Introduction
Ligustrazine (LZ), a bioactive component from the
traditional Chinese medicine ligusticum, is primarily used in China
as a vasodilator (1). In recent
years, it has been reported that LZ inhibits tumor metastasis and
improves the sensitivity of multidrug resistant tumor cells to
chemotherapeutic agents (2).
However, LZ is chemically unstable with a half-life of ~1.5 h
(3) and lacks a compatible drug
delivery system, which limits its potential as a chemotherapeutic
agent in the management of cancer. Our previous study demonstrated
that liposomes loaded with LZ enhanced the effect of LZ in
reversing multi-drug resistance (MDR) in K562/ADM cells (4). However, liposome is not an ideal
carrier for anticancer agents due to its low encapsulation
efficiency (39.5%) and lack of active targeting (5). Therefore, the current study
synthesized folate-conjugated chitosan nanoparticles (FA-CS-NPs)
loaded with LZ to enhance the targeting ability and
biocompatibility mediated by folate receptor.
Chitosan NPs are emerging as drug delivery system
due to its favorable characteristic features such as size,
biocompatibility, high drug encapsulation efficiency, controlled
drug release potential and long circulating half-life (6). Furthermore, due to the presence of
primary amino groups, CS-NPs are easily modified by various
ligands, including folate (7),
epidermal growth receptor (8) and
polypeptides (9). Thus,
modifications of CS-NPs with ligands specific for receptors on
tumor cells may enhance the specificity of the drugs delivered to
the tumor cells. Folate is an extensively studied ligand as it is
stable, inexpensive and has low immunogenicity (7). Furthermore, the expression of folate
receptor (FR) is higher in human cancer cells, including HeLa and
MCF-7 cells, than in normal cells (10,11).
FA-CS-NPs loaded with anticancer agents produced enhanced
intracellular accumulation of therapeutic agents, including
doxorubicin and gemcitabine, in FR-positive tumor cells, including
HeLa (12), B16F1 and SMMC-722192
skin melanoma cells (13), and
COLO357 pancreatic cancer cells (14). However, the use of LZ encapsulated
in FA-CS-NPs as a natural MDR reversal agent has not been
studied.
The aim of the current study was to develop a novel,
cost effective LZ-loaded NPs based drug delivery system to target
tumor metastasis and to counter MDR during cancer therapy. FA-CS
was synthesized by conjugating folate to chitosan via
amino-acylation reaction and FA-CS-LZ-NPs were prepared by
ionotropic gelation methods. Subsequently, the physical properties
and biological activity of FA-CS-LZ-NPs were characterized. In
addition, the cancer-targeting specificity of FA-CS-LZ-NPs was
determined using MCF-7 (FR-positive) and A549 (FR-negative)
cells.
Materials and methods
Reagents
Chitosan (50 kDa; degree of deacetylation, >90%),
folate, 1-(3-dimethylaminoproply)-3-ethylcarbodiimide hydrochloride
(EDC), phosphate buffered saline (PBS, pH 7.4),
3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazoliumbromide (MTT),
and dimethylsulfoxide (DMSO) were purchased from Sigma-Aldrich
(Merck KGaA, Darmstadt, Germany). Sodium tripolyphosphate (TPP) was
purchased from Kermel Chemical Reagent Co., Ltd., Tianjin, China).
LZ (2,3,5,6-tetramethylpyrazine) was purchased from Zelang
Pharmaceutical Co., Ltd. (Nanjing, China). Methyl alcohol
(chromatographic grade) was purchased from Tedia Company, Inc.
(Fairfield, OH, USA).
Cell lines
MCF-7 human breast carcinoma cell line and A549
human lung adenocarcinoma cell line were purchased from Blood
Research Administration (Tianjin, China). The cells were cultured
in Dulbeccos modified Eagle's medium (DMEM) and supplemented with
10% fetal bovine serum (both from Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA), 100 U/ml penicillin and 100 µg/ml
streptomycin at 37°C in a humidified atmosphere with 5%
CO2.
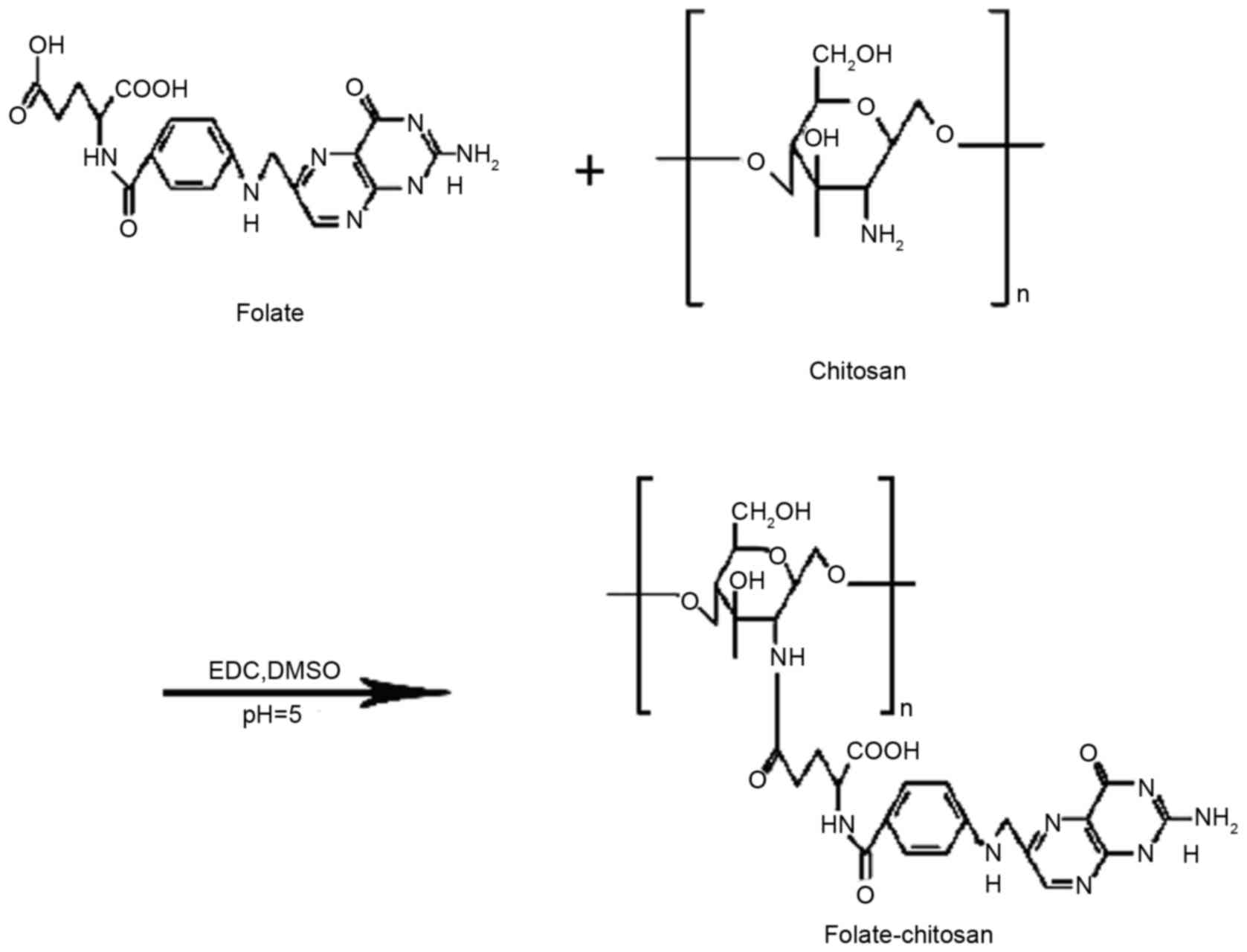
Conjugation and analysis of FA-CS
FA-CS was prepared through an amino-acylation
reaction (Fig. 1). Briefly,
different concentrations of folate were dissolved into anhydrous
DMSO with stirring. EDC (10 mol/ml) was added into the solution and
stirred at room temperature for 1 h. Subsequently, 5 ml chitosan
sodium acetate (pH 5.0: w/v 5%) was added to the solution, stirred
at 30°C in the dark overnight. The resultant mixture was adjusted
to pH 9.0 and then dialyzed against 0.1 M sodium phosphate buffer
(pH 7.4) for 3 days, followed by dialysis against water for 3 days
using a dialysis bag (molecular weight cut-off, 12 kDa). The
mixture was frozen at −48°C for 12 h, and it was subsequently
isolated using a FreeZone system (2.5L; Labconco, Kansas City, MO,
USA); the synthesized product was obtained following 24 h.
