Introduction
Osteoarthritis (OA) is a leading cause of pain and
disability in the aging population. A primary characteristic of OA
is angiogenesis, characterized by the formation and invasion of new
blood vessels into the hyaline cartilage (1). It has previously been demonstrated
that angiogenesis is important in the progression of cartilage
degradation, which results in the re-initiation of endochondral
bone formation and the subsequent increase in subchondral bone
density and cartilage thinning (2). Angiogenesis results in the
innervation of articular cartilage, therefore providing a potential
source of pain in OA (3). Healthy
cartilage is avascular and aneural, and the mechanisms underlying
blood vessel initiation and invasion into cartilage during OA
remain unknown.
The endogenous, antiangiogenic factor
chondromodulin-I (ChM-I) is specifically expressed in cartilage.
Previous studies have demonstrated that ChM-I may stimulate DNA
synthesis and growth of chondrocytes in culture (4,5), but
may inhibit DNA synthesis and growth of endothelial cells (5–7).
Furthermore, ChM-I may inhibit vascular endothelial growth
factor-A-stimulated chemotactic migration of endothelial cells
(8) and tube morphogenesis of
endothelial cells (5). The
expression of ChM-I is specific to the avascular zone of cartilage
in the developing bones of cattle (5), mice (9) and humans (10). These results suggested that ChM-I
may be involved in the antiangiogenic properties of cartilage, and
the absence of ChM-I expression may create a permissive
microenvironment for vascular invasion of cartilage under
physiological conditions.
Various studies have demonstrated that the loss of
ChM-I expression in articular cartilage may be partly responsible
for promoting the invasion of blood vessels into cartilage during
OA progression (11–13). However, the pattern of ChM-I
expression varies in different cartilage degeneration models. For
example, in a surgically induced rat knee OA model, ChM-I
expression was at first upregulated in the extracellular matrix
(ECM) and cytoplasm of chondrocytes and then the expression
decreased (11). In a study on
immobilized ankle joints of rats, the percentage of ChM-I-positive
cartilage was significantly decreased compared with normal ankle
joints (12). In a rat
temporomandibular joint OA model, the expression of ChM-I in the
cytoplasm was reported to first decrease, and then increase
(13).
The expression of ChM-I and its correlation with
angiogenesis in human cartilage remains to be elucidated. The
present study evaluated the mRNA and protein expression of ChM-I
the in articular cartilage of patients with OA, followed by an
examination of the association between ChM-I and angiogenesis in
non-calcified cartilage. In conclusion, the expression of ChM-I in
the cytoplasm initially decreased and was followed by an increase,
in line with cartilage degeneration. However, the ChM-I in the ECM
decreased gradually, and was correlated with angiogenesis. These
results suggest that maintaining ChM-I levels in the ECM may
improve the ability to resist vascular ingrowth of cartilage,
especially in mild osteoarthritis.
Materials and methods
Patients and samples
The present study was approved by the ethics
committee of The Southwest Hospital of The Third Military Medical
University (Chongqing, China), and each participant provided
written informed consent, according to the Declaration of Helsinki.
