Introduction
Ischemic heart disease is a pervasive health problem
worldwide. Severe ischemia or hypoxia induces activation of cell
death mechanisms including necrosis, apoptosis and autophagy.
Autophagy is an important process for the degradation and recycling
of long-lived proteins and cytoplasmic organelles, and is activated
in response to a variety of extracellular and intracellular stimuli
(1). Therefore, autophagy serves a
cell protective role under certain circumstances. For example,
transient and moderate ischemia induced autophagy via a 5′
adenosine monophosphate-activated protein kinase (AMPK)-dependent
mechanism. Glucose deprivation (GD), which mimics myocardial
ischemia, induced autophagy in cultured cardiac myocytes. Survival
of cardiac myocytes was decreased by 3-methyladenine, an inhibitor
of autophagy, suggesting that autophagy is protective against GD in
cardiac myocytes. GD-induced autophagy coincided with activation of
AMPK (2). However, autophagy
induced by acute ischemia is associated with reduced cell
viability, possibly due to the non-specific degradation of
cytoplasmic contents (3,4). Therefore, inhibition of autophagy
during severe hypoxia may be beneficial for the survival of
myocardial cells and may reduce cardiac injury.
Autophagy and apoptosis serve essential roles in
cell death and regulate cardiovascular disease. Studies have
demonstrated that following cardiac ischemia/reperfusion injury,
autophagy and apoptosis are stimulated to reduce cell survival via
activation of the mitochondrial c-Jun N-terminal kinase signaling
pathway (5–7). However, the association between
autophagy and apoptosis is complex. An early study revealed that in
response to stress stimuli, autophagy may trigger apoptosis and
lead to cell death (8). In
addition, the two pathways share common regulatory factors and each
may influence and alter the activity of the other (9). Therefore, it is important to
investigate the interaction between autophagy and apoptosis during
hypoxia in the heart, as the levels of the two processes must be
regulated to protect cells.
Berberine, an isoquinoline alkaloid, is derived from
herbs, including Hydrastis canadensis (goldenseal) and
Coptis chinensis (Coptis or goldenthread) from the
Ranunculaceae family, Arcangelisia flava from the
Menispermaceae family, and Berberis aquifolium (Oregon
grape) and Berberis aristata (tree turmeric) from the
Berberidaceae family (10,11). Berberine may have the potential to
treat a wide range of diseases, including endothelial dysfunction,
hyperlipemia and diabetes (12–14).
In addition, our previous study demonstrated that berberine may
significantly decrease infarct size and improve cardiac function
following ischemia/reperfusion-induced myocardial injury, and this
may involve the inhibition of excessive autophagy (15). Various studies have demonstrated
that berberine may protect against apoptosis following pathological
ischemia (16–18). However, the effect of berberine on
autophagy in myocardial cells during hypoxia remains to be
determined. The present study demonstrated that berberine treatment
significantly improved cell viability and suppressed autophagy and
apoptosis in hypoxia-induced myocardial cells. Additionally, the
underlying mechanism of berberine protection was investigated.
Materials and methods
Reagents
Dulbecco's modified Eagle's medium (DMEM), fetal
bovine serum (FBS) and penicillin/streptomycin (pen/strep; 10,000
U/ml each) were purchased from Gibco; Thermo Fisher Scientific,
Inc. (Waltham, MA, USA). Dimethyl sulfoxide (DMSO) and berberine
were obtained from Sigma-Aldrich; Merck KGaA (Darmstadt, Germany).
A rabbit antibody against β-actin (catalog no. ab8227) was obtained
from Abcam (Cambridge, UK). Rabbit anti-microtubule-associated
proteins 1A/1B light chain 3 (LC3) B (catalog no. 2775), rabbit
anti-B-cell lymphoma 2 (Bcl-2)/adenovirus E1B 19 kDa
protein-interacting protein 3 (BNIP3; catalog no. 12396), rabbit
anti-Bcl-2 (catalog no. 2870), rabbit anti-caspase-3 (catalog no.
9665) and rabbit anti-Bcl-2-associated X protein (Bax; catalog no.
2772) antibodies were purchased from Cell Signaling Technology,
Inc. (Danvers, MA, USA). A goat anti-rabbit secondary antibody
conjugated to Alexa Fluor® 680 (catalog no. A-21109) was obtained
from Invitrogen; Thermo Fisher Scientific, Inc.
Cell culture and treatment
The H9c2 rat myocardium-derived cell line was
purchased from the American Type Culture Collection (Manassas, VA,
USA). Cells were cultured in DMEM containing 4,500 mg/l glucose and
supplemented with 10% (v/v) FBS, 10 mM
4-(2-hydroxyethyl)-1-piperazinethanesulfonic acid (Sigma Aldrich;
Merck KGaA) and 1% pen/strep solution at 37°C in a 5%
CO2 incubator. To determine the optimal duration of
hypoxia, cells at ~90% confluence were resuspended in serum-free
and low glucose (1,500 mg/l) DMEM and incubated for 4 h.