The chemical structure of FA-CS was analyzed by
infrared spectroscopy (IR; WGH-30; Gang Dong Technology Co., Ltd.,
Tianjing, China) and 1H nuclear magnetic resonance (NMR;
in D2O, 500 MHz; Bruker Corporation, Billerica, MA,
USA). The coupled number (the number of folate molecules to CS) of
folate to chitosan was calculated based on the molar extinction
coefficient value, which was determined by UV-1601
spectrophotometer (Shimadzu Corporation, Kyoto, Japan) at 363
nm.
Preparation and physicochemical
characterization of FA-CS-LZ-NPs
FA-CS-LZ-NPs were prepared according to ionotropic
gelation method in the following steps. LZ (20 mg) was added to
different coupled numbers of FA-CS solution (2 mg/ml) in 20 ml 1%
(w/w) acetic acid (pH 5.0) at room temperature. TPP (1 mg/ml; 5 ml)
was added into the FA-CS solution and stirred using a magnetism
mixer (200 rpm/min; 2,000 × g) at room temperature for 1 h. The
mixture was then centrifuged at 100,000 × g for 30 min, the
supernatant was collected and the encapsulation efficiency (EE) and
loading efficiency (LE) were determined. The precipitate was
dispersed in 20 ml deionized water and adjusted 2 mg/ml, and
FA-CS-LZ-NPs were isolated by lyophilization. The particle sizes
and poly dispersities of FA-CS-LZ-NPs diluted in deionized water
were determined using dynamic light scattering system Zetasizer
Nano ZS90 (Malvern Instruments, Ltd., Malvern, UK) in
triplicate.
FA-CS-LZ-NPs were dissolved with deionized water to
1 mg/ml and adjusted to neutral pH. One-drop sample was placed on a
carbon coated film 300 mesh copper grid and allowed to sit for 5
min or until air-dried. The sample was stained with 1 M
phosphotungstic acetate solution for 5 min, and any excess
phosphotungstic acetate was removed with filter paper.
Morphological characteristics of the nanoparticles were examined
using a high resolution transmission electron microscope (TEM;
JEM-2000EX; JEOL, Ltd., Tokyo, Japan).
Determination of EE and LE
The supernatant collected during the final stages of
FA-CS-LZ-NPs synthesis as described above was analyzed to determine
the amount of free LZ by high performance liquid chromatography
(HPLC; programmable solvent module 125; Beckman Coulter, Inc.,
Brea, CA, USA). The chromatographic conditions used were as
follows: HypersilODS-C18 column (250×4.6 mm, 5 µm); A,
methanol; B, water (A:B=60:40, v:v, HPLC grade); wavelength, 280
nm; flow rate, 1.0 ml/min; and 25°C. EE and LE were calculated as
follows: LZ EE (%)=(WtotalLZ -Wfree
LZ)/WtotalLZ × 100; and LZ LE
(%)=(WtotalLZ-Wfree LZ)/WNPs ×
100. WtotalLZ was the total amount of added LZ;
Wfree LZ was the amount of free LZ in the supernatant;
and WNPs was the weight of FA-CS-LZ-NPs after
lyophilization.
In vitro LZ release
FA-CS-LZ-NPs (100 mg) was resuspended in 50 ml
deionized water for injection and dialyzed using a membrane
dialysis bag (molecular weight cut-off, 12 kDa) in PBS at pH 5.0,
7.4 and/or 9.0 at 37±0.5°C with continuous stirring. Dialysis
buffer (5 ml) was drawn out of the dialysis bag at regular
intervals. The concentration of the released LZ in the solutions
collected at different time interval was determined by HPLC.
Cytotoxicity assay
MCF-7 (FR-positive) (11) and A549 (FR-negative) cells were
seeded at a density of 1×104/well in 100 µl culture media in a
96-well culture plate and incubated for 24 h. Different
concentrations (1, 0.5, 0.25, 0.05 or 0.01 mg/ml) of FA-CS-LZ-NPs
were added to the cells and incubated for 48 h at 37°C. MTT
solution (0.5%, 10 µl) was added to the cells in each well and
incubated for another 4 h and 100 µl DMSO was added. Absorbance was
detected on a microplate reader at 492 nm. The cell viability (%)
was calculated as [optical density (OD) of treated group/OD of
control group] × 100.
Intracellular uptake of
FA-CS-LZ-NPs
MCF-7 or A549 cells were seeded at a density of
1×104/well in 12-well culture plate. FA-CS-LZ-NPs, CS-LZ-NPs and LZ
solution (the final LZ concentration of 50 µg/ml in each group)
were added to the wells and cultured for 1, 2, 4, 6 or 8 h.
Subsequently, the cells were rinsed with PBS three times, digested
with 0.25% trypsin, and centrifuged at 2,000 × g. Methanol (100 µl)
was added into cell suspension, the cell mixture was freeze-thawed
repeatedly. The LZ concentration of each sample was determined by
HPLC.
The autofluorescence intensity of LZ in the cells
was monitored by a SP5 laser scanning confocal microscopy
(NanoFocus AG, Oberhausen, Germany). The excitation and emission
wavelengths of LZ were 295 and 345 nm, respectively. MCF-7 and A549
cells were seeded into 35 mm cell culture plates at a density of
1×105/plate. The MCF-7 cells were added with 100 µl
FA-CS-LZ-NPs, CS-LZ-NPs or LZ solution (final LZ concentration, 50
µg/ml in each group) and cultured for 4 h, with A549 cells with 100
µl FA-CS-LZ-NPs (final LZ concentration, 50 µg/ml) as the negative
control. The green fluorescence of LZ was monitored at excitation
wavelength 295 nm.
FA-CS-LZ-NPs targeting in MCF-7
cells
MCF-7 and A549 cells were cultured in FA-depleted
DMEM for 1 week, seeded at a density of 1×105/well in a 12-well
plate and incubated for 24 h. Then, the cells were treated with
folate (0.2 µg) or untreated. Subsequently, MCF-7 cells were
treated with 100 µl FA-CS-LZ-NPs, CS-LZ-NPs or LZ solution at 50
µg/ml for 4 h. A549 cells were treated with 100 µl FA-CS-LZ-NPs (50
µg/ml) for 4 h. The LZ concentration for each sample was determined
by HPLC.
Statistical analysis
All experiments were repeated at least three times
and the data are presented as the mean ± standard deviation.
Tukey's test was performed to determine statistical significance
and a P<0.05 was considered to indicate a statistically
significant difference.
Ethical statement
The experimental protocol was approved by the Ethics
Committee of the Second Hospital of Dalian Medical University
(Dalian, China).
Results
Chemical structure of FA-CS
The functional groups of FA-CS were prepared by an
amino-acylation reaction and characterized by infrared
spectroscopy. Folate-modified chitosan (Fig. 2A) was contrasted to either folate
or chitosan alone (Fig. 2B and C).