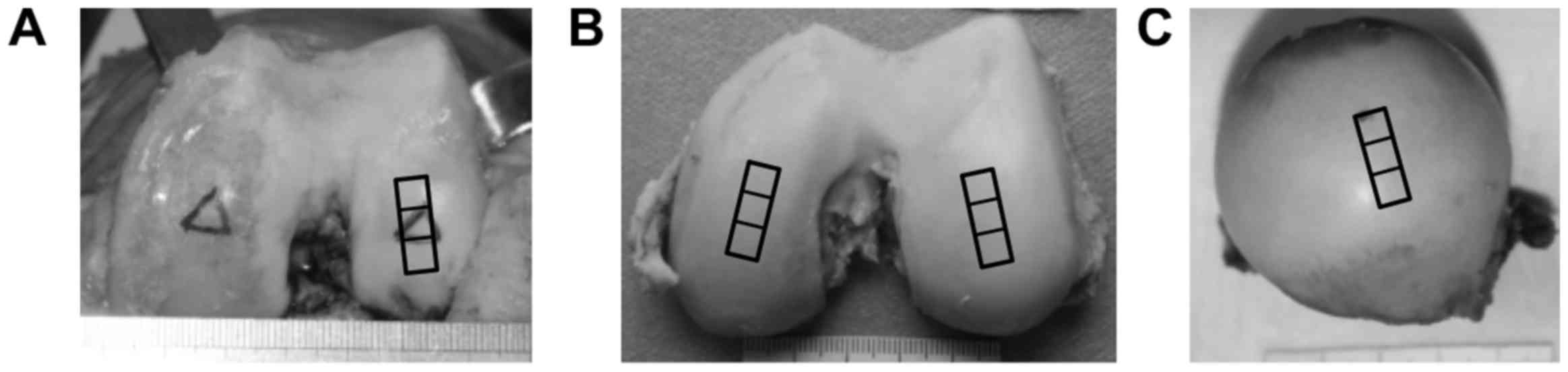
In the OA group, osteochondral samples (1.5×0.5×1.0 cm) were
collected from the weight-bearing area of the lateral femur condyle
of 27 patients (3 males, 24 females, aged 55–60 years) with OA who
were undergoing total knee arthroplasty (Fig. 1A). In the young group, a total of 6
normal cartilage samples were obtained from the weight-bearing area
of the medial and lateral femur condyle of 3 young patients (1
males, 2 females, aged 18–30 years) that had previously undergone
amputative procedures (Fig. 1B).
In the aged group, 7 additional normal cartilage samples were
obtained from the weight-bearing area of the femur head of 7 aged
patients (2 males, 5 females, aged 65–72 years) that had previously
undergone total hip arthroplasty for femoral neck fracture
(Fig. 1C). In the OA group, any
patients with lower extremity trauma or other joint diseases were
excluded. In the young group and the aged group, any patients with
arthropathy were excluded. Each cartilage sample was subdivided
into three parts for subsequent immunohistology, western blotting
and reverse transcription-quantitative polymerase chain reaction
(RT-qPCR) analysis.
Histology
Tissue blocks were decalcified in 10% EDTA for 14
days at 4°C and then embedded in paraffin. Sections (5 µm) were cut
from each paraffin block using an automatic microtome. Following
deparaffinization, sections were stained with hematoxylin for 3 min
and differentiated in 1% acid alcohol for 15s. Sections were
subsequently stained in 0.02% aqueous Fast Green for 3 min, washed
in 1% acetic acid for 15 s to remove remnant stain, and
counterstained in 0.1% Safranin-O for 3 min. Sections were
dehydrated through serial dilutions of ethanol, cleared in xylene,
and mounted using neutral gum. Sections were scored according to
the Osteoarthritis Research Society International grading system
(14) by two different observers
(blinded to the study). The cartilage samples were classified as
normal (G0), mild OA (G1), moderate OA (G2) or severe OA (G3).
According to the method described by Fransès et al (15), osteochondral vascular density was
determined as the number of vascular channels that terminate in the
non-calcified cartilage divided by the section length. A DP26
colored CCD camera (Olympus Corporation, Tokyo, Japan) mounted onto
an Olympus BX51-PMS binocular light microscope (Olympus
Corporation) and the cellSens Life Science Imaging Software
(Olympus Corporation) were used for digital image evaluation. The
results of the evaluation were consistent between the two observers
(r>0.9). The mean values of the two measurements were used for
statistical analysis.
Immunohistochemistry
Immunohistochemical staining was performed on
adjacent sections using the SABC-POD Immunohistochemistry Staining
kit (Boster Systems, Inc., Pleasanton, CA, USA), according to the
manufacturer's protocol. Briefly, sections were deparaffinized in
xylene and rehydrated in graded ethanol and water, and incubated
with 3% H2O2 at room temperature for 10 min.
The slides were washed several times with PBS, pre-incubated with
5% BSA (Beijing Solarbio Science & Technology, Co., Ltd.,
Beijing, China) at room temperature for 20 min, followed by
incubation with rabbit anti-ChM-I antibody (sc-33563; 1:100; Santa
Cruz Biotechnology Inc., Dallas, TX, USA) at 4°C overnight.
