Introduction
Tuberculosis is a chronic, transmittable disease
caused by Mycobacterium tuberculosis. Approximately
one-third of the world's population is estimated to be infected
with tuberculosis (1–3). Osteoarticular tuberculosis is
recognized as the most common site of extrapulmonary tuberculosis,
accounting for ~35–50% of extrapulmonary tuberculosis and 3–5% of
the total tuberculosis (4,5). The traditional oral anti-tuberculosis
agents do not result in satisfactory treatment of osteoarticular
tuberculosis, due to the low drug concentration achieved locally in
the tuberculosis lesion and a series of side effects (6,7).
The combination of drug-delivery systems and bone
tissue repair appears to be the most promising option for
advancement in osteoarticular tuberculosis treatment (8). At present, the reconstruction
implants used for the treatment of bone defects include autogenous
bone, allograft bone and artificial bone. However, autogenous bone
and allograft bone have clinical limitations in the treatment of
osteoarticular tuberculosis (9).
For example, they exhibit low drug loading capacity and have
limited tendency to release the drugs in a sustained manner. To
overcome these disadvantages, researchers developed artificial bone
materials, which have become commonly used carriers for
anti-tuberculosis drugs in the treatment of osteoarticular
tuberculosis (9).
Poly(lactic-co-glycolic acid) (PLGA) is widely used
as a carrier material for controlled drug release (10). It exhibits good biocompatibility
and biodegradability, and delays the release of drugs. Rifapentine
is a highly effective, first-line anti-tuberculosis drug. It is
synthesized in one step from rifampin. Rifapentine has an
antibacterial spectrum similar to that of rifampin; however, it has
a stronger antibacterial activity to M. tuberculosis, a
milder adverse reaction and its drug elimination half-life is
longer than rifampin (11–14). Rifapentine is able to effectively
penetrate into infected bone, dead bone, inflammatory cells and
biofilms formed by bacteria to eradicate bacterial infections
(15). Furthermore, rifapentine
prevents drug resistance (15). An
in vitro study from Wu et al (16) reported that rifapentine-loaded PLGA
microspheres (RPMs) are bioactive and efficient controlled-release
delivery systems, with great promise towards the treatment of
osteoarticular tuberculosis (16).
Hydroxyapatite (HA) is a widely-used replacement
material for bone tissue repairing (17,18).
Bone-like hydroxyapatite (BHA) is a type of carbonate HA, that is
similar to the composition and structure of normal bone. BHA
exhibits good biocompatibility, safety and osteogenic activity
(19–21). Compared with HA, BHA has stronger
biological activity and faster degradability (19). However, pure BHA is not conducive
to processing. When BHA is combined with poly amino acid (PAA),
which has a similar molecular structure to collagen, its strength
and mechanical properties are enhanced. A previous study from our
group has demonstrated that BHA/PAA is effective in osteogenesis
and reconstruction of long segmental bone defects both in
vivo and in vitro (22). Recently, Yan et al (23) reported that RPM-loaded BHA/PAA is
effective in the treatment of rabbit chronic osteomyelitis induced
by Staphylococcus aureus. However, the effects of RPM-loaded
BHA/PAA on osteoarticular tuberculosis have not been examined to
date.
In the present study, the in vivo drug
release characteristics of RPM-loaded BHA/PAA were evaluated on
rabbit model of bone defects. Furthermore, the osteogenesis
induction ability and the side effects of RPM-loaded BHA/PAA were
investigated in vivo. The present study may provide a novel
approach for the clinical treatment of osteoarticular
tuberculosis.
Materials and methods
Preparation of materials
RPMs were prepared through an oil-in-water emulsion
solvent evaporation method, as previously described (16,23).
Briefly, 50 mg of rifapentine (Jinan Mingxin Pharmaceutical Co.,
Ltd., Sichuan, China) was dissolved into the polymer solution and
200 mg of PLGA (Jinan Daigang Biomaterial Co., Ltd., Jinan, China)
was dissolved in 10 ml of dichloromethane. RPMs were prepared with
an entrapment efficiency of 85.78±2.00%, an actual drug loading of
17.16±0.40% and a mean diameter of 25.267 µm. The BHA/PAA materials
(National Nanomaterial Company of Sichuan University, Chengdu,
China) were prepared using the standard atmospheric pressure
solution method (24). RPMs were
composited with BHA/PAA to make RPM-loaded BHA/PAA, with a size of
15×5×5 mm3, a weight of 750.50±8.54 mg and an aperture size of
100–500 µm.
Rabbit model with bone defects
The animal experiments were approved by the Animal
Care Committee of Chongqing Medical University (Chongqing, China).
