567, 95% CI: 0.382–0.841, P=0.005), but not in
receive model or homozygote comparison. A significantly lower
prevalence of A allelic frequency (P<0.001, OR=0.520, 95% CI:
0.489–0.554) was observed in EH than in the control group, which
suggested that the A allele may be a protective factor for the EH
in the northeastern Han Chinese population. When subdivided for
sex, the situation was the same in the females in total, but not in
males. As there was a rare frequency of AA in males, logistic
regression analysis was not performed in receive model or
homozygote comparison. The P-value of rs73079108 genotype-sex
interaction was significant (P=0.015). For the rs74148971
polymorphism, significant association could only be found in
recessive model, but no significant association was identified in
other genetic models.
 | Table V.Logistic regression analysis of
additive genetic model comparison for each single-nucleotide
polymorphism genotype associated with essential hypertension in the
northeastern Han Chinese population. |
Table V.
Logistic regression analysis of
additive genetic model comparison for each single-nucleotide
polymorphism genotype associated with essential hypertension in the
northeastern Han Chinese population.
|
| Overall | Male | Female |
|---|
|
|
|
|
|
|---|
| SNP | Contrast | OR | 95% CI | P-value | OR | 95% CI | P-value | OR | 95% CI | P-value |
|---|
| rs73079108 | AA:AG:GG | 0.604 | 0.418–0.873 | 0.007 | 0.702 | 0.407–1.210 | 0.203 | 0.551 | 0.328–0.926 | 0.024 |
|
| AA+GA:GG | 0.567 | 0.382–0.841 | 0.005 | 0.806 | 0.461–1.409 | 0.450 | 0.410 | 0.229–0.735 | 0.003 |
|
| AA:GA+GG | 0.548 | 0.142–2.111 | 0.382 | – | – | – | 1.605 | 0.327–7.885 | 0.560 |
|
| AA:GG | 3.592 | 0.765–16.865 | 0.105 | – | – | – | 2.004 | 0.379–10.587 | 0.413 |
| rs74148971 | AA:AG:GG | 1.184 | 0.836–1.677 | 0.342 | 1.149 | 0.699–1.889 | 0.585 | 1.175 | 0.717–1.925 | 0.522 |
|
| AA+GA:GG | 1.210 | 0.798–1.834 | 0.369 | 1.123 | 0.617–2.042 | 0.704 | 1.273 | 0.705–2.298 | 0.424 |
|
| AA:GA+GG | 1.508 | 1.298–1.752 | <0.001 | 1.255 | 1.033–1.526 | 0.022 | 2.105 | 1.615–2.743 | <0.001 |
|
| AA:GG | 1.328 | 0.495–3.564 | 0.573 | 2.552 | 0.758–8.591 | 0.130 | 0.346 | 0.063–1.899 | 0.222 |
Interactive effect of rs73079108
polymorphism on EH
To examine whether there were associations between
C4BPA rs73079108, obesity and EH, all subjects were
subdivided into the obese and non-obese subgroup according to BMI
and the analysis was further conducted.
Genotype distributions and allele frequencies of EH
in the non-obese cases and non-obese female were significantly
different from the control subjects (Table VI). Following logistic regression
analysis, rs73079108 was indicated to be significantly related to
the prevalence of EH both in the non-obese (P=0.001, OR=0.374,
95%CI [0.205–0.682]) and in the non-obese female (P=0.001,
OR=0.251, 95%CI [0.110–0.573]). Whereas in obese group, no
significant associations could be identified between rs73079108 and
hypertension risk (Table VII).
The P-value for rs73079108 genotype-BMI interaction was 0.042. The
results indicated that there was a significant correlation between
rs73079108 genotypes and obesity on EH, especially in women.
 | Table VI.The genotype distributions and allele
frequencies of the C4BPA gene rs73079108 polymorphism in obese and
non-obese samples. |
Table VI.