Following washes with PBS, the sections were incubated with the
secondary goat anti-rabbit immunoglobulin G HRP antibody (SPN-9001;
1:300; ZSGB-BIO, Beijing, China) at 37°C for 30 min. The color
reaction was developed with 3,3′-diaminodenzidine and
counterstained with hematoxylin. Normal rabbit serum served as a
negative control in place of the anti-ChM-I antibody. Cytoplasmic
immunostaining was graded on a scale of 0–3, where 0=no staining;
1=weak staining; 2=moderate staining; 3=strong staining.
Quantitative analysis of ChM-I immunostaining intensity in ECM was
performed by analysis of the computer gray scan, using Image-Pro
Plus 5.0 (Media Cybernetics, Inc., Rockville, MD, USA).
Western blotting
In western blot analysis, total protein from OA
cartilage and normal cartilage specimens were extracted using a
Total Protein Extraction kit (Nanjing KeyGen Biotech, Co., Ltd.,
Nanjing, China). Protein concentrations were determined with the
Pierce BCA Protein Assay kit (Pierce; Thermo Fisher Scientific,
Inc., Waltham, MA, USA). Total proteins (40 µg) were separated by
8% SDS-PAGE, and transferred to a polyvinylidene difluoride
membrane. Membranes were blocked with 5% skimmed milk at room
temperature for 2 h, and incubated with rabbit polyclonal
anti-ChM-I antibody (1:1,000; sc-33563; Santa Cruz Biotechnology
Inc, Dallas, TX, USA) at 4°C overnight. The membrane was washed
with TBS + 0.1% Tween (TBST) three times for 10 min each, and
incubated with the secondary goat anti-rabbit-IgG-horseradish
peroxidase antibody (1:5,000; ZDR-5306; ZSGB-BIO) at room
temperature for 90 min. The membrane was subsequently washed with
TBST four times for 20 min each. Blots were stripped and reprobed
with mouse monoclonal anti-GAPDH antibody (1:1,000; G8795;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) to confirm
equivalence in loading. Analysis of absorbance was performed using
the Quantity One v4.6.7 (Bio-Rad Laboratories, Inc., Hercules, CA,
USA).
RT-qPCR
Total RNA was extracted with Cartilage RNAout kit
(Beijing Tiandz Gene Technology Co., Ltd., Beijing, China) and
reverse transcribed into cDNA using the PrimeScript RT reagent kit
with gDNA Eraser (Takara Bio, Inc., Otsu, Japan). Target gene
primers were designed as follows: ChM-I, forward
5′-GAAGGCTCGTATTCCTGAGG-3′, reverse 5′-GGCATGATCTTGCCTTCCAG-3′; and
GAPDH (used as an endogenous control), forward
5′-GCACCGTCAAGGCTGAGAA-3′, reverse 5′-TGGTGAAGACGCCAGTGGA-3′. qPCR
was performed in a reaction volume of 25 µl with the QuantiTect
SYBR-Green PCR kit (Qiagen, Inc., Valencia, CA, USA). The cycling
program was performed under the following conditions: 5 min at
95°C, followed by 40 cycles of 10 sec at 95°C, 30 sec at 60°C.
Assays were performed in triplicate on the Applied Biosystems 7500
Real-Time PCR machine (Applied Biosystems; Thermo Fisher
Scientific, Inc.). The expression of ChM-I was normalized to that
of GAPDH using the 2−ΔΔCq method (16).
Statistical analysis
Data were analyzed using the Statistical Package for
Social Scientists (SPSS) version 14.0.1 (SPSS Inc., Chicago, IL,
USA). The categorical variables were reported as absolute values
and the continuous variables as the mean ± standard deviation.
Pearson's χ2 test was used to compare the categorical variables
between the frequencies, corrected for continuity. The normality of
the distribution for continuous variables was examined with the
Kolmogorov-Smirnov test. One-way analysis of variance followed by
the Fisher's least significant difference test was used to compare
the different study groups for normally distributed continuous
variables. The Kruskal-Wallis H test was performed, followed by the
Mann-Whitney U-test and Bonferroni correction if data were not
normally distributed. Correlation between two parameters was
identified by Spearman rank correlation analysis. P<0.05 was
considered to indicate a statistically significant difference. Each
test was repeated six times.