The basic characteristics of IR bands of chitosan at 3,372 cm-1 was
attributed to the stretching vibration of O-H and N-H (Fig. 2B). The strong bands observed at
1642 and 1,600 cm-1 were attributed to the bending vibration of N-H
(Fig. 2C). Not only the
characteristic bands of the original chitosan but also the
characteristic bands of folate were observed by the FA-CS IR
spectrum (Fig. 2A). Compared with
the chitosan spectrum, a new IR band at 1,560 cm-1, which shows an
amide linkage was identified in folate modified chitosan.
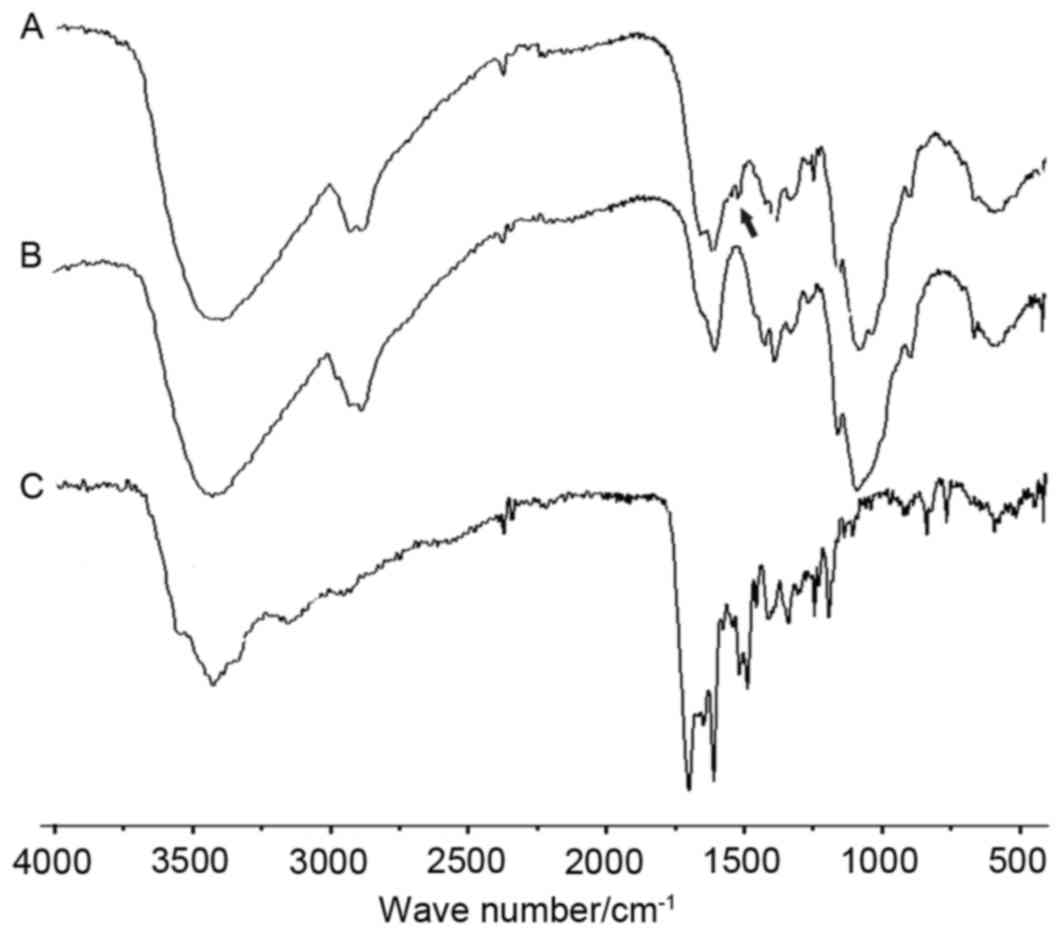 | Figure 2.IR spectrum of (A) FA-CS, (B) CS and
(C) FA. IR (KBr): 3,372 cm−1 (O-H, N-H stretching
vibration), 2,924, 2,880 cm−1 (C-H stretching
vibration), 1,642, 1,600 cm−1 (N-H in-plane bending
vibration), 1,420 cm−1 (−CH2, -CH3
deformation vibration), 1,560 cm−1 (O=C-N stretching
vibration), 1,070 cm−1 (O-H deformation vibration),
1,028 cm−1 (C-O stretching vibration), 1,158, 896
cm−1 (β-glycosidic bond). FA-CS had a new band at 1,560
cm−1, which stands for amide linkage. FA, folate; CS,
chitosan; IR, infrared spectroscopy. |
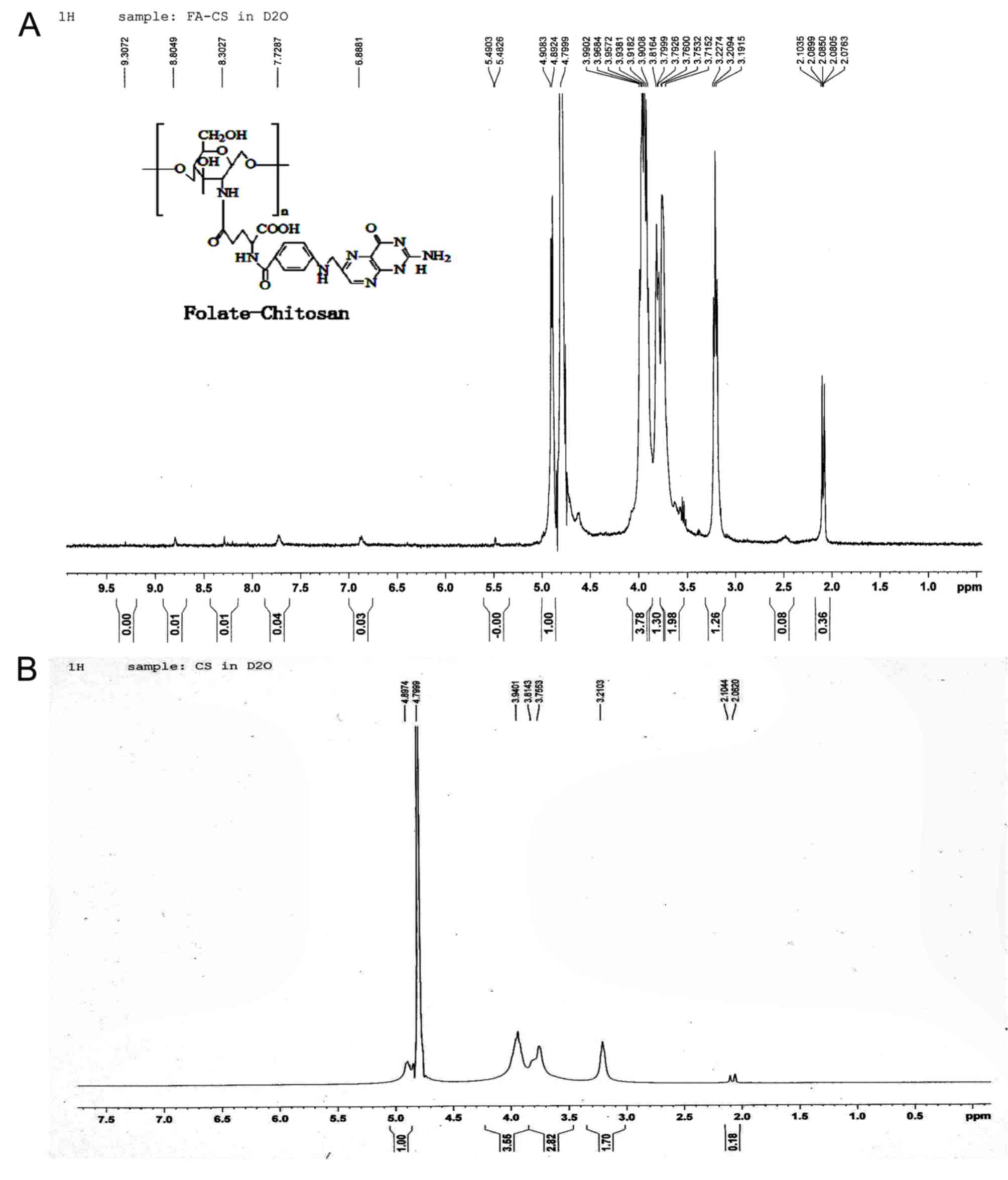
The 1H NMR spectrum of FA-CS (Fig. 3A) and chitosan (Fig. 3B) were compared, and observed that
the 1H NMR (D2O) δ spectrum of FA-CS
exhibited the new peaks at 2.5 (br s, β and γ-CH2-groups, 4H), 7.7
ppm (benzene ring), 6.9 (s, aromatic H of pteridine) and 8.8 (s,
OH, 1H) as presented in Fig. 3.