A total of 66 male New Zealand white rabbits at the age of 4 months
and weighing 2.5–3 kg were obtained from the Experimental Animal
Center of Chongqing Medical University. All rabbits were housed at
17–21°C, with a 12-h light/dark cycle and 30–70% relative humidity.
They had free access to water and food. The rabbits were
anesthetized by intravenous injection of pentobarbital (30 mg/kg)
in the ear margin. The bilateral femoral condyle was shaved and
disinfected. An ~5 mm-depth hole was made by a 4 mm drilling bit
from the femoral lateral condyle to the interior condyle. Rabbits
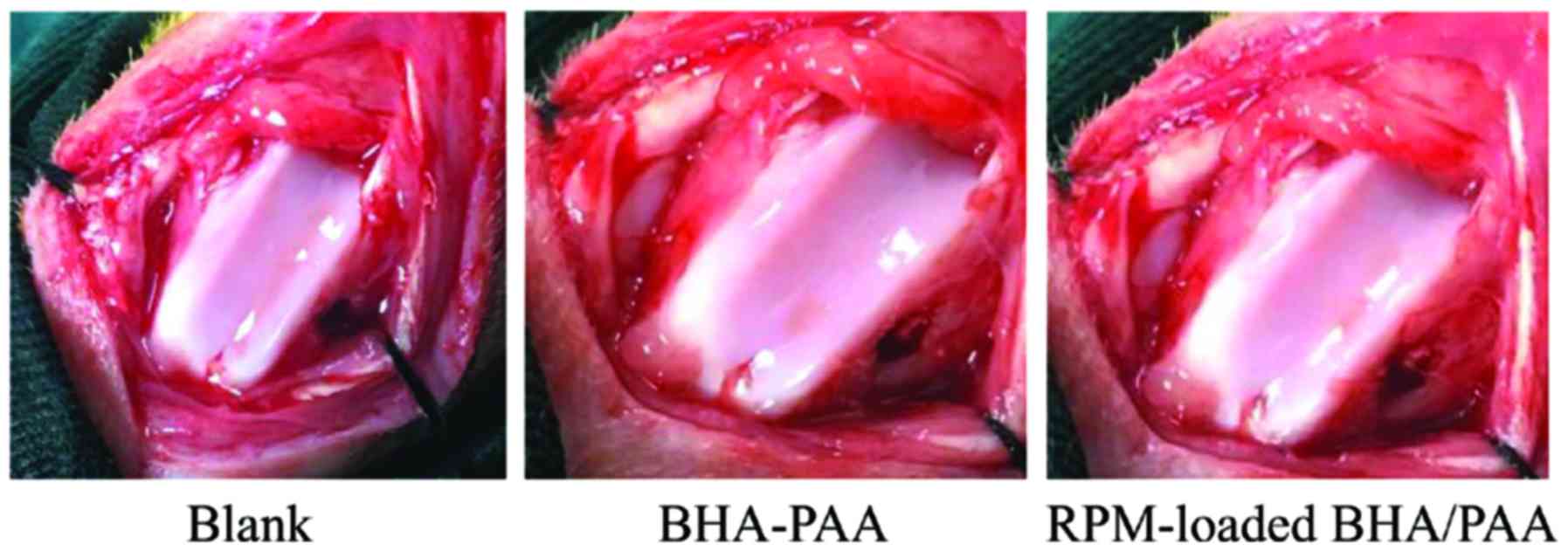
in the BHA/PAA group (n=6) or RPM-loaded BHA/PAA group (n=48) were
implanted with BHA/PAA or RPM-loaded BHA/PAA materials in the
bilateral bone holes, while rabbits in the blank group (n=6) were
not treated with any materials (Fig.
1). Rabbits in the normal group (n=6) did not receive any
treatment. Following washing with saline, the wounds were closed
with sutures layer by layer.
Hematoxylin and eosin (H&E)
staining
A total of 6 rabbits from each group was sacrificed
at the indicated times. Then, the bone, heart, liver and kidney
tissues were collected and fixed in 10% paraformaldehyde overnight,
followed by embedding in paraffin. The paraffin-embedded tissues
were cut into 5 µm thick sections and immersed in xylene followed
by a graded series of ethanol to remove the paraffin. The sections
were then stained with H&E (Beyotime Institute of
Biotechnology, Shanghai, China) and observed under a light
microscope (Nikon Corporation, Tokyo, Japan). Three sections were
prepared and viewed for each tissue sample.