Results
General condition
The present study evaluated age, sex, side and body
mass index of 37 donors (Table I).
No statistically significant differences were identified between
these variables (P>0.05), except for the age of donors
(P<0.001).
 | Table I.Clinical parameters of donors. |
Table I.
Clinical parameters of donors.
| Grade | n | Sex (M/F) | Sides (L/R) | Age (years) | BMI
(kg/m2) |
|---|
| G0 (Y) | 6 | 2/4 | 2/4 | 26.2±3.4 | 22.6±3.6 |
| G0 (A) | 7 | 2/5 | 2/5 | 69.8±2.2 | 26.3±1.8 |
| G1 | 7 | 0/7 | 3/4 | 57.1±1.2 | 25.0±2.4 |
| G2 | 10 | 2/8 | 6/4 | 56.8±1.1 | 25.2±3.0 |
| G3 | 10 | 1/9 | 6/4 | 57.0±1.6 | 25.5±3.1 |
|
F/χ2 |
| 3.554a | 2.802a | 48.243b | 7.965b |
| P-value |
| 0.470 | 0.591 | 0.000 | 0.093 |
ChM-I expression in normal cartilage
of young and aged donors
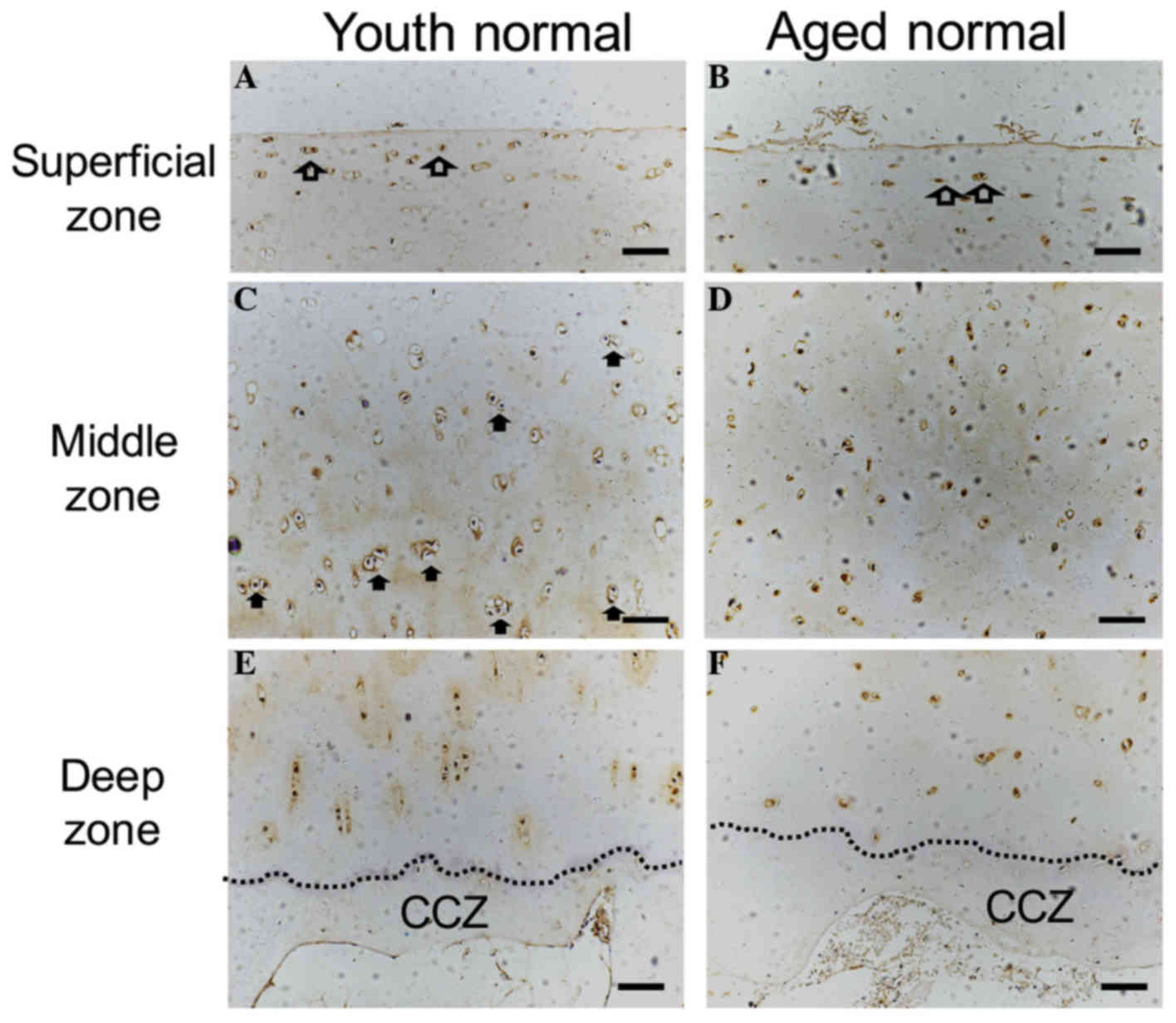
The surface of normal cartilage from both young and
aged donors was observed to be smooth and intact. Flattened cells
were observed in the superficial zone, and ChM-I immunostaining was
positive (Fig. 2A and B). In the
middle zone, mitotic activity appeared to be higher in the young
human cartilage compared with the aged cartilage specimen. ChM-I