The two peaks 4.3 (br s, NH2, 2H) and 4.5 (d, -NH-, 1H) of FA-CS
are not vivid in the spectrum because of the high molecular weight
of FA-CS (50 kDa). The appearance of these peaks confirmed the
successful conjugation of folate with chitosan.
The degree of the substitution of folate on chitosan
was calculated by UV spectrophotometry. The folate concentration
conjugated with chitosan was directly proportional to the coupled
ratio and coupled number (Table
I). Coupled ratio of folate and chitosan of 170:1 (mol/mol),
the number of folate in each chitosan (molecular weight
5×104 Da), achieved the highest coupled number of
34.
 | Table I.Coupled number of folate on chitosan
at different ratios. |
Table I.
Coupled number of folate on chitosan
at different ratios.
| Folate/mol | Chitosan/mol | Coupled ratio | Coupled number |
|---|
|
9 | 1 | 0.035 | 4 |
| 17 | 1 |
0.1023 | 12 |
| 45 | 1 |
0.1936 | 22 |
| 56 | 1 |
0.1862 | 21 |
| 113 | 1 |
0.2432 | 27 |
| 170 | 1 |
0.2929 | 34 |
Based on the results of IR spectroscopy,
1H NMR spectrums and UV spectrophotometer, it was
concluded that folate was conjugated to chitosan.
Physicochemical characterization of
FA-CS-LZ-NPs
FA-CS-LZ-NPs were prepared with different coupled
number of folate-modified chitosan. The EE, LE and mean diameter
size (MD) of FA-CS-LZ-NPs were decreased with the increasing
coupled numbers in FA-CS (Table
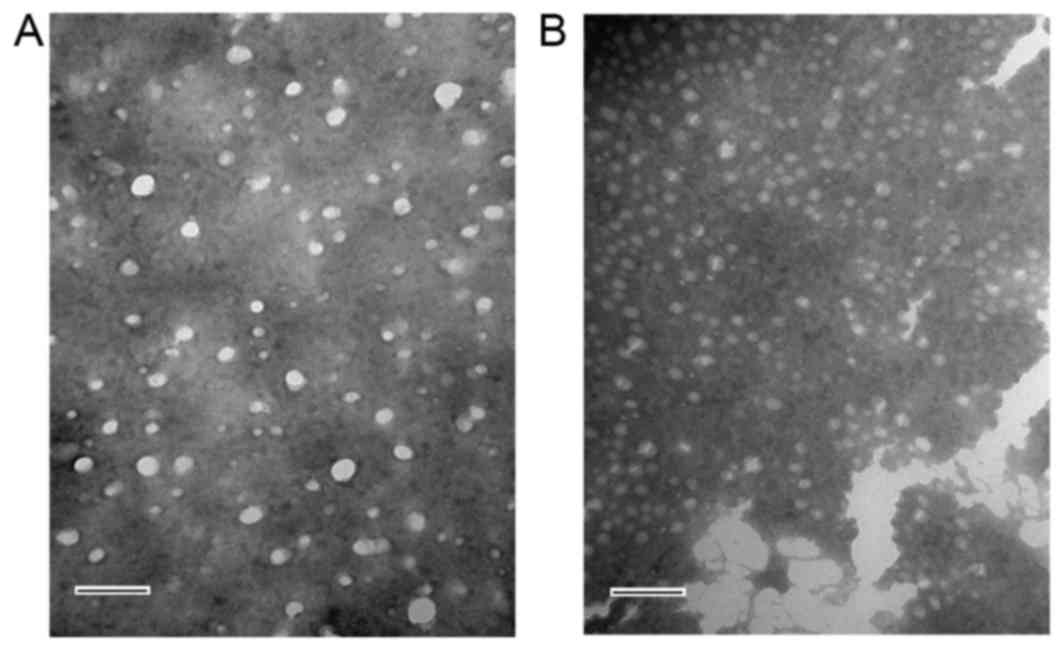
II). The morphology of FA-CS-LZ-NPs was determined by TEM
(Fig. 4). FA-CS-LZ-NPs and
FA-CS-NPs had uniform spherical morphology, and the distribution of
particle size was in the range of 100–200 nm. These results were in
agreement with the MDs of the prepared nanoparticles as determined
by dynamic light scattering measurements using a Zetasizer Nano
ZS90.
 | Table II.EE, LE and MD of different coupled
number FA-CS-LZ-NPs (n=3). |
Table II.
EE, LE and MD of different coupled
number FA-CS-LZ-NPs (n=3).
| Coupled number | EE (%) | LE (%) | MD (nm) |
|---|
| 4 | 67.4±0.97 | 18.1±0.68 | 221.8±1.22 |
| 12 | 63.8±0.34 | 16.9±0.21 | 194.0±0.70 |
| 22 | 59.6±0.23 | 15.3±0.16 | 182.7±0.56 |
| 34 | 55.7±0.27 | 13.8±0.18 | 174.9±0.91 |
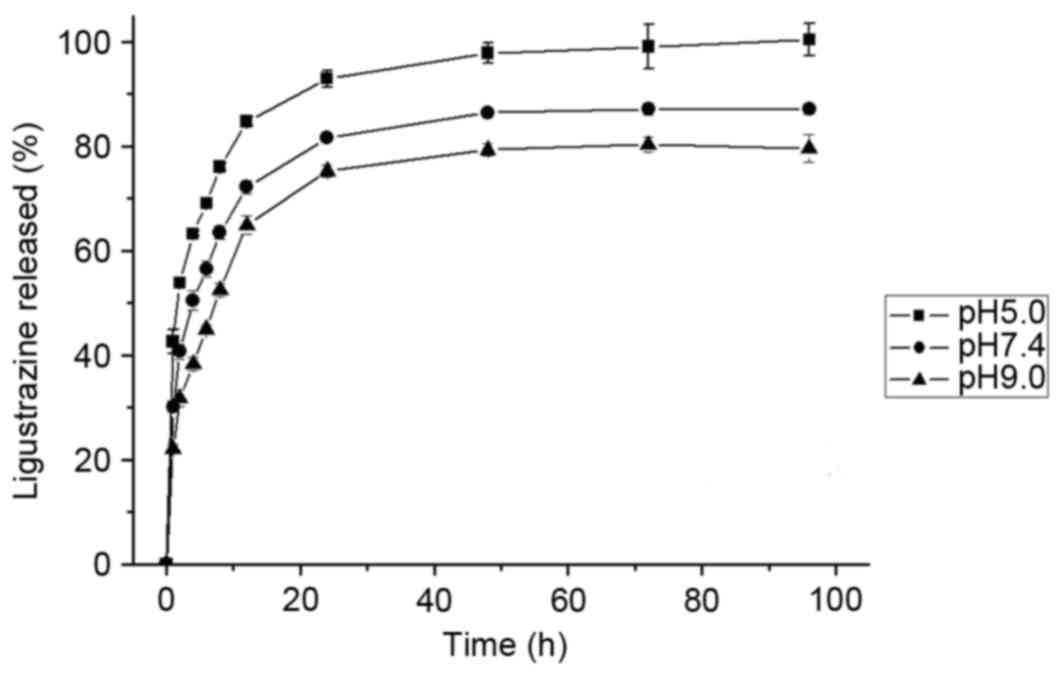
In vitro release of LZ
The release of LZ from FA-CS-LZ-NPs in PBS at pH
5.0, 7.4 or 9.0 was investigated. The percentages of LZ released
are presented in Fig. 5. The LZ
released reached equilibrium at the end of 4 days. The cumulative
release proportion was ~95%. The pH value of dissolution medium was
found to affect the release rate of FA-CS-LZ-NPs. The LZ release
rate from the nanoparticles increased with decrease in pH of
dissolution medium, suggesting that LZ release from FA-CS-LZ-NPs
may be higher in in a weak acidic tumor microenvironment.