Subsequently, cells were cultured under hypoxic conditions (1%
O2, 5% CO2 and 94% N2) for 1 to 12
h. Berberine (Sigma Aldrich; Merck KGaA; 5, 10 or 25 µM) dissolved
in DMSO, 5 mM 3-methyladenine (Sigma Aldrich; Merck KGaA) dissolved
in PBS or 10 nM rapamycin (Cell Signaling Technology, Inc.,
Danvers, MA, USA) dissolved in PBS, 10 µM Compound C (Sigma
Aldrich; Merck KGaA) dissolved in DMSO, were added to cells, which
were seeded in 6-well plates at 1×106 cells/well, following a 4-h
incubation in serum-free low glucose DMEM, and incubated under
normoxic conditions (21% O2, 5% CO2 and 74%
N2) for 1 h prior to culturing under hypoxic conditions
(1% O2, 5% CO2 and 94% N2) for 6
h. For the control group, cells were cultured constantly under
normoxic condition.
Determination of cell viability
The
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay was utilized to assess cell viability. Cells were seeded in
96-well culture plates (3×103 per well). Following treatment, cells
were incubated with 0.5 mg/ml MTT (Roche Applied Science, Penzberg,
Germany) in DMEM for 4 h. The blue formazan crystals produced from
viable cells were dissolved in DMSO. Absorbance was measured
spectrophotometrically at a wavelength of 570 nm.
Protein isolation and western blot
analysis
Following treatment, cells were washed with cold PBS
(pH 7.4) and lysed for 30 min with lysis buffer [0.5% Nonidet 40,
50 mmol/l Tris-HCl (pH 7.5), 1 mmol/l EDTA, 1 mmol/l EGTA and 150
mmol/l NaCl, containing 10% glycerol, 50 mmol/l sodium fluoride, 10
mmol/l sodiumpyrophosphate, 1 mmol/l sodium orthovanadate, 80
µmol/l β-glycerophosphate, 1 mmol/l phenylmethanesulfonyl fluoride,
10 µg/ml aprotinin, 100 µg/ml soybean trypsin inhibitor and 10
µg/ml leupeptin] and centrifuged for 10 min at 12,000 × g at 4°C.
Subsequently, protein concentrations were measured using a
Bicinchoninic Acid assay (Pierce; Thermo Fisher Scientific, Inc.).
Cell lysates (20 µg) were loaded onto 10% polyacrylamide SDS gels
and subjected to electrophoresis. Proteins were subsequently
transferred onto polyvinylidene difluoride membranes (EMD
Millipore, Billerica, MA, USA). Membranes were blocked with 5%
skimmed milk in TBS containing 0.05% Tween-20 (TBST) for 1 h at
room temperature, and probed with anti-LC3B, anti-Bcl-2, anti-Bax,
anti-cleaved caspase-3, anti-BNIP3 (diluted 1:1,000 in TBST) or
anti-β-actin (diluted 1:5,000 in TBST) primary antibodies for 24 h
at 4°C, followed by a goat anti-rabbit secondary antibody (diluted
1:1,000 in TBST) for 1 h at 25°C, conjugated to the
far-red-fluorescent Alexa Fluor 680 dye. Signals were detected by
the Odyssey® Imaging system (LI-COR Biosciences, Lincoln, NE, USA).
Densitometric analysis was performed using Quantity One software
version 4.6.2 (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Annexin V/propidium iodide (PI) double
staining and flow cytometry
Apoptosis was measured by flow cytometry, which was
performed using Annexin V, FITC Apoptosis Detection kit (Dojindo
Molecular Technologies, Inc., Kumamoto, Japan) according to the
manufacturer's protocol. Cells seeded at 1×106, were incubated with
5 µl Annexin V-FITC and 5 µl PI at room temperature for 15 min in
the dark and apoptotic cells were detected by flow cytometry. The
mean fluorescence intensity of Annexin V/PI staining in the
myocytes was analyzed using the BD FACSCalibur™ (BD Biosciences,
Franklin Lakes, NJ, USA) and FlowJo software version 7.61 (Stanford
University, CA, USA) was used to analyze the results.
Statistical analysis
Three or more groups were compared by one-way
analysis of variance followed by the Student-Newman-Keuls and
Dunnett post hoc tests. Data are expressed as the mean ± standard
deviation and were analyzed using SPSS software version 18.0 (SPSS,
Inc., Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference. Experiments were performed at
least three times.