protein expression was detected in the cytoplasm and in the
surrounding ECM of the articular cartilage (Fig. 2C and D). Vascular channels were not
observed in the deep zone of either of the two groups, and ChM-I
expression was decreased in the ECM of the lower deep zone and in
the calcified cartilage zone compared with the middle zone
(Fig. 2E and F). ChM-I expression
in the deep zone appeared to be lower in the aged samples compared
with the youth samples, however, no statistical significance
(P>0.05) was identified for ChM-I protein in the cytoplasm of
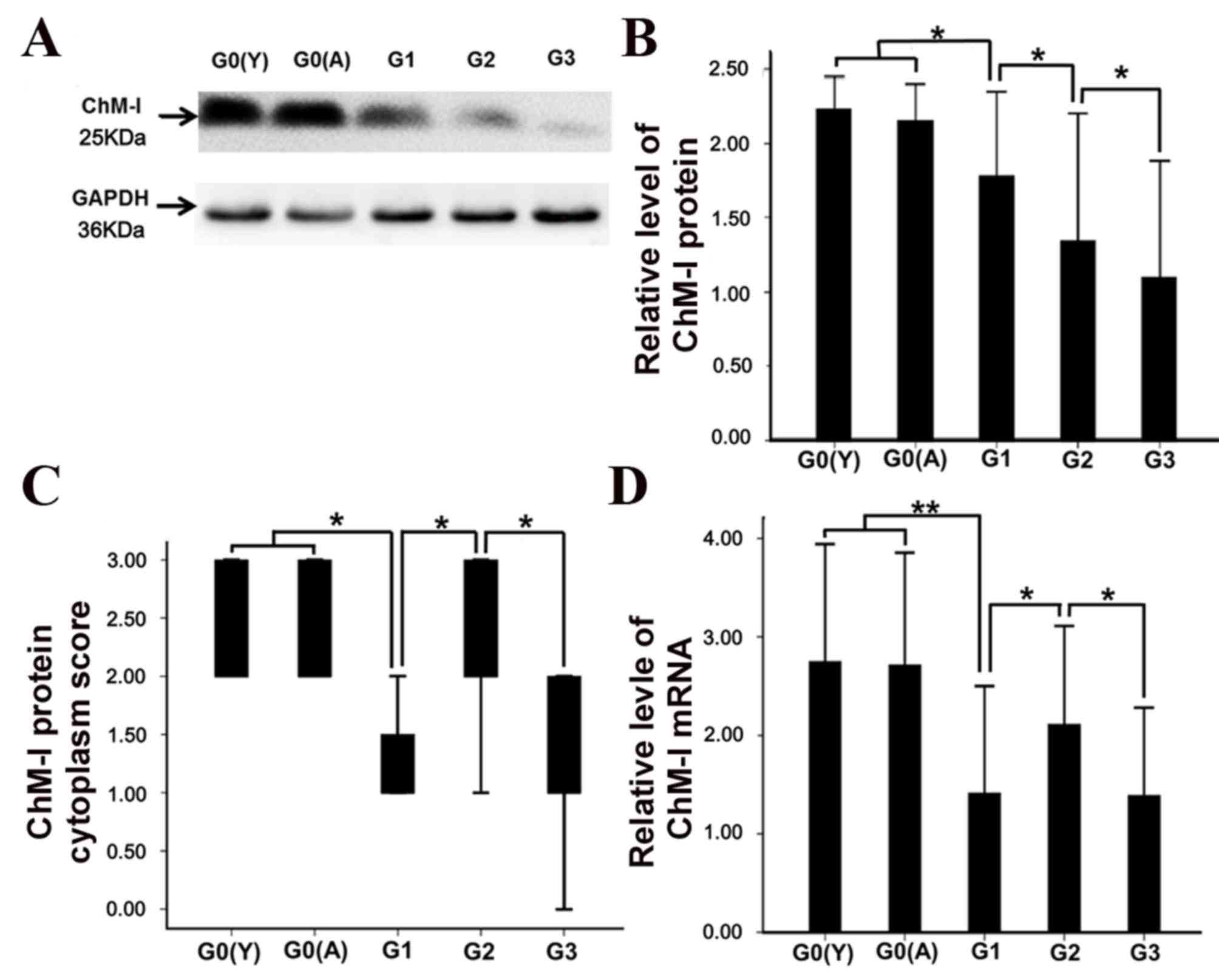
whole cartilage (Fig. 3C).
Furthermore, there was no significant difference between the young
and aged normal cartilage samples in protein (Fig. 3A, B) and mRNA (Fig. 3D) expression levels.
ChM-I expression in OA cartilage
ChM-I expression in cartilage at different stages of
OA was observed by immunostaining, and the results are presented in
Fig. 4. In mild OA cartilage,
chondrocyte fibrosis was observed by Safranin-O/Fast green and
ChM-I protein expression in the cytoplasm was significantly
decreased in the superficial zone, compared with the superficial