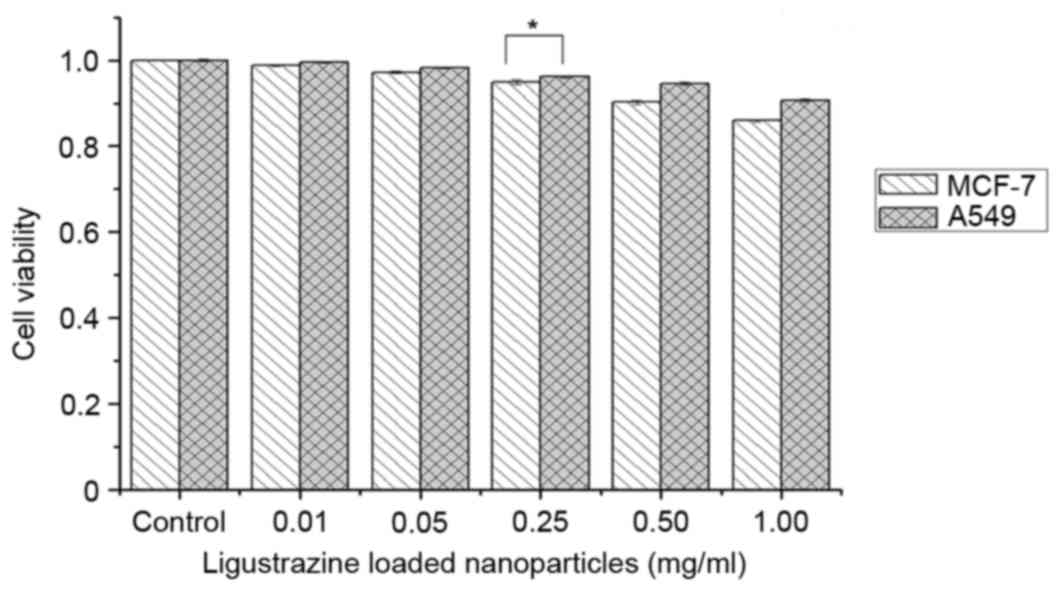
In vitro toxicity assessment
Cytotoxicity of the FA-CS-LZ-NPs in MCF-7 and A549
cells was evaluated using MTT assay. The non-toxic dose was
determined as the concentration at which cell viability was
>95%. The non-toxic dose of FA-CS-LZ-NPs in MCF-7 cells was 0.25
mg/ml, and the non-toxic dose of LZ in the two cell types was 50
µg/ml. Therefore, these dosages were chosen for all subsequent
experiments. The toxicity of FA-CS-LZ-NPs was not significantly
different between the cell lines (Fig.
6).
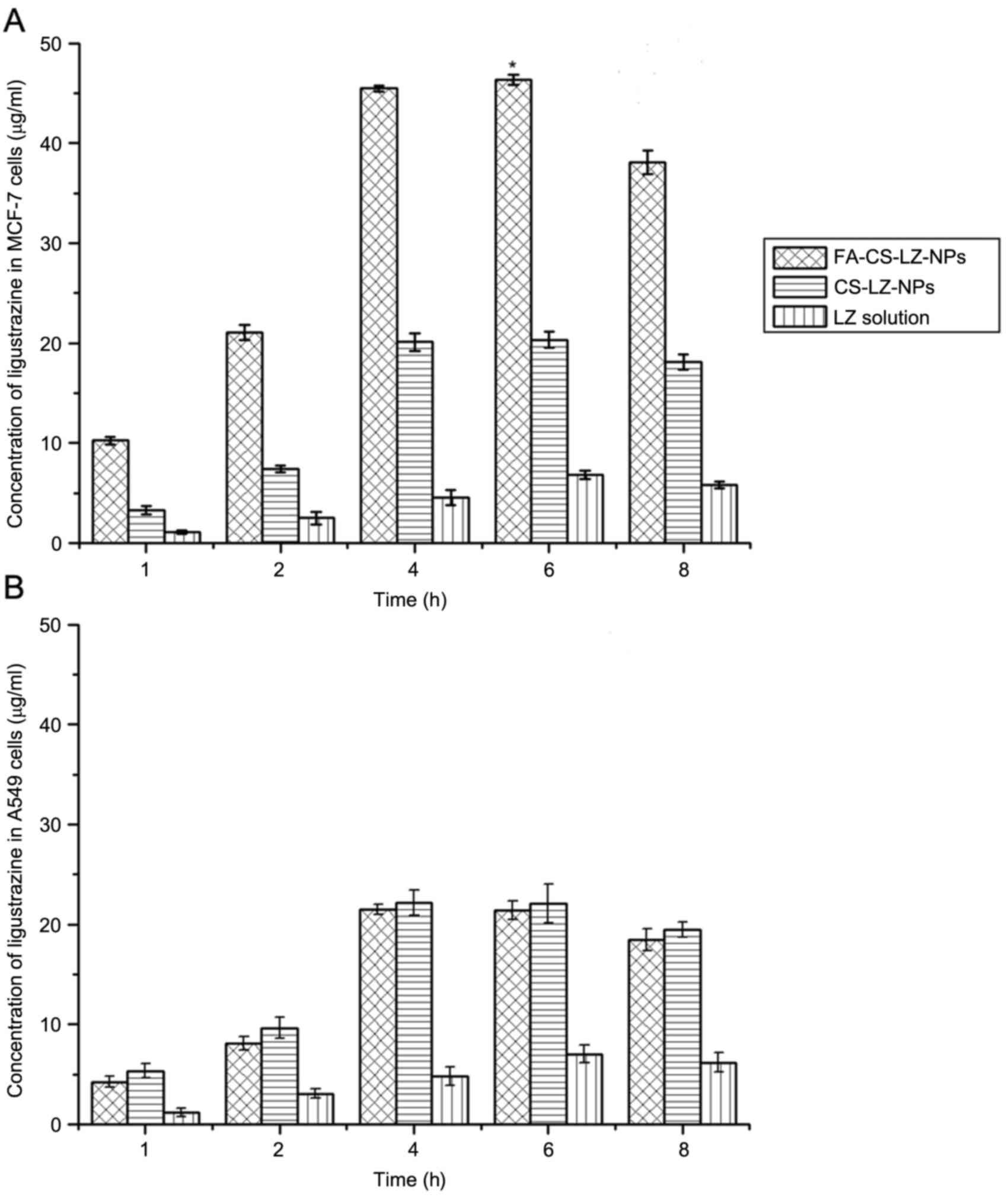
Intracellular uptake of
FA-CS-LZ-NPs
MCF-7 and A549 cells were incubated with
FA-CS-LZ-NPs, CS-LZ-NPs or LZ alone solution, at a concentration of
50 µg/ml LZ in each group. The LZ concentration of each sample was
determined by HPLC in at 1, 2, 4, 6, or 8 h after incubation with
MCF-7 and A549 cells. The intracellular concentration of LZ in
cells treated with FA-CS-LZ-NPs was significantly higher than cells
treated with CS-LZ-NPs or the LZ solution only. The LZ
concentration increased between 1 and 4 h, and reached a peak at 6
h (Fig. 7A); however, no
significant difference in the intracellular LZ concentration was
observed between the FA-CS-LZ-NP and CS-LZ-NP-treated A549 cells
(Fig. 7B). Additionally, at 4 h,
the intracellular LZ (45.47±0.32 µg/ml) in MCF-7 cells treated with
the FA-CS-LZ-NPs was significantly higher than that in the A549
cells (20.10±0.92 µg/ml). These results indicated that FA-CS-LZ-NPs
were able to specifically target and deliver LZ into FR-positive
MCF-7 cells.