Results
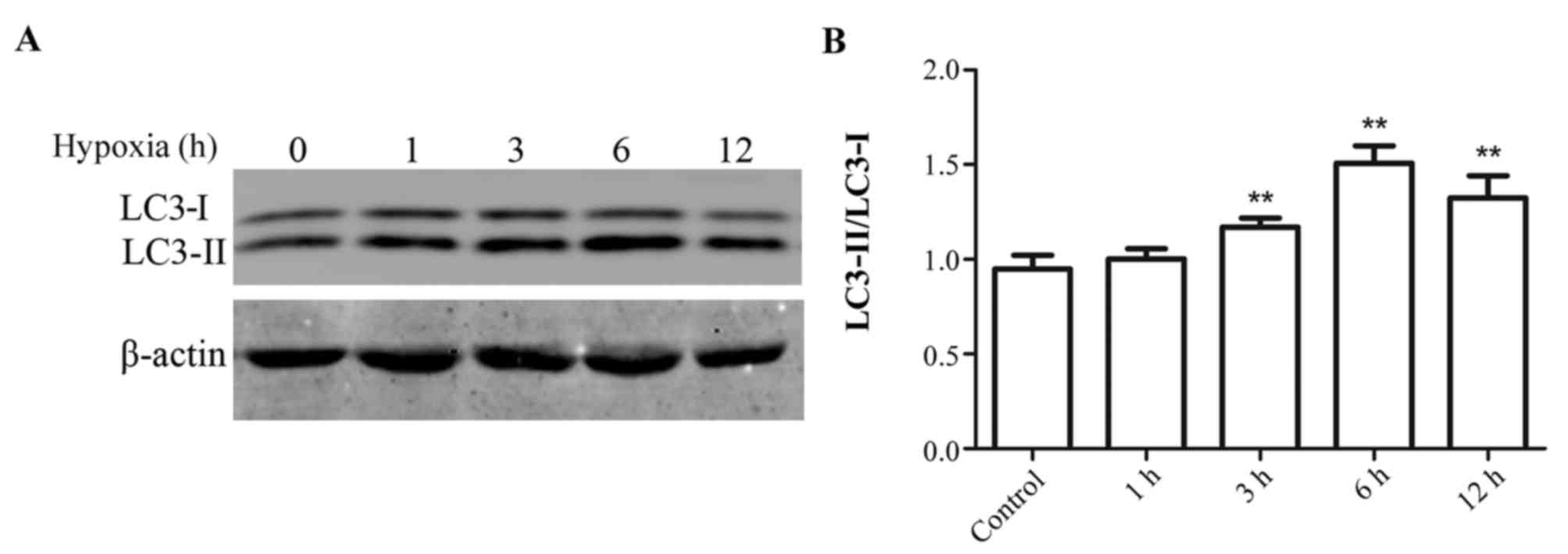
Effect of hypoxia duration on
autophagy in myocytes
H9c2 myocytes were exposed to hypoxic conditions for
1 to 12 h. Subsequently, protein expression levels of LC3-I and
LC3-II were measured by western blotting. The greatest increase in
the ratio of LC3-II/LC3-I expression levels compared with the
control group was observed in cells subjected to hypoxia for 6 h.
Therefore the following experiments were all performed with cells
subjected to hypoxia for 6 h (Fig.
1).
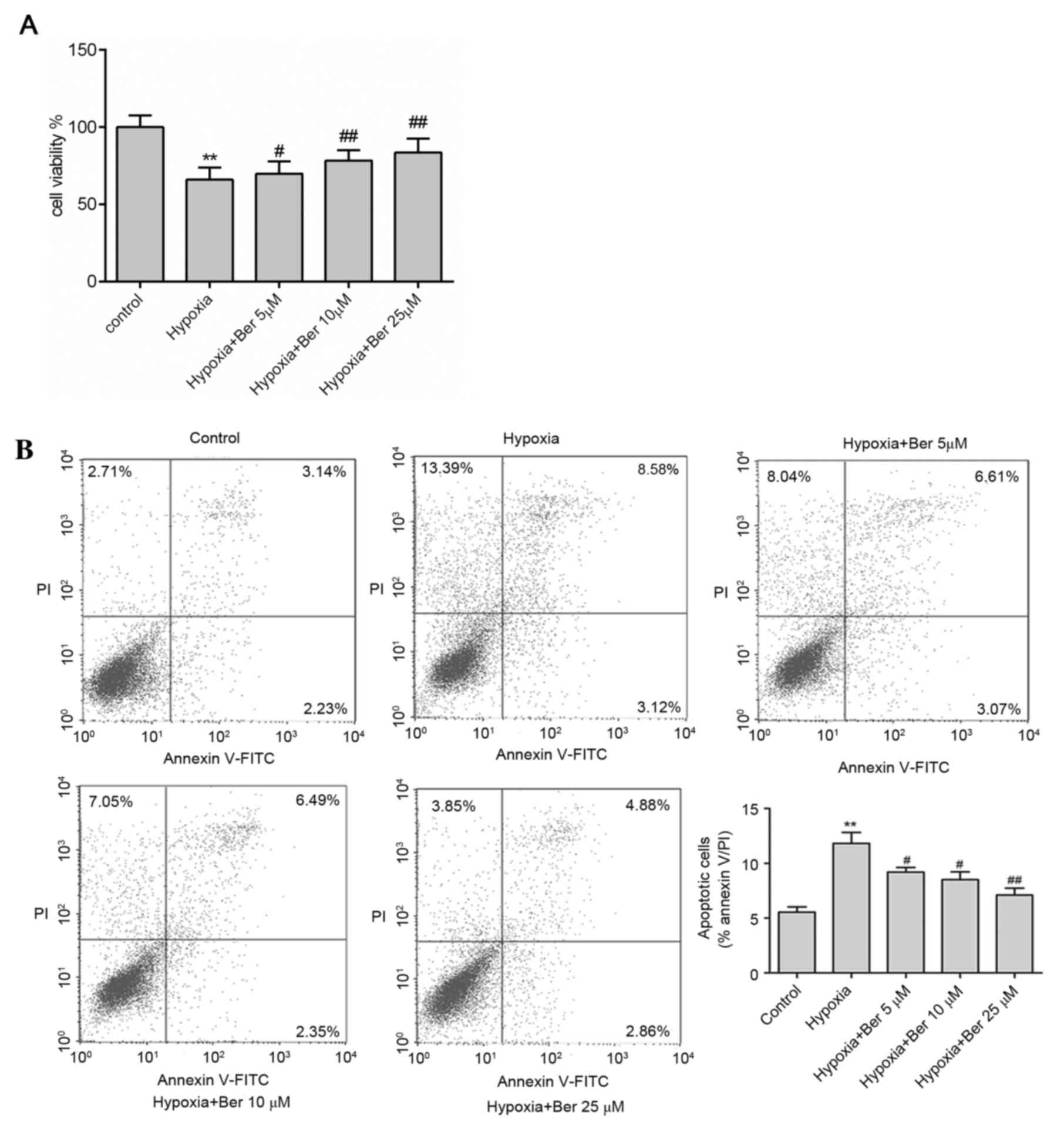
Berberine attenuates hypoxia-induced
autophagy and apoptosis in H9c2 myocytes
The effect of berberine on autophagy and apoptosis
in hypoxic myocytes was investigated. H9c2 cells were pretreated
with 5–25 µM berberine for 1 h. Hypoxia exposure reduced cell
viability compared with the control group; this effect was
abrogated by berberine treatment in dose-dependent manner, as
determined by an MTT assay (Fig.