High-performance liquid chromatography
(HPLC)
Concentrations of rifapentine in the plasma and the
local muscle tissues were determined using HPLC. All 48 rabbits in
the RPM-loaded BHA/PAA group were subjected to this assay. A total
of 4 ml blood was collected from the ear margin vein and
centrifuged at 10,000 × g for 5 min at room temperature. The
supernatant (100 µl) was mixed with 1 ml of methanol, followed by
centrifugation at 10,000 × g for 10 min at 4°C. Finally, 20 µl of
the supernatant was collected for the drug concentration assay.
Muscle tissue (~1 g) around the material implanting site was
collected and homogenized, and 500 µl of the homogenate was mixed
with 1.5 ml formaldehyde and vortexed for 2 min. Following
centrifugation at 10,000 × g for 10 min at 4°C, 20 µl of the
supernatant was collected for the drug concentration assay. The
internal standard rifapentine was obtained from the National
Institute for the Control of Pharmaceutical and Biological Products
(Beijing, China). HPLC analysis was performed using a C18 column
(250×4.6 mm; Waters Corporation, Milford, MA, USA) with the mobile
phase of methanol-acetonitrile (5:4 v/v) on a D-2000 HPLC system
(Hitachi, Ltd., Tokyo, Japan). The temperature was set at 40°C, the
flow rate was 0.6 ml/min and the wavelength was 268 nm.
Biochemical analyses
The blood samples (n=6 for each group) were
centrifuged at 1,000 × g and 4°C for 10 min, and the serum was
extracted and stored at −20°C. The concentrations of alanine
aminotransferase (ALT), aspartate aminotransferase (AST), blood
urea nitrogen (BUN) and creatinine (Cr) in the serum were measured
using an Olympus AU2700 Automated Chemistry Analyzer (Olympus
Corporation, Tokyo, Japan).
Statistical analysis
Statistical analysis was performed using SPSS
version 19.0 statistical software (IBM Corp., Armonk, NY, USA) and
the data were expressed as the mean ± standard deviation. The
significance of differences between two groups was analyzed using