The genotype distributions and allele
frequencies of the C4BPA gene rs73079108 polymorphism in obese and
non-obese samples.
|
|
|
| Genotype
(frequency, %) |
| Allele (frequency,
%) |
|
|---|
|
|
|
|
|
|
|
|
|---|
| rs73079108
samples | Group | n | AA | GA | GG |
P-valuea | A allele | G allele |
P-valueb |
|---|
| Obese n=338 | EH (Total) | 191 | 3 | 26 | 162 | 0.931 | 32 | 350 | 0.784 |
|
| Control
(Total) | 147 | 3 | 21 | 123 |
| 27 | 267 |
|
|
| EH (Male) | 127 | 0 | 17 | 110 | 0.093 | 17 | 237 | 0.307 |
|
| Control (Male) | 107 | 3 | 14 | 90 |
| 20 | 194 |
|
|
| EH (Female) | 64 | 3 | 9 | 52 | 0.212 | 15 | 113 | 0.644 |
|
| Control
(Female) | 40 | 0 | 7 | 33 |
| 7 | 73 |
|
| Non-obese
n=482 | EH (Total) | 178 | 0 | 18 | 160 | <0.001 | 18 | 338 | <0.001 |
|
| Control
(Total) | 304 | 6 | 71 | 227 |
| 83 | 525 |
|
|
| EH (Male) | 87 | 0 | 10 | 77 | 0.331 | 10 | 164 | 0.448 |
|
| Control (Male) | 13 | 2 | 17 | 111 |
| 21 | 239 |
|
|
| EH (Female) | 91 | 0 | 8 | 83 | <0.001 | 8 | 174 | <0.001 |
|
| Control
(Female) | 174 | 4 | 54 | 116 |
| 62 | 286 |
|
 | Table VII.Logistic regression analysis of the
rs73079108 polymorphism associated with essential hypertension in
obese and non-obese samples. |
Table VII.
Logistic regression analysis of the
rs73079108 polymorphism associated with essential hypertension in
obese and non-obese samples.
|
|
| Overall | Male | Female |
|---|
|
|
|
|
|
|
|---|
| Sample groups | Contrast | OR | 95% CI | P-value | OR | 95% CI | P-value | OR | 95% CI | P-value |
|---|
| Non-obese | AA:AG:GG | 0.374 | 0.205–0.682 | 0.001 | 1.587 | 0.577–4.369 | 0.371 | 0.251 | 0.110–0.573 | 0.001 |
| Obese | AA:AG:GG | 0.961 | 0.568–1.628 | 0.883 | 0.723 | 0.359–1.455 | 0.363 | 0.737 | 0.289–1.878 | 0.522 |
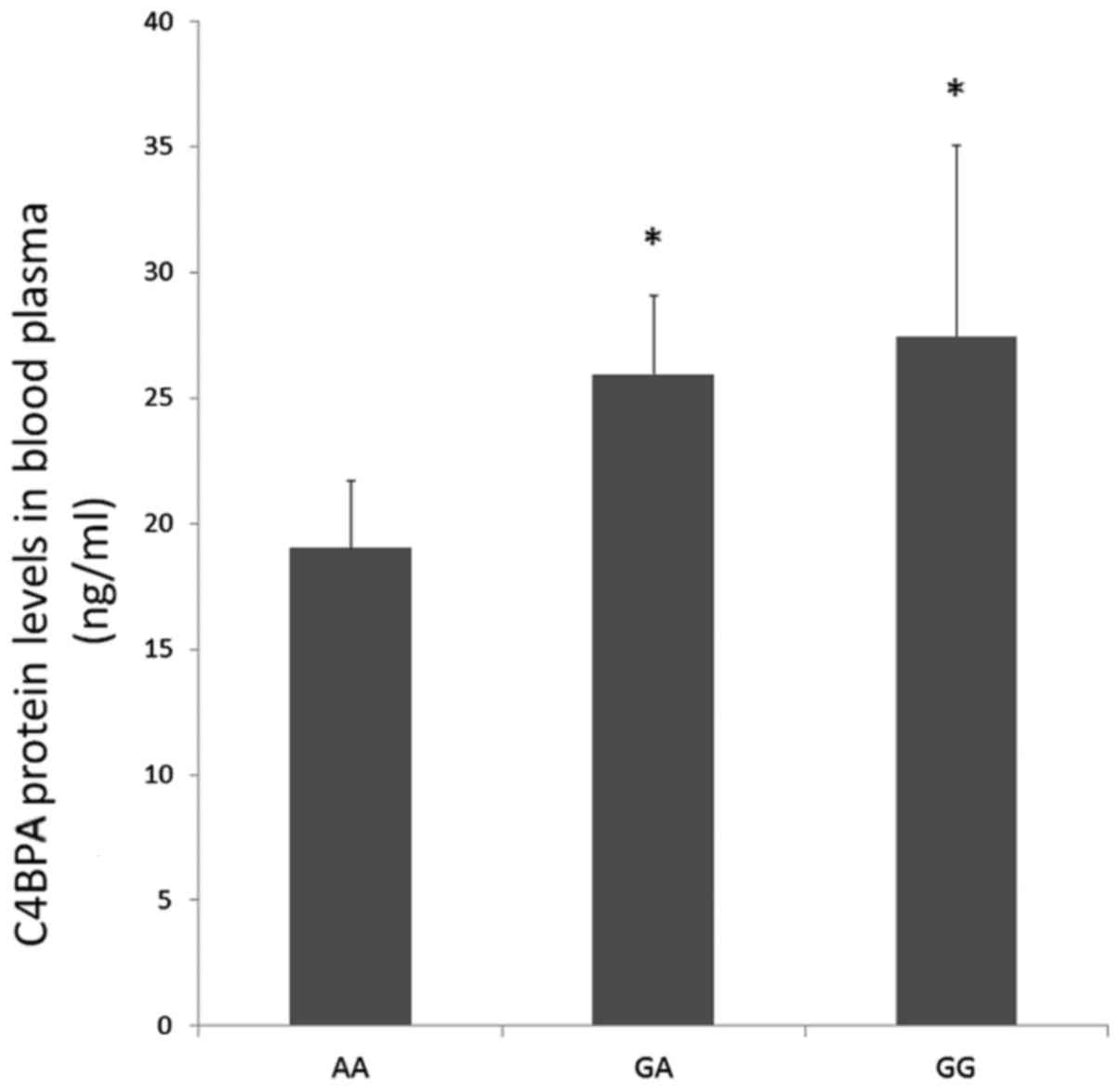