2A). In addition, there was a marked increase in apoptosis
following hypoxia, as determined by the increased percentage of
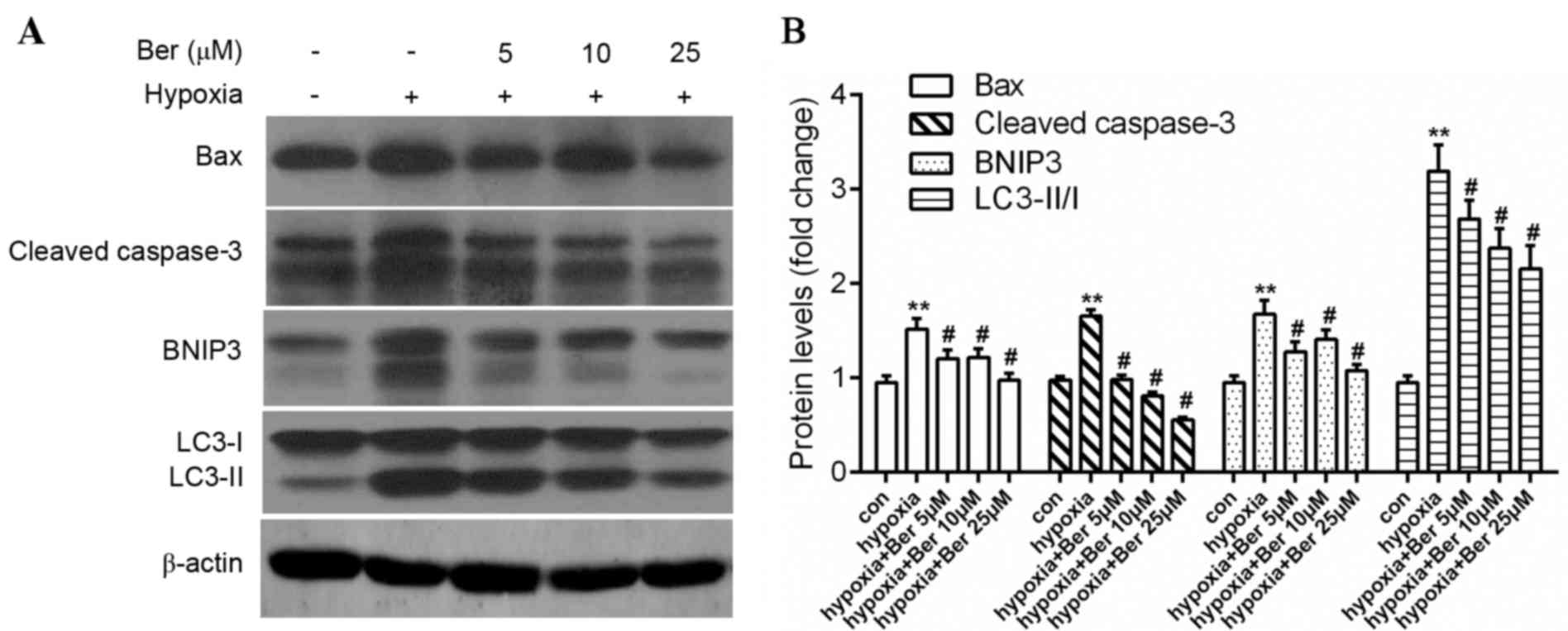
Annexin V/PI double positive cells (Fig. 2B), upregulation of the
pro-apoptotic proteins Bax and caspase-3 (Fig. 3). A similar effect on autophagy was
observed, which was demonstrated by the ratio of LC3-II/LC3-I and
BNIP3 protein expression levels (Fig.
3). Treatment with berberine reversed this effect in a
dose-dependent manner. This data suggested that berberine enhanced
cell survival via inhibition of proteins involved in autophagy and
apoptosis.