Student's t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
General observation after bone
defect
Following surgery, all the rabbits suffered from
lackluster behavior, lameness and decline in appetite; the wound
was red and swollen, and the rabbits lost weight. However, no
mortality was observed. One week following implantation, the
rabbits gradually recovered. The weight of the rabbits in the
RPM-loaded BHA/PAA group increased and returned to the normal
weight after two weeks. The weights of the rabbits in the blank and
BHA/PAA group also increased, but remained lower than normal
(Table I). After 12 weeks, normal
gait was restored in the BHA/PAA and RPM-loaded BHA/PAA groups;
however, the rabbits in the blank group still suffered from
lameness.
 | Table I.The weight of rabbits following
surgery, kg. |
Table I.
The weight of rabbits following
surgery, kg.
|
| 1 week | 2 weeks | 3 weeks | 4 weeks | 8 weeks | 12 weeks |
|---|
| Normal | 2.9±0.1 | 3.1±0.1 | 3.3±0.2 | 3.6±0.3 | 4.3±0.3 | 4.7±0.5 |
| Blank | 2.4±0.2a | 2.7±0.1 | 3.1±0.2 | 3.3±0.3 | 3.8±0.3 | 4.4±0.2 |
| BHA-PAA | 2.3±0.3a | 2.9±0.2 | 3.1±0.1 | 3.2±0.2 | 3.6±0.3 | 4.5±0.3 |
| RPM-loaded
BHA/PAA | 2.3±0.2a | 2.8±0.1 | 3±0.1 | 3.3±0.2 | 3.9±0.4 | 4.2±0.3 |
H&E staining of bone
specimens
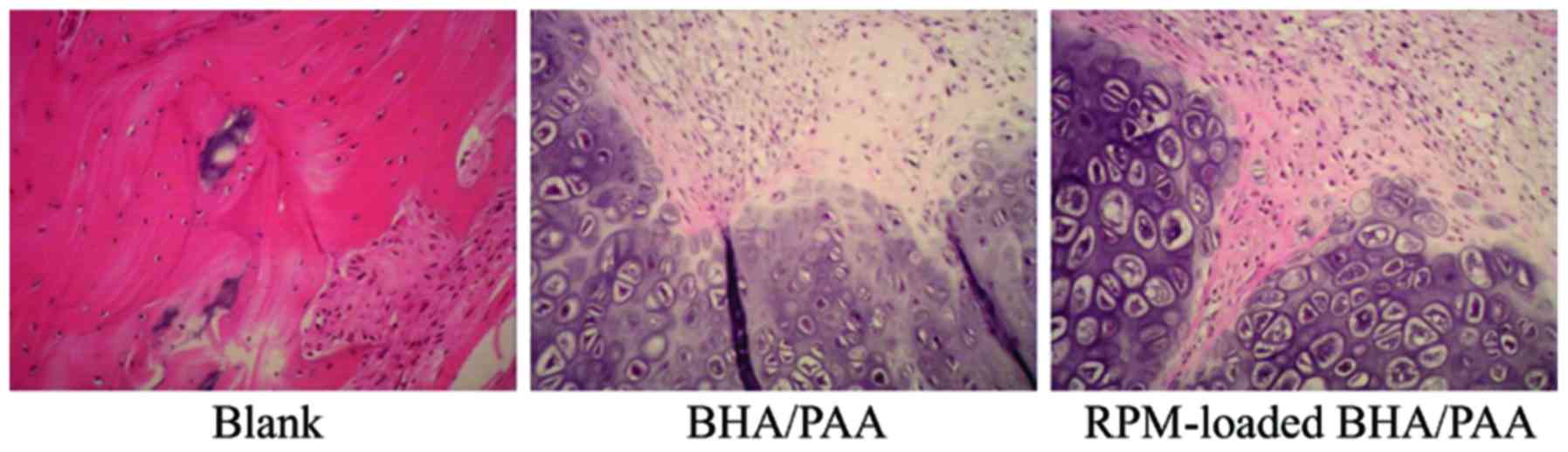
At 12 weeks following implantation, bone specimens
were collected and stained with H&E. As demonstrated in
Fig. 2, a large amount of fibrous
tissue was observed in the blank group. In the BHA/PAA and
RPM-loaded BHA/PAA groups, the implanted materials were degraded
and absorbed, and new bone had formed.
Concentrations of rifapentine in the
plasma and the local muscle tissues
The concentrations of rifapentine detected in the
plasma and muscle tissues of the RPM-loaded BHA/PAA group are
listed in Table II. Rifapentine
concentration in the plasma samples reached the peak level at day 1
following implantation; however, it decreased significantly at day