rs73079108 affects C4BPα protein level
in control samples
To determine the effect of rs73079108 on C4BPα
protein level, 100 control samples were randomly selected and the
expression of C4BPα in the blood plasma was detected. Through ELISA
detection, a significant increase in C4BPα protein level was
observed in GA and GG carriers compared to the AA carriers
(P<0.05; Fig. 4). In addition,
it presented an increase trend in the GG carriers compared to the
GA carriers, but the difference was not statistically
significant.
Discussion
In the present study, the authors verified the
elevated expression of C4BPA in the peripheral blood both at
the transcriptional and translational level, which was consistent
with their previous work through microarray analysis, suggesting a
positive relationship between C4BPA and EH. A total of four
SNPs in the 5′ upstream region were identified and genotyping were
further performed and analyzed. Univariate analysis demonstrated
that elevated age, BMI, HR, TG, TC, FBG and weight may be risk
factors of EH. The chi-squared test and logistic regression
analysis, by taking the confounding factors together with the SNP
into the regression model, were performed and the association of
C4BPA rs73079108 with EH in the northeastern Han Chinese
population was identified. The rs73079108-A allele may be a
protective marker for EH in total and in females, and rs73079108
was indicated to affect C4BPα protein level in control samples. In
addition, although the distribution of rs74148971 genotypes was not
significantly different in both groups, the receive model indicates
the association of rs74148971 and AA genotype may be a risk factor.
The authors further carried on the stratification analysis by sex
and BMI and discussed the possible relationship of EH,
C4BPA, sex and BMI.