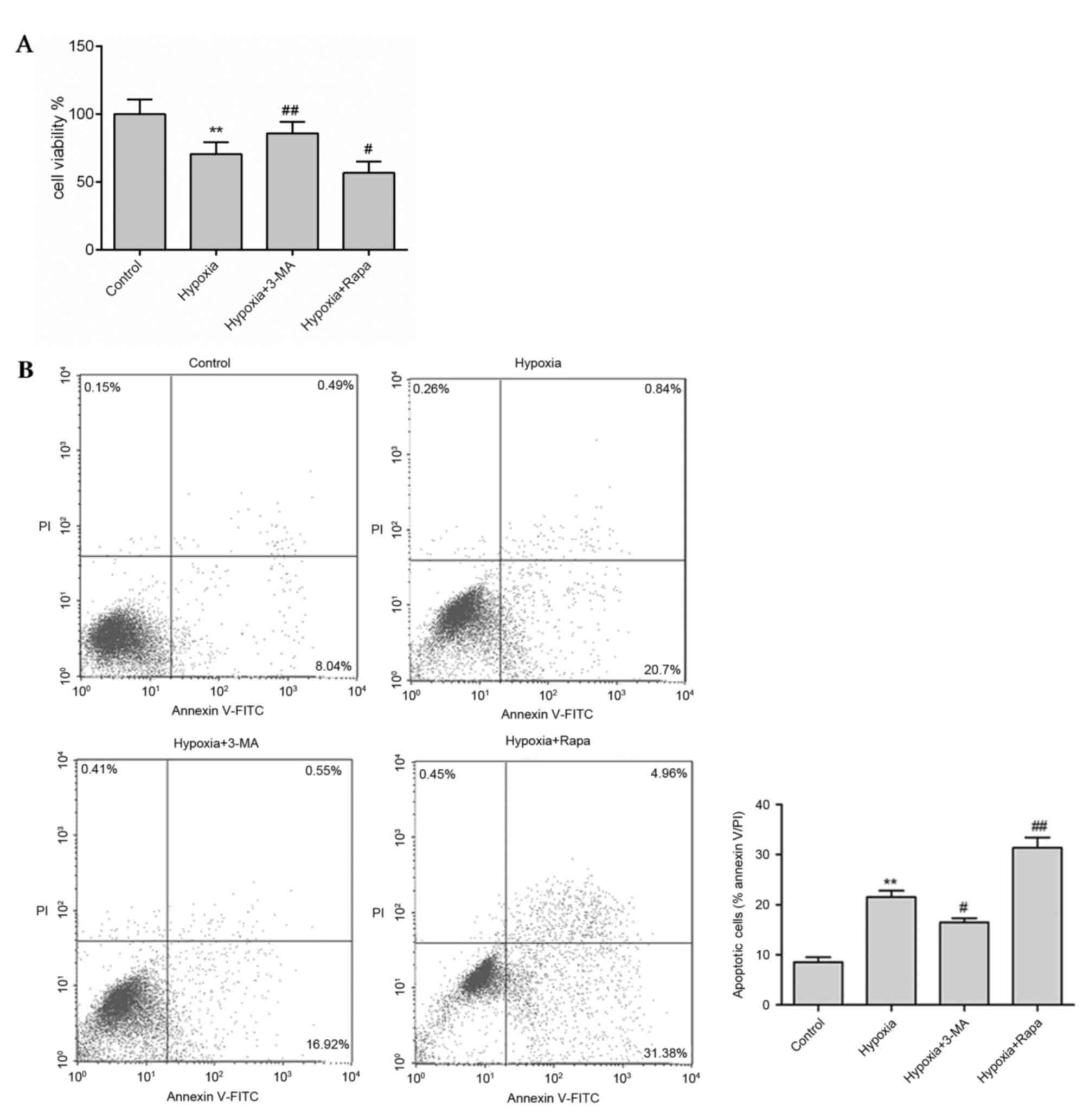
Inhibition of autophagy reduces
apoptosis in hypoxic H9c2 cells
As berberine significantly blocked apoptosis and the
expression levels of autophagy-associated proteins in hypoxic H9c2
cells, the association between autophagy and apoptosis was
investigated using the autophagy inhibitor 3-MA and the autophagy
inducer rapamycin. Hypoxia treatment markedly reduced H9c2 cell
viability (Fig. 4A) and enhanced
apoptosis (Fig. 4B) compared with
the control. However, in the presence of 3-MA, cell viability was
enhanced and apoptosis was reduced compared with the hypoxia group,
whereas rapamycin treatment had the opposite effect (Fig. 4). In addition, similar results were
observed in protein expression levels as measured by western
blotting. Expression levels of the autophagy markers LC3-II and
BNIP3, the pro-apoptosis proteins Bax and cleaved caspase-3 were
significantly reduced by 3-MA treatment (P<0.01), whereas the
anti-apoptosis protein Bcl-2 was significantly enhanced (P<0.01;
Fig. 5), compared with the hypoxic
group. However, rapamycin treatment significantly increased the
expression levels of LC3-II, BNIP3 and Bax, and reduced Bcl-2
expression compared with the control group (P<0.01; Fig. 5). These results suggested that
inhibition of autophagy reduced apoptosis and increased cell
viability in hypoxic myocytes.