3 and was undetectable at 2 weeks following implantation (Table II). The concentration of
rifapentine in the muscle tissues around the material implanting
site was also examined. The results revealed that rifapentine
concentration in the muscle tissues was higher than the minimum
inhibitory concentration of rifapentine against M.
tuberculosis (0.12–0.25 mg/l) (25) for at least 12 weeks following
implantation (Table II).
 | Table II.Concentrations of rifapentine in
plasma and local muscle tissues in the RPM-loaded BHA/PAA
group. |
Table II.
Concentrations of rifapentine in
plasma and local muscle tissues in the RPM-loaded BHA/PAA
group.
|
| Concentration of
rifapentine, µg/ml |
|---|
|
|
|
|---|
| Sample | 1 day | 3 days | 1 week | 2 weeks | 3 weeks | 4 weeks | 8 weeks | 12 weeks |
|---|
| Plasma | 5.2 | 1.1 | 0.2 | 0 | 0 | 0 | 0 | 0 |
| Muscle tissues | 12.2 | 8.5 | 8 | 7.5 | 6.3 | 5.1 | 3.8 | 3.1 |
Side effects of RPM-loaded BHA/PAA on
heart, liver and kidney
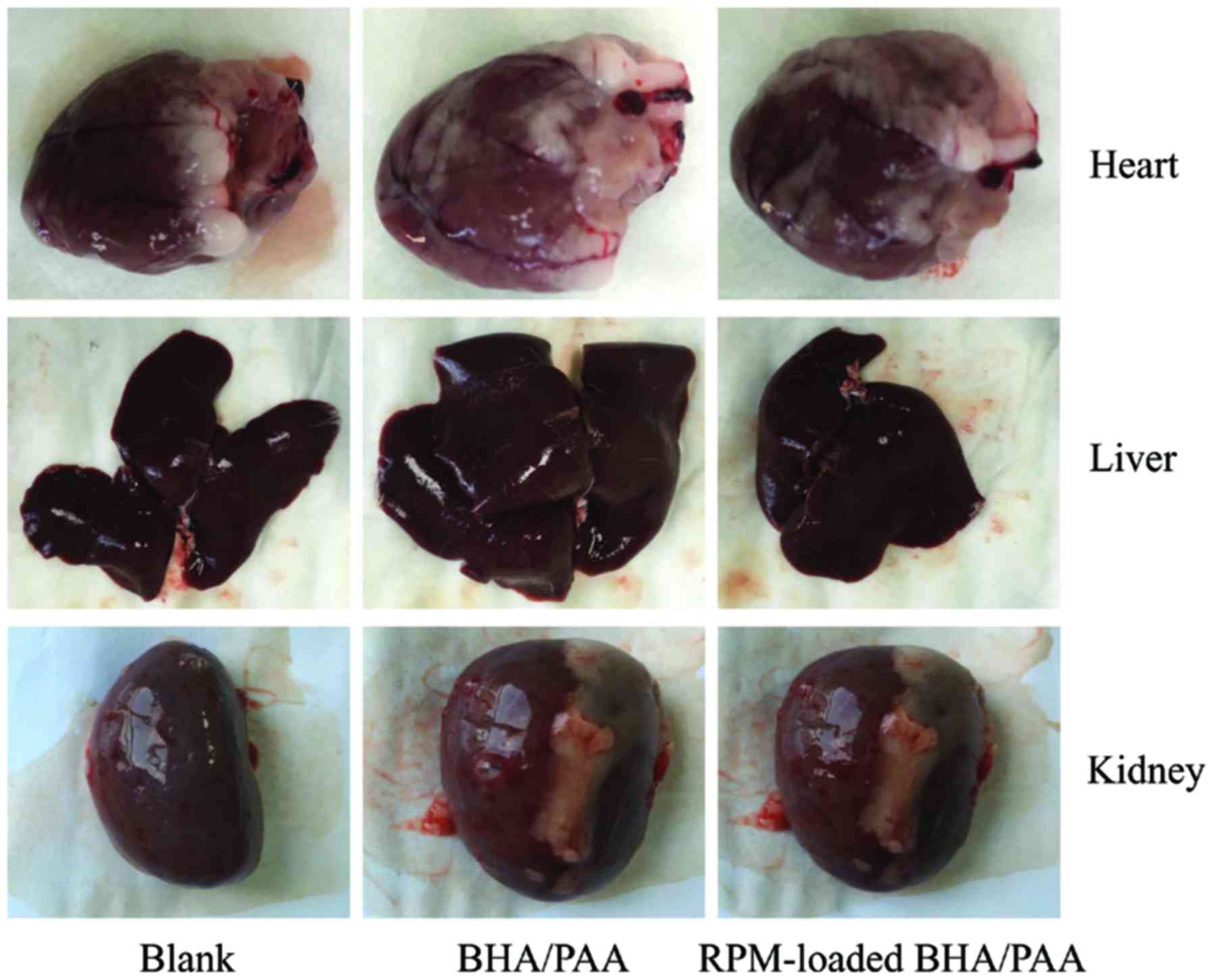
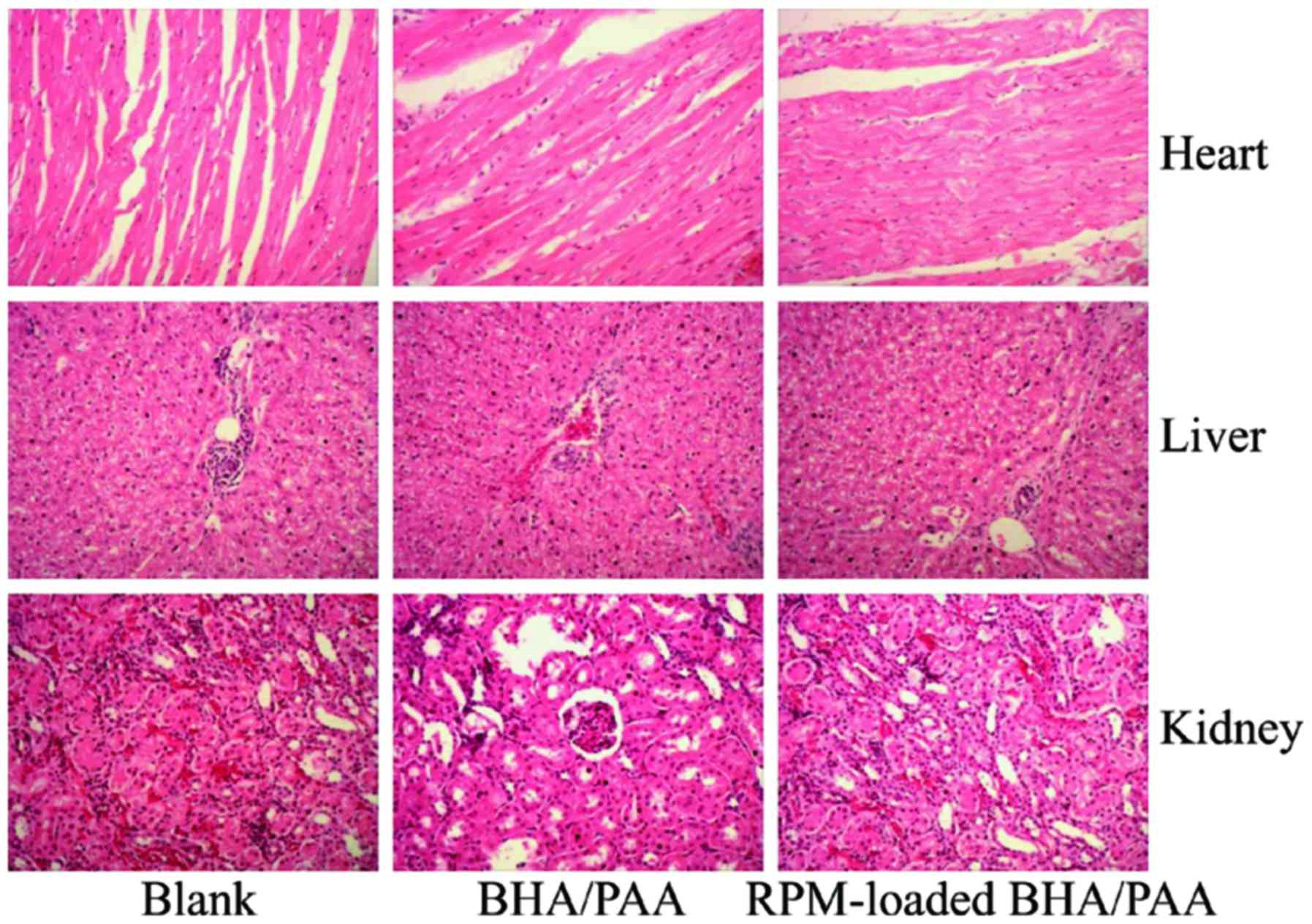
The heart, liver and kidney tissues were collected
at 12 weeks following implantation. As illustrated in Fig. 3, no significant changes were
observed in the appearance of heart, liver and kidney amongst the
three groups. In addition, H&E staining revealed no
histopathological abnormalities in the heart, liver and kidneys of
the treated rabbits (Fig. 4).
Biochemical analyses for markers of liver and kidney functions
revealed that the concentrations of ALT, AST, BUN and Cr in the
serum were significantly increased at day 1 and 3 following
implantation; however, serum concentrations for all four markers
returned to normal levels within 1 week (Table III).
 | Table III.Concentrations of ALT, AST, BUN and Cr
in serum. |
Table III.
Concentrations of ALT, AST, BUN and Cr
in serum.
|
| Experimental
group | ALT (U/l) | AST (U/l) | BUN (mmol/l) | Cr (µmol/l) |
|---|
| 1 day | Blank | 48.82±7.05 | 55.65±9.09 | 7.78±1.25 | 131.97±25.62 |
|