C4BP is an acute phase protein and it increases in
concentration upon inflammation (22). The C4BP levels have been proven to
be strongly and directly correlated to hs-CRP, which has been
proved to be associated with EH in a previous report (23). C4BPA or C4BP have also been
reported to be associated with myocardial infarction (23), atherosclerosis of the descending
thoracic aorta (24), preeclampsia
(25), venous thrombosis (15), schizophrenia (26), non-small cell lung cancer (27), joint hypermobility syndrome
(28), triglyceride levels, as
well as platelet count and warfarin treatment (29), but no evidence has been proved
their direct association with EH. To the best of the authors'
knowledge, the present study is the first time to identify the
possible positive association between C4BPA and EH. C4BPA
may contribute to the EH etiology in at least three ways: (a)
Inhibition of complement activation by C4BP contributes to the
endothelial dysfunction (30),
which is now considered as essential element of EH etiology; (b)
C4BPA may indirectly influence blood pressure through
combination with low-density lipoprotein receptor-related protein 6
and regulate glucose metabolism (31). In the current study, the authors
further proved the association between glucose and C4BPA and
that FBG levels varied significantly among different genotypes of
rs73079108 (Table IV; (c) The
association of atherosclerosis and triglycerides with C4BPA
also indicated the potential role of C4BPA in EH.
In a previous study, the authors already
demonstrated that there was upregulated expression of C4BPA
in EH (4), and the protective
factor of rs73079108-A allele. In addition, rs73079108 was
identified to affect the C4BPa level in controls. Based on the
results, the authors inferred that the mutation from G allele to A
allele in the 5′upstream region may possibly change the DNA
response elements and effect the transcription and consequently
reduced C4BPA expression, which could attenuate the effects
of C4BPA on EH. The present study then examined C4BPα
protein level with different rs73079108 genotypes. A significant
increase in C4BPα protein levels was identified in GA and GG
carriers relative to the AA carriers. This data suggested that the
A allele of rs73079108 influenced C4BPα levels and further proved
the association with EH.
Sexual bias has long been recognized in
hypertension. Here, stratification analysis by sex indicated that
the association of rs73079108 was EH were female-specific. It may
be caused by differences in lifestyle, social stress, hormonal
system and genetic determinants (32). Conversely, C4BPα may bind
efficiently to LRP (10) and
effect the LRP functions in regulation of lipid homeostasis, LDL
uptake and body fat mass (31).
The significant differences of LDL-C and TG between EH and controls
in females may partly contribute to the different association in
sex-subgroups. Still, the different results between male and female
as well as the P-value for genotype-sex interaction should be
treated cautiously, as the female subjects were considerably fewer
than males, which may be a limiting factor to detect the difference
of OR estimates between the subgroups.
Following stratification analysis on obesity,
association of rs73079108 and EH risk was shown in the non-obese
subjects and non-obese women. Significant association between
rs73079108 genotypes and BMI on EH risk could be identified. These
findings suggested a potential effect of obesity on the association
between hypertension risk and genetic factors.
In the current study, all participants were enrolled
from the northeastern Han ethnic group and strict inclusive and
exclusive criteria were made to reduce population stratification on
some level. However, there were still some limitations, as
demonstrated by the following: (a) Given that no clues implying
correlation between C4BPA and hypertension biomarkers, the authors
did not examine the association between rs73079108 and biomarkers.
In the following study, they will focus on the downstream
regulators of C4BPA involved in the regulation of EH to see whether
the potential biomarkers of hypertension can be identified. (b) Due
to relatively small number of the study samples, and strong genetic
influences from other populations caused by migration in history,
our understanding of the role of C4BPA gene polymorphisms in the
development of EH was limited. Additional studies with a larger
sample size in more diverse areas are required to further verify
the association found in the present study. (c) Four SNPs were
preliminarily examined in the present study, and only rs73079108
was further analyzed, whereas other SNPs are still worthy of
study.
To the best of the authors' knowledge, the present
study is the first study to examine the association between the
rs73079108 polymorphism and EH in the northeastern Han Chinese
population, to identify rs73079108-A as a protective factor of EH
and to verify the association between C4BPA with EH.
Subgroup analysis by sex and BMI demonstrated specificity in female
and non-obese samples. Further functional studies of C4BPA
in EH and studies in different populations are needed to confirm
the discovery.