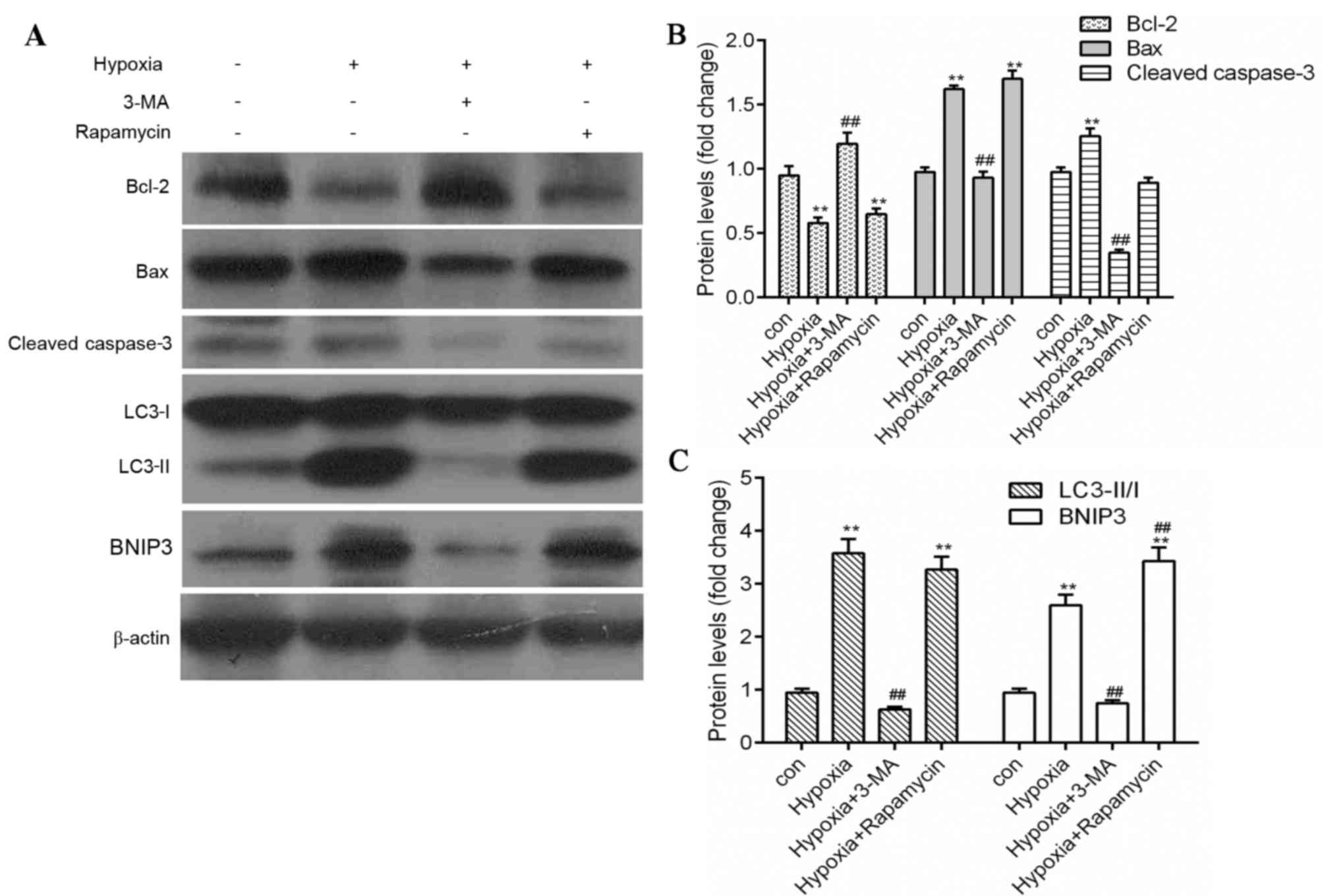 | Figure 5.Inhibition of autophagy reduces
apoptosis in hypoxia-induced myocytes. (A) Protein expression
levels of Bax, Bcl-2, cleaved caspase-3, BNIP3, LC3 and β-actin
were measured by western blot analysis. Quantification of (B)
Bcl-2, Bax and cleaved caspase-3, and (C) LC3-II/LC3-I and BNIP3
protein expression levels by densitometry. β-actin served as the
loading control. Data are expressed as the mean ± standard
deviation and were obtained from three independent experiments.
**P<0.01 vs. control group; ##P<0.01 vs. hypoxia
group. Bax, B-cell lymphoma 2-associated X protein; Bcl-2, B-cell
lymphoma 2; BNIP3, B-cell lymphoma 2/adenovirus E1B 19 kDa
protein-interacting protein 3; LC3, microtubule-associated proteins
1A/1B light chain 3; 3-MA, 3-methyladenine. |
AMPK signaling pathway activation may
be involved in the regulation of apoptosis by berberine in hypoxic
H9c2 cells
To confirm the effect of berberine on the inhibition
of apoptosis, the specific AMPK inhibitor, Compound C, was
utilized. Compound C treatment significantly reduced cellular
apoptosis during hypoxia, compared with hypoxia treatment alone.
The reduction in apoptosis by Compound C was comparable to that of
berberine treatment or berberine and Compound C in combination,
suggesting that AMPK activation may be involved in the regulation
of apoptosis in hypoxic H9c2 cells, and the inhibitory effect of
berberine on apoptosis may be associated with the inhibition of
AMPK activation.
Discussion
The present study demonstrated that berberine
effectively reduced the levels of apoptosis and the protein
expression levels of autophagy markers, and enhanced cell survival
in hypoxic myocardial cells. In addition, the inhibition of
autophagy reduced apoptosis and further improved cell viability.
These observations suggested that berberine increases cell survival
by decreasing autophagy to reduce cell apoptosis in hypoxic
myocytes. Furthermore, inhibition of AMPK activation may be
involved in the regulation of apoptosis by berberine.
Autophagy is involved in various physiological and
pathological cellular processes, including development,
differentiation, inflammation, immunity, metabolism and cell death
(19–21). Studies have suggested that
autophagy serves a protective role in cells during ischemia. For
example, Yan et al (22)
demonstrated that autophagy may be activated by repetitive
myoca+rdial ischemia in pigs, and may serve as a homeostatic
mechanism to suppress apoptosis and to protect myocardium from the
deleterious effects of chronic ischemia. In addition, the
protective effect of autophagy was demonstrated in models of brain
(23) and renal (24) ischemia. Studies by Hamacher-Brady
et al (25) and Dosenko
et al (26) supported these
findings, which suggested that regulated autophagy serves a role in
cell survival. However, autophagy is a double-edged sword that
mediates cell death under specific circumstances (9). Excessive autophagy leads to cell
death via destruction of the cytosol and organelles (27). Evidence suggests that an
uncontrolled and excessive induction of autophagy during
reperfusion may lead to cellular dysfunction in cardiomyocytes, and
eventually to autophagic cell death (2,3,28).