| BHA/PAA |
88.56±6.08b |
92.37±10.46a |
15.11±2.05b |
245.11±25.95b |
|
| RPM-loaded
BHA/PAA |
98.76±11.68b |
113.67±18.21b |
15.92±1.84b |
258.12±30.57b |
| 3 days | BHA/PAA |
81.58±11.67a |
82.05±7.05a | 10.07±2.12 |
218.91±30.34a |
|
| RPM-loaded
BHA/PAA |
85.05±9.05b |
85.34±7.02a | 11.15±2.01 |
222.14±25.98a |
| 1 week | BHA/PAA | 60.66±7.25 | 70.02±7.11 | 9.21±1.88 | 200.74±28.79 |
|
| RPM-loaded
BHA/PAA | 65.28±7.88 | 73.27±8.05 | 9.35±2.05 | 198.12±25.67 |
| 2 weeks | BHA/PAA | 54.25±6.92 | 54.25±5.88 | 7.15±1.68 | 142.28±29.22 |
|
| RPM-loaded
BHA/PAA | 52.43±7.02 | 60.13±7.67 | 8.02±1.22 | 155.28±20.95 |
Discussion
BHA/PAA is a polar polymer which has great potential
as a bone repair material. It combines the biocompatibility and
bone conduction of BHA with the mechanical properties of PAA, to
induce osteogenesis. Previous studies have reported that the polar
groups in BHA/PAA, including-OH,-NH2 and -COOH, promote
the colonization of bone cells on the material (26). In addition, the porous network
structure and surface roughness of BHA/PAA provide a biochemical
environment to promote osteoblast proliferation, differentiation,
migration, adhesion and promote bone repair and regeneration
(27–29). A previous study by Yan and Jiang
(22) confirmed that BHA/PAA
exhibits good osteogenesis activity both in vitro and in
vivo; in vitro, BHA/PAA promoted the growth, adhesion
and calcium nodule formation of MG63 bone osteosarcoma cells, while
in an animal model of radial bone defect, the implanted BHA/PAA
material could be fused with the host bone and new bone gradually
formed. In the present study, the BHA/PAA material was completely
degraded and absorbed at 12 weeks following implantation and new
trabecular bone and cartilage had formed. The present results were
consistent with previous studies from our group (22,23)
and confirmed that BHA/PAA is a good bone graft substitute material
with good biological compatibility and capable of osteogenic
induction.