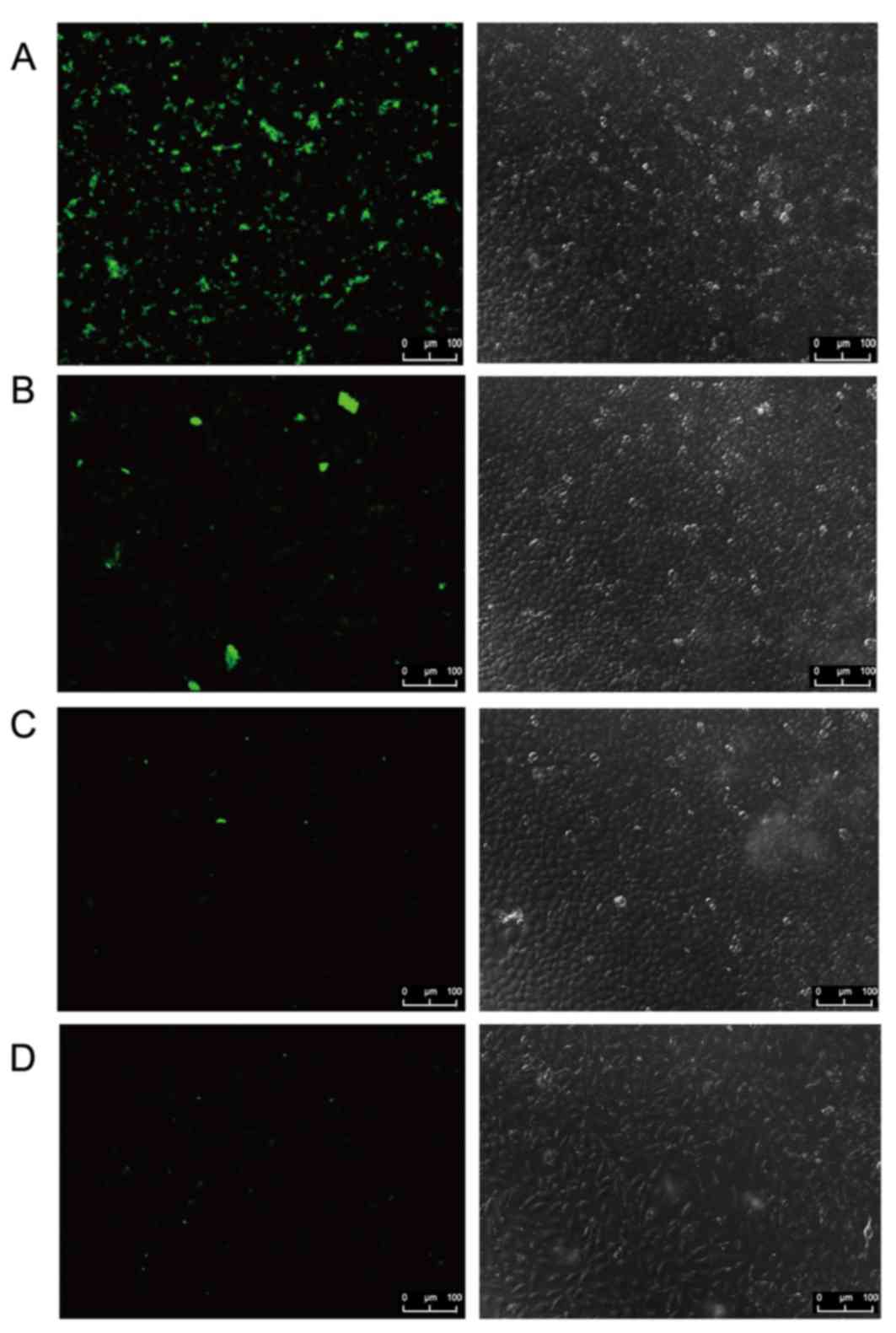
The intracellular uptake efficiency of LZ in MCF-7
cells and A549 cells was also investigated using laser confocal
microscopy. Fluorescence intensity of LZ in MCF-7 cells treated
with FA-CS-LZ-NPs was higher than that in MCF-7 cells treated with
CS-LZ-NPs, LZ solution and the FR-negative A549 cells treated with
FA-CS-LZ-NPs, indicating that FA-CS-LZ-NPs facilitated
intracellular uptake of LZ (Fig.
8).
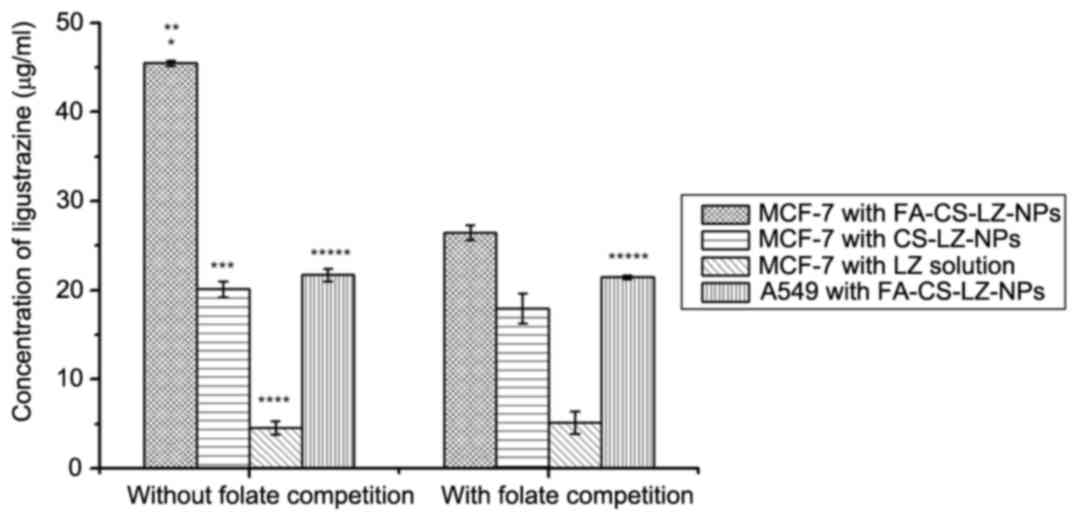
FR specific targeting of MCF-7 cells
by FA-CS-LZ-NPs
To further explore the mechanism by which
FA-CS-LZ-NPs target the tumor cells, a folate competition assay was
performed and the intracellular concentration of LZ was determined
by HPLC (Fig. 9). Under folate
deficient conditions, the intracellular concentration of LZ in
MCF-7 cells treated with FA-CS-LZ-NPs was significantly higher than
that of the CS-LZ-NPs and LZ solution group. By contrast, the LZ
concentration in the MCF-7 cells cultured with folate and treated
with FA-CS-LZ-NPs markedly decreased compared to MCF-7 cells with
FA-CS-LZ-NPs in folate deficient conditions. However, these
differences were not observed in FR-negative A549 cells.