The present study investigated the effect of autophagy during
hypoxia in H9c2 cells. The maximum level of autophagy was observed
following 6 h of hypoxia, and the inhibition of autophagy by 3-MA
markedly improved cell viability. This data suggested that the
reduction of autophagy may benefit cell survival in certain
circumstances, and that autophagy may be detrimental to the cell
during hypoxia. Notably, Aki et al (29) reported similar results following
glucose starvation.
There is considerable crosstalk between autophagy
and apoptosis pathways. One molecule that may connect autophagy and
apoptosis is BNIP3, which is a BH3-domain-containing member of the
mitochondrial pro-apoptotic Bcl-2 family (30). It has been reported that in
hypoxia-induced neonatal myocytes, upregulated expression of BNIP3
resulted in mitochondrial dysfunction and subsequent apoptosis
(31–33). Wang et al (34) demonstrated that during ischemia,
BNIP3 was highly upregulated, which subsequently stimulated
mitochondrial perturbations, autophagy and cell death. In the
present study, hypoxic H9c2 cells pretreated with 3-MA expressed
significantly decreased levels of BNIP3, and reduced levels of the
pro-apoptotic proteins Bax and caspase-3. Therefore, these findings
suggested that inhibition of autophagy may reduce cellular
apoptosis and enhance cell survival in hypoxic H9c2 cells.
Our previous study revealed that berberine
significantly alleviated reperfusion injury, and enhanced the
survival of myocytes via inhibition of excessive autophagy.
However, it is unclear what role berberine serves during hypoxia in
myocardial cells. The findings of the present study revealed that
berberine markedly inhibited the ratio of LC3-II/LC3-I and the
protein expression levels of BNIP3, Bax and cleaved caspase-3. This
suggested that berberine attenuates cell injury during hypoxia via
inhibition of autophagy and apoptosis in H9c2 cells. In conclusion,
the results of the present study suggested that berberine treatment
protects against hypoxia-induced myocardial injury by selectively
inhibiting excessive autophagy and reducing cell apoptosis. Given
that cells pre-treated with berberine prior to hypoxia could
suppress excessive autophagy, berberine may be a potential
therapeutic agent for preventing myocardial ischemia injury, but
further in vivo studies are required.
Acknowledgements
The present study was supported by the Traditional
Chinese Medicine Administration of Zhejiang Province (grant no.
2016ZA137) and Wenzhou Science & Technology Bureau (grant nos.
Y20150036 and Y20150035).
References
|
1
|
Kelekar A: Autophagy. Ann N Y Acad Sci.
1066:259–271. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Matsui Y, Takagi H, Qu X, Abdellatif M,
Sakoda H, Asano T, Levine B and Sadoshima J: Distinct roles of
autophagy in the heart during ischemia and reperfusion: Roles of
AMP-activated protein kinase and Beclin 1 in mediating autophagy.
Circ Res. 100:914–922. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Valentim L, Laurence KM, Townsend PA,
Carroll CJ, Soond S, Scarabelli TM, Knight RA, Latchman DS and
Stephanou A: Urocortin inhibits Beclin1-mediated autophagic cell
death in cardiac myocytes exposed to ischaemia/reperfusion injury.
J Mol Cell Cardiol. 40:846–852. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mizushima N, Levine B, Cuervo AM and
Klionsky DJ: Autophagy fights disease through cellular
self-digestion. Nature. 451:1069–1075. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xu J, Qin X, Cai X, Yang L, Xing Y, Li J,
Zhang L, Tang Y, Liu J, Zhang X and Gao F: Mitochondrial JNK
activation triggers autophagy and apoptosis and aggravates
myocardial injury following ischemia/reperfusion. Biochim Biophys
Acta. 1852:262–270. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ferrandi C, Ballerio R, Gaillard P,
Giachetti C, Carboni S, Vitte PA, Gotteland JP and Cirillo R:
Inhibition of c-Jun N-terminal kinase decreases cardiomyocyte
apoptosis and infarct size after myocardial ischemia and
reperfusion in anaesthetized rats. Br J Pharmacol. 142:953–960.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chambers JW, Pachori A, Howard S, Iqbal S
and LoGrasso PV: Inhibition of JNK mitochondrial localization and
signaling is protective against ischemia/reperfusion injury in
rats. J Biol Chem. 288:4000–4011. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Espert L, Denizot M, Grimaldi M,
Robert-Hebmann V, Gay B, Varbanov M, Codogno P and Biard-Piechaczyk
M: Autophagy is involved in T cell death after binding of HIV-1
envelope proteins to CXCR4. J Clin Invest. 116:2161–2172. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Maiuri MC, Zalckvar E, Kimchi A and
Kroemer G: Self-eating and self-killing: Crosstalk between
autophagy and apoptosis. Nat Rev Mol Cell Biol. 8:741–752. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Peng WH, Wu CR, Chen CS, Chen CF, Leu ZC
and Hsieh MT: Anxiolytic effect of berberine on exploratory
activity of the mouse in two experimental anxiety models:
Interaction with drugs acting at 5-HT receptors. Life Sci.