RPM was first generated by our research group
(16) and preliminary experiments
have characterized the physiochemical properties of RPMs. RPMs were
spherical with rough surfaces. The in vitro drug release
studies revealed that following an initial rapid drug release,
rifapentine release gradually decreased and ~80% of the
encapsulated rifapentine was released following ~4 weeks of
incubation. Furthermore, RPMs were able to eliminate S. aureus
in vitro. These results indicated that RPMs were bioactive and
efficient controlled-release delivery systems, and may exhibit a
great potential in the treatment of osteoarticular tuberculosis
(16). In the present study,
BHA/PAA was used as a carrier of RPM to prepare a novel
sustained-release drug material.
A previous study has demonstrated the curative
effect of RPM-loaded BHA/PAA in the treatment of rabbit chronic
osteomyelitis induced by S. aureus (23). The in vitro experiments
demonstrated that RPM-loaded BHA/PAA was able to slowly release
antibiotics and inhibited bacterial growth effectively for up to 5
weeks. In vivo, RPM-loaded BHA/PAA effectively eradicated
the bacterial infection, induced osteogenesis and promoted new bone
formation while the material was gradually degraded and absorbed.
The present study aimed to investigate the curative effect of
RPM-loaded BHA/PAA in the treatment of osteoarticular tuberculosis.
The in vivo release tests demonstrated that RPM-loaded
BHA/PAA exhibited sustained release profiles of rifapentine and the
drug concentration in the muscle tissues remained higher than the
minimum inhibitory concentration of rifapentine against M.
tuberculosis for as long as 12 weeks. Furthermore, the H&E
staining and biochemical analyses indicated that RPM-loaded BHA/PAA
had no long-term side effects to the heart, liver and kidneys of
the treated rabbits.
In conclusion, the present study revealed for the
first time that RPM-loaded BHA/PAA may be a useful material for
treating osteoarticular tuberculosis. RPM-loaded BHA/PAA promoted
new bone formation, while it was gradually degraded and absorbed.
Furthermore, rifapentine was released in a sustained manner from
this material and no side effects were observed in the heart, liver
and kidney of the treated animals. The present results may
therefore provide useful new tools towards improving the treatment
of osteoarticular tuberculosis.
Acknowledgements
This research was supported by the National Natural
Science Foundation of China (no. 81171685).
References
|
1
|
Dye C and Williams BG: The population
dynamics and control of tuberculosis. Science. 328:856–861. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen M, Gan H and Remold HG: A mechanism
of virulence: Virulent Mycobacterium tuberculosis strain H37Rv, but
not attenuated H37Ra, causes significant mitochondrial inner
membrane disruption in macrophages leading to necrosis. J Immunol.
176:3707–3716. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Barry CE III, Boshoff HI, Dartois V, Dick
T, Ehrt S, Flynn J, Schnappinger D, Wilkinson RJ and Young D: The
spectrum of latent tuberculosis: Rethinking the biology and
intervention strategies. Nat Rev Microbiol. 7:845–855.
2009.PubMed/NCBI
|
|
4
|
Nagashima H, Yamane K, Nishi T, Nanjo Y
and Teshima R: Recent trends in spinal infections: Retrospective
analysis of patients treated during the past 50 years. Int Orthop.
34:395–399. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
WHO global tuberculosis control report
2010. Summary. Cent Eur J Public Health. 18:2372010.PubMed/NCBI
|
|
6
|
Sequeira W, Co H and Block JA:
Osteoarticular tuberculosis: Current diagnosis and treatment. Am J
Ther. 7:393–398. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ge Z, Wang Z and Wei M: Measurement of the
concentration of three antituberculosis drugs in the focus of
spinal tuberculosis. Eur Spine J. 17:1482–1487. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Saifullah B, Hussein MZ and Al Ali SH
Hussein: Controlled-release approaches towards the chemotherapy of
tuberculosis. Int J Nanomedicine. 7:5451–5463. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Moore WR, Graves SE and Bain GI: Synthetic
bone graft substitutes. ANZ J Surg. 71:354–361. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Freitas S, Merkle HP and Gander B:
Microencapsulation by solvent extraction/evaporation: Reviewing the
state of the art of microsphere preparation process technology. J
Control Release. 102:313–332. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Aristoff PA, Garcia GA, Kirchhoff PD and
Showalter HD: Rifamycins-obstacles and opportunities. Tuberculosis
(Edinb). 90:94–118. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Keung A, Eller M, McKenzie K and Weir S:
Single and multiple dose pharmacokinetics of rifapentine in man:
Part II. Int J Tuberc Lung Dis. 3:437–444. 1999.PubMed/NCBI
|
|
13
|
Bemer-Melchior P, Bryskier A and Drugeon
HB: Comparison of the in vitro activities of rifapentine and
rifampicin against Mycobacterium tuberculosis complex. J Antimicrob
Chemother. 46:571–576. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Assandri A, Ratti B and Cristina T:
Pharmacokinetics of rifapentine, a new long lasting rifamycin, in
the rat, the mouse and the rabbit. J Antibiot (Tokyo).