Furthermore, addition of folate did not obviously affect the
intracellular uptake LZ in cells that were treated with CS-LZ-NPs
or LZ solutions. These results suggested that FA-CS-LZ-NPs were
internalized via FR.
Discussion
NPs have been extensively investigated as a delivery
system to achieve site-specific delivery of cytotoxic anticancer
agents. Chitosan was selected as the nanoparticle carrier material
in the present study as its amino groups can conjugate with
moieties specific for a cell type or tumor cells (15). Furthermore, chitosan modified with
special materials, including folate and arginylglycylaspartic acid
polypeptides, functions as an active receptor-mediated targeted
drug delivery system. Folate is a stable, inexpensive and poorly
immunogenic chemical with a high affinity for FR (16). FA-CS-NPs have been loaded with
anticancer agents, such as doxorubicin (17), 5-fluorouracil (18) and used as DNA (19) delivery vehicle, in previous
studies. LZ has been reported to have anticoagulation,
antioxidation and calcium antagonism properties, thus, is widely
used in the management of cardiovascular and cerebrovascular
diseases (20). In addition, a
number of studies have reported that LZ has an important role in
reversing MDR (2,21). In the current study, FA-CS-NPs were
used as a vehicle to deliver a natural product (LZ) with the
potential to reverse MDR.
In this study, chitosan NPs were modified by folate
and loaded with LZ to improve the targeting specificity of NPs to
FR-positive cells. The synthesized FA-CS-LZ-NPs had desirable
diameter, encapsulation efficiency and uniform spherical
morphology. The average size of FA-CS-LZ-NPs with 22
folate-modified chitosan was 182.7±0.56 nm and those with 34 folate
modified chitosan were 174.9±0.91 nm. The size and surface charge
of nanoparticles could be changed by the concentration ratio of
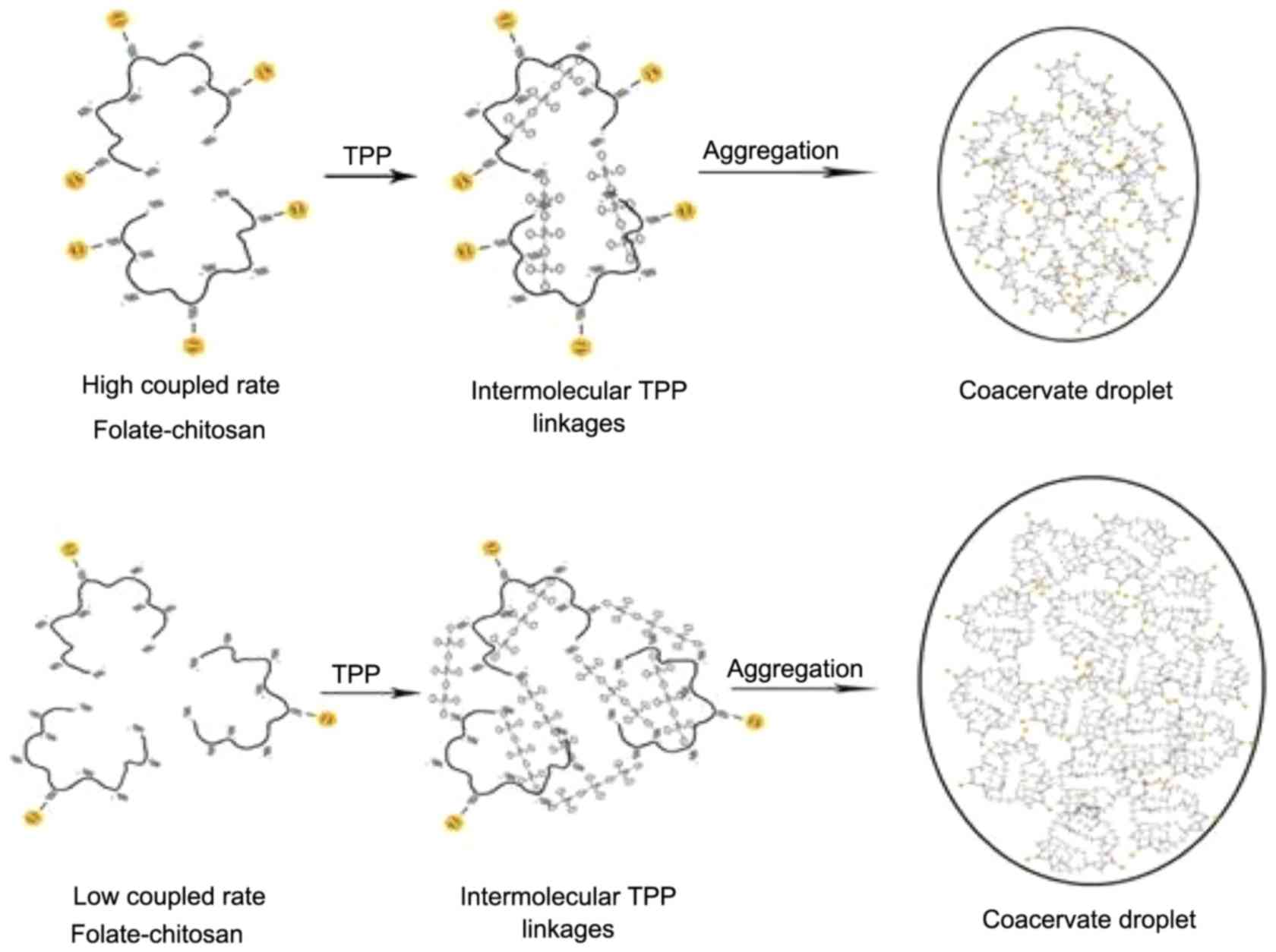
chitosan, folate and TPP. Fewer folate on one chitosan chain
increased the size of nanoparticles because of the free amino
groups and low steric hindrance of the folate (Fig. 10). The size of FA-CS-LZ-NPs (182.7
nm) were smaller than folate-modified carboxymethyl chitosan NPs
diameter (267.8 nm) (22) and
folic acid-conjugated human serum albumin nanoparticles diameter
(239 nm) (23). The size of
FA-CS-NPs (182.7 nm) is appropriate for LZ delivery, in contrast
with folate-conjugated hyaluronic acid polymeric micelles at size
191.9 nm are effective in paclitaxel delivery (24). It was observed that pH affects the
release rate of LZ from FA-CS-LZ-NPs. The faster drug release rate
at lower pH may be due to the stronger protonation of amino groups
of chitosan which caused the loose nanoparticle structure and
higher solubility of LZ at lower pH. The LZ release rate from the
nanoparticles increased with a decrease in pH (5.0) of the
dissolution medium, suggesting that LZ release from FA-CS-LZ-NPs
may be higher in a weak acidic microenvironment. As the tumor
microenvironment is slightly acidic (25), FA-CS-LZ-NPs may have potential as
an anticancer system. The cell viability profile assessed by MTT
assay demonstrated that FA-CS-NPs had no significant cytotoxicity
at concentrations as high as 1 mg/ml, so FA-CS-LZ-NPs may be a safe
tool that could potentially overcome MDR during cancer therapy.
FA-CS-NPs targeted MCF-7 cells via FR without obvious cytotoxicity.
In fact, chitosan NPs were demonstrated to be a suitable and
controlled drug delivery system without significant cytotoxicity in
liver cells (26), and folate gold
nanoparticles do not induce significant cytotoxicity in MCF-7 cells
(27). In this study, internalized
FA-CS-LZ-NPs detected in MCF-7 cells cultured with folate may be
attributed to the fact that folate at 0.2 µg/ml occupied all the
folate receptors on the cells. The intracellular concentration of
LZ in MCF-7 cells following exposure to FA-CS-LZ-NPs was
significantly higher than that of cells exposed to CS-LZ-NPs and LZ
alone. Folate and FR may be the main cause of FA-CS-LZ-NP cellular
uptake. FA-CS-NPs may be a more suitable vehicle, compared with
CS-NPs, for overcoming tumor drug resistance due to its high
cellular uptake specificity by FR-expressing cells.