Acknowledgements
The present study was supported by the Natural
Science Foundation of China (grant no. 81570360) and the Graduate
Innovation Fund of Jilin University (grant no. 2012112).
References
|
1
|
Liu X, Meng F and Yang P: Association
study of CD36 single nucleotide polymorphisms with essential
hypertension in the Northeastern Han Chinese. Gene. 527:410–415.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mancia G, de Backer G, Dominiczak A,
Cifkova R, Fagard R, Germano G, Grassi G, Heagerty AM, Kjeldsen SE,
Laurent S, et al: 2007 Guidelines for the management of arterial
hypertension: the task force for the management of arterial
hypertension of the european society of hypertension (ESH) and of
the European society of cardiology (ESC). J Hypertens.
25:1105–1187. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tanira MO and Al Balushi KA: Genetic
variations related to hypertension: A review. J Hum Hypertens.
19:7–19. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Korkor MT, Meng FB, Xing SY, Zhang MC, Guo
JR, Zhu XX and Yang P: Microarray analysis of differential gene
expression profile in peripheral blood cells of patients with human
essential hypertension. Int J Med Sci. 8:168–179. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Suankratay C, Mold C, Zhang Y, Lint TF and
Gewurz H: Mechanism of complement-dependent haemolysis via the
lectin pathway: Role of the complement regulatory proteins. Clin
Exp Immunol. 117:442–448. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Scharfstein J, Ferreira A, Gigli I and
Nussenzweig V: Human C4-binding protein. I. Isolation and
characterization. J Exp Med. 148:207–222. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ogata RT, Mathias P, Bradt BM and Cooper
NR: Murine C4b-binding protein. Mapping of the ligand binding site
and the N-terminus of the pre-protein. J Immunol. 150:2273–2280.
1993.PubMed/NCBI
|
|
8
|
de Frutos P Garcia and Dahlbäck B:
Interaction between serum amyloid P component and C4b-binding
protein associated with inhibition of factor I-mediated C4b
degradation. J Immunol. 152:2430–2437. 1994.PubMed/NCBI
|
|
9
|
Hessing M, Vlooswijk RA, Hackeng TM,
Kanters D and Bouma BN: The localization of heparin-binding
fragments on human C4b-binding protein. J Immunol. 144:204–208.
1990.PubMed/NCBI
|
|
10
|
Westein E, Denis CV, Bouma BN and Lenting
PJ: The alpha-chains of C4b-binding protein mediate complex
formation with low density lipoprotein receptor-related protein. J
Biol Chem. 277:2511–2516. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Blom AM, Berggârd K, Webb JH, Lindahl G,
Villoutreix BO and Dahlbäck B: Human C4b-binding protein has
overlapping, but not identical, binding sites for C4b and
streptococcal M proteins. J Immunol. 164:5328–5336. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ram S, Cullinane M, Blom AM, Gulati S,
McQuillen DP, Monks BG, O'Connell C, Boden R, Elkins C, Pangburn
MK, et al: Binding of C4b-binding protein to porin: A molecular
mechanism of serum resistance of Neisseria gonorrhoeae. J Exp Med.
193:281–295. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Antoniades C, Bakogiannis C, Tousoulis D,
Antonopoulos AS and Stefanadis C: The CD40/CD40 ligand system:
Linking inflammation with atherothrombosis. J Am Coll Cardiol.
54:669–677. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ridker PM and Silvertown JD: Inflammation,
C-reactive protein, and atherothrombosis. J Periodontol. 79 8
Suppl:S1544–S1551. 2008. View Article : Google Scholar
|
|
15
|
Buil A, Trégouët DA, Souto JC, Saut N,
Germain M, Rotival M, Tiret L, Cambien F, Lathrop M, Zeller T, et
al: C4BPB/C4BPA is a new susceptibility locus for venous thrombosis
with unknown protein S-independent mechanism: Results from
genome-wide association and gene expression analyses followed by
case-control studies. Blood. 115:4644–4650. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Georges AB, Benayoun BA, Caburet S and
Veitia RA: Generic binding sites, generic DNA-binding domains:
Where does specific promoter recognition come from? FASEB J.