75:2451–2462. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang F, Zhao G, Cheng L, Zhou HY, Fu LY
and Yao WX: Effects of berberine on potassium currents in acutely
isolated CA1 pyramidal neurons of rat hippocampus. Brain Res.
999:91–97. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang M, Wang CM, Li J, Meng ZJ, Wei SN,
Li J, Bucala R, Li YL and Chen L: Berberine protects against
palmitate-induced endothelial dysfunction: Involvements of
upregulation of AMPK and eNOS and downregulation of NOX4. Mediators
Inflamm. 2013:2604642013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Heidarian E, Rafieian-Kopaei M, Khoshdel A
and Bakhshesh M: Metabolic effects of berberine on liver
phosphatidate phosphohydrolase in rats fed on high lipogenic diet:
An additional mechanism for the hypolipidemic effects of berberine.
Asian Pac J Trop Biomed. 4 Suppl 1:S429–S435. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Vuddanda PR, Chakraborty S and Singh S:
Berberine: A potential phytochemical with multispectrum therapeutic
activities. Expert Opin Investig Drugs. 19:1297–1307. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Huang Z, Han Z, Ye B, Dai Z, Shan P, Lu Z,
Dai K, Wang C and Huang W: Berberine alleviates cardiac
ischemia/reperfusion injury by inhibiting excessive autophagy in
cardiomyocytes. Eur J Pharmacol. 762:1–10. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pires EN Simoes, Frozza RL, Hoppe JB, Bde
M Menezes and Salbego CG: Berberine was neuroprotective against an
in vitro model of brain ischemia: Survival and apoptosis pathways
involved. Brain Res. 1557:26–33. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Visnagri A, Kandhare AD and Bodhankar SL:
Renoprotective effect of berberine via intonation on apoptosis and
mitochondrial-dependent pathway in renal ischemia
reperfusion-induced mutilation. Ren Fail. 37:482–493. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen K, Li G, Geng F, Zhang Z, Li J, Yang
M, Dong L and Gao F: Berberine reduces ischemia/reperfusion-induced
myocardial apoptosis via activating AMPK and PI3K-Akt signaling in
diabetic rats. Apoptosis. 19:946–957. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mizushima N and Levine B: Autophagy in
mammalian development and differentiation. Nat Cell Biol.
12:823–830. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Deretic V, Saitoh T and Akira S: Autophagy
in infection, inflammation and immunity. Nat Rev Immunol.
13:722–737. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rabinowitz JD and White E: Autophagy and
metabolism. Science. 330:1344–1348. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yan L, Vatner DE, Kim SJ, Ge H, Masurekar
M, Massover WH, Yang G, Matsui Y, Sadoshima J and Vatner SF:
Autophagy in chronically ischemic myocardium. Proc Natl Acad Sci
USA. 102:13807–13812. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Carloni S, Buonocore G and Balduini W:
Protective role of autophagy in neonatal hypoxia-ischemia induced
brain injury. Neurobiol Dis. 32:329–339. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jiang M, Liu K, Luo J and Dong Z:
Autophagy is a renoprotective mechanism during in vitro hypoxia and
in vivo ischemia-reperfusion injury. Am J Pathol. 176:1181–1192.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hamacher-Brady A, Brady NR and Gottlieb
RA: Enhancing macroautophagy protects against ischemia/reperfusion
injury in cardiac myocytes. J Biol Chem. 281:29776–29787. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dosenko VE, Nagibin VS, Tumanovska LV and
Moibenko AA: Protective effect of autophagy in anoxia-reoxygenation
of isolated cardiomyocyte? Autophagy. 2:305–306. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nishida K, Yamaguchi O and Otsu K:
Crosstalk between autophagy and apoptosis in heart disease. Circ
Res. 103:343–351. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ma X, Liu H, Foyil SR, Godar RJ,
Weinheimer CJ, Hill JA and Diwan A: Impaired autophagosome
clearance contributes to cardiomyocyte death in
ischemia/reperfusion injury. Circulation. 125:3170–3181. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Aki T, Yamaguchi K, Fujimiya T and
Mizukami Y: Phosphoinositide 3-kinase accelerates autophagic cell
death during glucose deprivation in the rat cardiomyocyte-derived
cell line H9c2. Oncogene. 22:8529–8535. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang K, Yin XM, Chao DT, Milliman CL and
Korsmeyer SJ: BID: A novel BH3 domain-only death agonist. Genes
Dev. 10:2859–2869. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Regula KM, Ens K and Kirshenbaum LA:
Inducible expression of BNIP3 provokes mitochondrial defects and
hypoxia-mediated cell death of ventricular myocytes. Circ Res.
91:226–231. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bruick RK: Expression of the gene encoding
the proapoptotic Nip3 protein is induced by hypoxia. Proc Natl Acad
Sci USA. 97:9082–9087. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kubasiak LA, Hernandez OM, Bishopric NH
and Webster KA: Hypoxia and acidosis activate cardiac myocyte death
through the Bcl-2 family protein BNIP3. Proc Natl Acad Sci USA.
99:12825–12830. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang EY, Gang H, Aviv Y, Dhingra R,
Margulets V and Kirshenbaum LA: p53 mediates autophagy and cell
death by a mechanism contingent on Bnip3. Hypertension. 62:70–77.
2013. View Article : Google Scholar : PubMed/NCBI
|