In conclusion, the FA-CS-LZ-NPs developed in the
current study had suitable diameter, high EE and LE, uniform
spherical morphology, and slow release in vitro, but no
cytotoxicity. FA-CS-LZ-NPs may be a potential tool to target
FR-positive cancer cells and thus, a promising candidate for
overcoming MDR.
Acknowledgements
This work was funded by the Science and Technology
Planning Project of Liaoning Province (grant no. 2012225020).
References
|
1
|
Ji XX, Song XL, Qian W, Yu XL and Zhu JY:
Effects and mechanism of action of ligustrazine on
isoprenaline-induced cardiomyocyte hypertrophy. Cell Biochem
Biophys. 70:1513–1518. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen J, Wang W, Wang H, Liu X and Guo X:
Combination treatment of ligustrazine piperazine derivate DLJ14 and
adriamycin inhibits progression of resistant breast cancer through
inhibition of the EGFR/PI3K/Akt survival pathway and induction of
apoptosis. Drug Discov Ther. 8:33–41. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xu K, Wang P, Xu X, Chu F, Lin J, Zhang Y
and Lei H: An overview on structural modifications of ligustrazine
and biological evaluation of its synthetic derivatives. Res Chem
Intermed. 41:1385–1411. 2015. View Article : Google Scholar
|
|
4
|
Fan Q, Fan GJ, Zhao JY and Yang PM: Study
on effect reversing MDR of ligustrazine liposomes on human
erythroleukemia-cell-line K562/ADM. China Pharmacist. 7:753–755.
2004.
|
|
5
|
Fan GJ, Fan Q and Leng DW: Preparation and
quality evaluation of ligustrazine liposomes. Pharm Care Res.
8:453–455. 2008.
|
|
6
|
Yao Q, Liu W, Gou XJ, Guo XQ, Yan J, Song
Q, Chen FZ, Zhao Q, Chen C and Chen T: Preparation,
characterization, and cytotoxicity of various chitosan
nanoparticles. J Nanomater. 2013:142013. View Article : Google Scholar
|
|
7
|
Li P, Wang Y, Zeng F, Chen L, Peng Z and
Kong LX: Synthesis and characterization of folate conjugated
chitosan and cellular uptake of its nanoparticles in HT-29 cells.
Carbohydr Res. 346:801–806. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Milane L, Duan ZF and Amiji M:
Pharmacokinetics and biodistribution of lonidamine/paclitaxel
loaded, EGFR-targeted nanoparticles in an orthotopic animal model
of multi-drug resistant breast cancer. Nanomedicine. 7:435–444.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Danhier F, Vroman B, Lecouturier N,
Crokart N, Pourcelle V, Freichels H, Jérôme C, Marchand-Brynaert J,
Feron O and Préat V: Targeting of tumor endothelium by RGD-grafted
PLGA-nanoparticles loaded with paclitaxel. J Control Release.
140:166–173. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shen Z, Li Y, Kohama K, Oneill B and Bi J:
Improved drug targeting of cancer cells by utilizing actively
targetable folic acid-conjugated albumin nanospheres. Pharmacol
Res. 63:51–58. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang H, Zhao P, Liang X, Gong X, Song T,
Niu R and Chang J: Folate-PEG coated cationic modified
chitosan-cholesterol liposomes for tumor-targeted drug delivery.
Biomaterials. 31:4129–4138. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shi Z, Guo R, Li W and Zhang Y, Xue W,
Tang Y and Zhang Y: Nanoparticles of deoxycholic acid, polyethylene
glycol and folic acid-modified chitosan for targeted delivery of
doxorubicin. J Mater Sci Mater Med. 25:723–731. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Song H, Su C, Cui W, Zhu B, Liu L, Chen Z
and Zhao L: Folic acid-chitosan conjugated nanoparticles for
improving tumor-targeted drug delivery. Biomed Res Int.
2013:7231582013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhou J, Wang J, Xu Q, Xu S, Wen J, Yu Z
and Yang D: Folate-chitosan-gemcitabine core-shell nanoparticles
targeted to pancreatic cancer. Chin J Cancer Res. 25:527–535.
2013.PubMed/NCBI
|
|
15
|
Nagpal K, Singh SK and Mishra DN: Chitosan
nanoparticles: A promising system in novel drug delivery. Chem
Pharm Bull (Tokyo). 58:1423–1430. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hou Z, Zhan C, Jiang Q, Hu Q, Li L, Chang
D, Yang X, Wang Y, Li Y, Ye S, et al: Both FA- and mPEG-conjugated
chitosan nanoparticles for targeted cellular uptake and enhanced
tumor tissue distribution. Nanoscale Res Lett. 6:5632011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee KD, Choi SH, Kim DH, Lee HY and Choi
KC: Self-organized nanoparticles based on chitosan-folic acid and
dextran succinate-doxorubicin conjugates for drug targeting. Arch
Pharm Res. 37:1546–1553. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li HL, He YX, Gao QH and Wu GZ:
Folate-polyethylene glycol conjugated carboxymethyl chitosan for
tumor-targeted delivery of 5-fluorouracil. Mol Med Rep. 9:786–792.
2014.PubMed/NCBI
|
|
19
|
Mansouri S, Cuie Y, Winnik F, Shi Q,
Lavigne P, Benderdour M, Beaumont E and Fernandes JC:
Characterization of folate-chitosan-DNA nanoparticles for gene
therapy. Biomaterials. 27:2060–2065. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen L, Lu Y, Wu JM, Xu B, Zhang LJ, Gao
M, Zheng SZ, Wang AY, Zhang CB, Zhang WW and Lei N: Ligustrazine
inhibits B16F10 melanoma metastasis and suppresses angiogenesis
induced by vascular endothelial growth factor. Biochem Biophys Res
Commun. 386:374–379. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang XG and Jiang C: Ligustrazine as a
salvage agent for patients with relapsed or refractory
non-Hodgkin's lymphoma. Chin Med J (Engl). 123:3206–3211.
2010.PubMed/NCBI
|
|
22
|
Tan YL and Liu CG: Preparation and
characterization of self-assembled nanoparticles based on folic
acid modified carboxymethyl chitosan. J Mater Sci Mater Med.
22:1213–1220. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ulbrich K, Michaelis M, Rothweiler F,
Knobloch T, Sithisarn P, Cinatl J and Kreuter J: Interaction of
folate-conjugated human serum albumin (HSA) nanoparticles with
tumour cells. Int J Pharm. 406:128–134. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu Y, Sun J, Cao W, Yang J, Lian H, Li X,
Sun Y, Wang Y, Wang S and He Z: Dual targeting folate-conjugated
hyaluronic acid polymeric micelles for paclitaxel delivery. Int J
Pharm. 421:160–169. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Du J, Lane LA and Nie S:
Stimuli-responsive nanoparticles for targeting the tumor
microenvironment. J Control Release. 219:205–214. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Loh JW, Yeoh G, Saunders M and Lim LY:
Uptake and cytotoxicity of chitosan nanoparticles in human liver
cells. Toxicol Appl Pharmacol. 249:148–157. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shakeri-Zadeh A, Mansoori GA, Hashemian
AR, Eshghi H, Sazgarnia A and Montazerabadi AR: Cancerous cells
targeting and destruction using folate conjugated gold
nanoparticles. Dyn Biochem Process Biotechnol Mol Biol. 4:6–12.
2010.
|