24:346–356. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yu JC, Hsiung CN, Hsu HM, Bao BY, Chen ST,
Hsu GC, Chou WC, Hu LY, Ding SL, Cheng CW, et al: Genetic variation
in the genome-wide predicted estrogen response element-related
sequences is associated with breast cancer development. Breast
Cancer Res. 13:R132011. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gu D, Su S, Ge D, Chen S, Huang J, Li B,
Chen R and Qiang B: Association study with 33 single-nucleotide
polymorphisms in 11 candidate genes for hypertension in Chinese.
Hypertension. 47:1147–1154. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ge D, Huang J, He J, Li B, Duan X, Chen R
and Gu D: beta2-Adrenergic receptor gene variations associated with
stage-2 hypertension in northern Han Chinese. Ann Hum Genet.
69:36–44. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Smith SC Jr, Clark LT, Cooper RS, Daniels
SR, Kumanyika SK, Ofili E, Quinones MA, Sanchez EJ, Saunders E and
Tiukinhoy SD: American Heart Association Obesity, Metabolic
Syndrome, and Hypertension Writing Group: Discovering the full
spectrum of cardiovascular disease: Minority health summit 2003:
Report of the obesity, metabolic syndrome, and hypertension writing
group. Circulation. 111:e134–e139. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Funakoshi M, Sasaki J and Arakawa K:
Proline-rich protein is a glycoprotein and an acute phase reactant.
Biochim Biophys Acta. 963:98–108. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Trouw LA, Okroj M, Kupreishvili K,
Landberg G, Johansson B, Niessen HW and Blom AM: C4b-binding
protein is present in affected areas of myocardial infarction
during the acute inflammatory phase and covers a larger area than
C3. PloS One. 3:e28862008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kimoto K, Inoue T, Oku K, Mori T, Kusuda
M, Handa K, Sakata N, Sasaki J and Arakawa K: Relation of
C4b-binding protein to athero-sclerosis of the descending thoracic
aorta. Artery. 22:101–114. 1996.PubMed/NCBI
|
|
25
|
Joyama S, Yoshida T, Koshikawa M, Sawai K,
Yokoi H, Tanaka A, Gotoh M, Ueda S, Sugawara A and Kuwahara T: C4d
and C4bp deposition along the glomerular capillary walls in a
patient with preeclampsia. Am J Kidney Dis. 37:E62001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang S, Lu H, Ni J, Zhang J, Tang W, Lu W,
Cai J and Zhang C: An evaluation of association between common
variants in C4BPB/C4BPA genes and schizophrenia. Neurosci Lett.
590:189–192. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Luo X, Liu Y, Wang R, Hu H, Zeng R and
Chen H: A high-quality secretome of A549 cells aided the discovery
of C4b-binding protein as a novel serum biomarker for non-small
cell lung cancer. J Proteomics. 74:528–538. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Watanabe A, Satoh K, Maniwa T and
Matsumoto K: Proteomic analysis for the identification of serum
diagnostic markers for joint hypermobility syndrome. Int J Mol Med.
37:461–467. 2016.PubMed/NCBI
|
|
29
|
Martin M, Gottsäter A, Nilsson PM, Mollnes
TE, Lindblad B and Blom AM: Complement activation and plasma levels
of C4b-binding protein in critical limb ischemia patients. J Vasc
Surg. 50:100–106. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wu F, Zou Q, Ding X, Shi D, Zhu X, Hu W,
Liu L and Zhou H: Complement component C3a plays a critical role in
endothelial activation and leukocyte recruitment into the brain. J
Neuroinflammation. 13:232016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Go GW: Low-density lipoprotein
receptor-related protein 6 (LRP6) is a novel nutritional
therapeutic target for hyperlipidemia, non-alcoholic fatty liver
disease and atherosclerosis. Nutrients. 7:4453–4464. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rana BK, Insel PA, Payne SH, Abel K,
Beutler E, Ziegler MG, Schork NJ and O'Connor DT: Population-based
sample reveals gene-gender interactions in blood pressure in White
Americans. Hypertension. 49:96–106. 2007. View Article : Google Scholar : PubMed/NCBI
|