37:1066–1075. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chan JG, Bai X and Traini D: An update on
the use of rifapentine for tuberculosis therapy. Expert Opin Drug
Deliv. 11:421–431. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu J, Zuo Y, Hu Y, Wang J, Li J, Qiao B
and Jiang D: Development and in vitro characterization of drug
delivery system of rifapentine for osteoarticular tuberculosis.
Drug Des Devel Ther. 9:1359–1366. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gentile P, Chiono V, Boccafoschi F, Baino
F, Vitale-Brovarone C, Vernè E, Barbani N and Ciardelli G:
Composite films of gelatin and hydroxyapatite/bioactive glass for
tissue-engineering applications. J Biomater Sci Polym Ed.
21:1207–1226. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Roohani-Esfahani SI, Nouri-Khorasani S, Lu
Z, Appleyard R and Zreiqat H: The influence hydroxyapatite
nanoparticle shape and size on the properties of biphasic calcium
phosphate scaffolds coated with hydroxyapatite-PCL composites.
Biomaterials. 31:5498–5509. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wu Y and Bose S: Nanocrystalline
hydroxyapatite: Micelle templated synthesis and characterization.
Langmuir. 21:3232–3234. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mastrogiacomo M, Scaglione S, Martinetti
R, Dolcini L, Beltrame F, Cancedda R and Quarto R: Role of scaffold
internal structure on in vivo bone formation in macroporous calcium
phosphate bioceramics. Biomaterials. 27:3230–3237. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Turco G, Marsich E, Bellomo F, Semeraro S,
Donati I, Brun F, Grandolfo M, Accardo A and Paoletti S:
Alginate/Hydroxyapatite biocomposite for bone ingrowth: A
trabecular structure with high and isotropic connectivity.
Biomacromolecules. 10:1575–1583. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yan L and Jiang DM: Study of bone-like
hydroxyapatite/polyamino acid composite materials for their
biological properties and effects on the reconstruction of long
bone defects. Drug Des Devel Ther. 9:6497–6508. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yan L, Jiang DM, Cao ZD, Wu J, Wang X,
Wang ZL, Li YJ and Yi YF: Treatment of Staphylococcus
aureus-induced chronic osteomyelitis with bone-like
hydroxyapatite/poly amino acid loaded with rifapentine
microspheres. Drug Des Devel Ther. 9:3665–3676. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Peng XL, Li YB, Wang XJ, Yan YG, Wei J and
Zhang L: Study on the soaking behaviors of
nano-hydroxyapatite/polyamide 66 biomedical composite in vitro.
Functional Mater. 2:253–255. 2004.
|
|
25
|
Rastogi N, Goh KS, Berchel M and Bryskier
A: Activity of rifapentine and its metabolite
25-O-desacetylrifapentine compared with rifampicin and rifabutin
against Mycobacterium tuberculosis, Mycobacterium africanum,
Mycobacterium bovis and M. bovis BCG. J Antimicrob Chemother.
46:565–570. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bos R, van der Mei HC and Busscher HJ:
Physico-chemistry of initial microbial adhesive interactions-its
mechanisms and methods for study. FEMS Microbiol Rev. 23:179–230.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wei G and Ma PX: Structure and properties
of Nano-hydroxyapatite/polymer composite scaffolds for bone tissue
engineering. Biomaterials. 25:4749–4757. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Owen GR, Jackson J, Chehroudi B, Burt H
and Brunette DM: A PLGA membrane controlling cell behaviour for
promoting tissue regeneration. Biomaterials. 26:7447–7456. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Huang J, Zhao D, Dangaria SJ, Luan X,
Diekwisch TG, Jiang G, Saiz E, Liu G and Tomsia AP: Combinatorial
design of hydrolytically degradable, bone-like biocomposites based
on PHEMA and hydroxyapatite. Polymer (Guildf). 54:909–919. 2013.
View Article : Google Scholar : PubMed/NCBI
|