zone of normal cartilage (Fig.
4A). In the middle zone, there were no changes in cell
morphology and ChM-I expression compared with the middle zone of
normal cartilage (Fig. 4C). In the
deep zone, vascular vessels were observed invading the
non-calcified cartilage and ChM-I expression was decreased compared
with the deep zone of normal cartilage (Fig. 4F). The mRNA levels of ChM-I were
decreased in mild OA compared with normal cartilage (Fig. 3D; P<0.05).
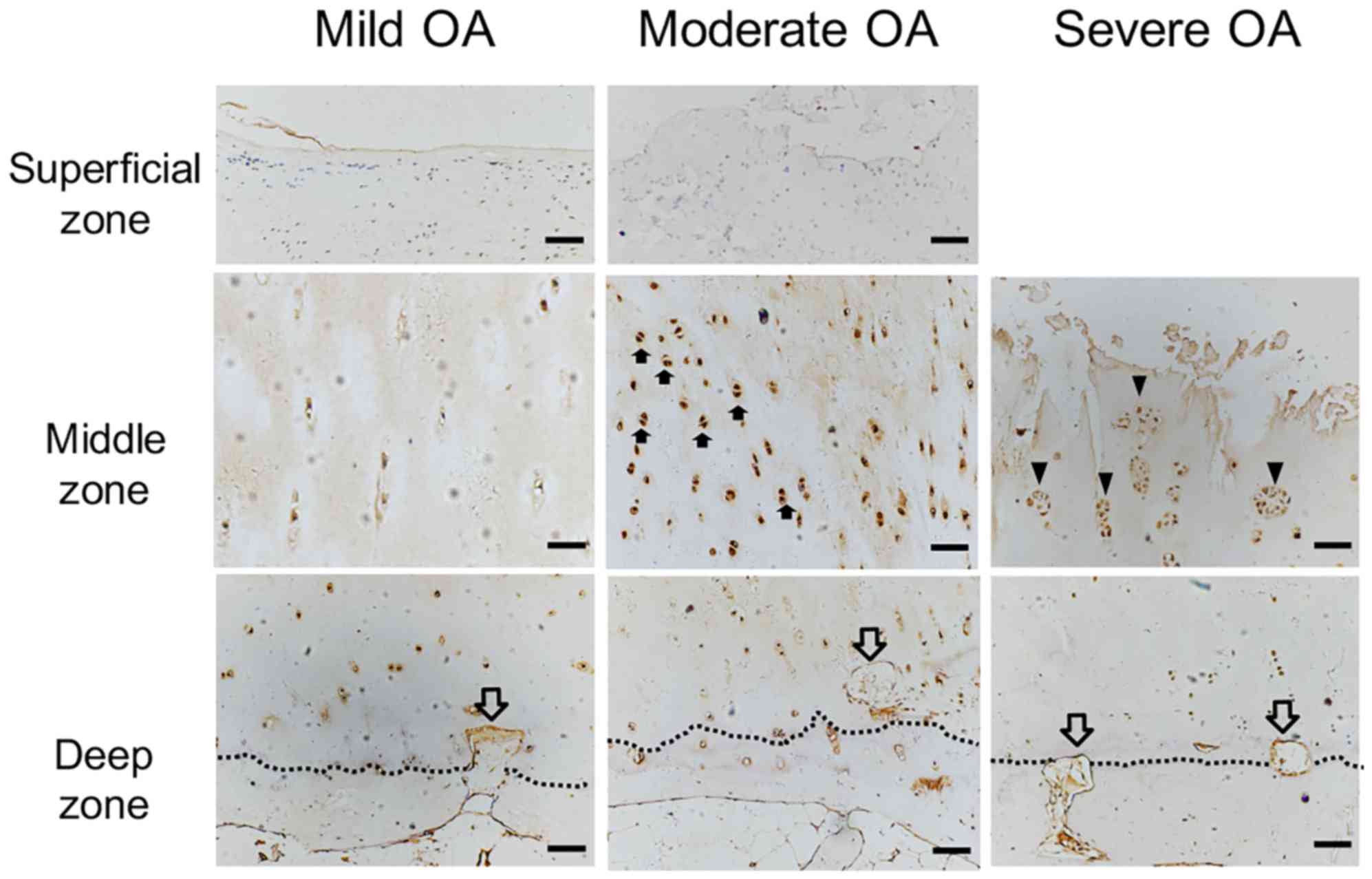 | Figure 4.ChM-I protein expression localization
in OA cartilage. ChM-I protein expression was detected by
immunostaining (brown). ChM-I protein expression was markedly
decreased in the cytoplasm and ECM in the superficial zone of mild
OA cartilage compared with normal cartilage, and vascular channels
(hollow arrow) invaded the non-calcified cartilage in the deep
zone, where ChM-I expression was decreased. Compared with mild OA
cartilage, the mitotically active cells with ChM-I protein
expression (solid arrow) were significantly increased in the
cytoplasm of the moderate OA cartilage middle zone, however there
were no differences in the superficial and deep zones. Vascular
channels were present in the deep zone. In severe OA, ChM-I
expression was primarily observed in the ECM surrounding the
cluster-forming chondrocytes (arrowheads), whereas ChM-I expression
was reduced in the ECM in all other zones of OA cartilage. Scale
bar, 200 µm. ChM-I, chondromodulin-I; ECM, extracellular matrix;
OA, osteoarthritis. |
In moderate OA cartilage, small cracks were observed
in the cartilage surface and ChM-I expression was markedly
decreased in the cytoplasm and ECM of the superficial zone
(Fig. 4B). In the middle zone, the
mitotic activity of chondrocytes appeared to be greater compared
with the young and aged group. ChM-I immunostaining was stronger in
the cytoplasm of moderate OA chondrocytes compared with the mild OA
cartilage (Fig. 4D). In the deep
zone, compared with the normal cartilage, a further decline in the
number of chondrocytes and cytoplasmic ChM-I expression was
observed (Fig. 4G). ChM-I mRNA
expression levels in moderately degenerated cartilage were
significantly higher compared with mildly degenerated cartilage
(Fig. 3D; P<0.05).
In all severe OA cartilage specimens, the cartilage
surface was severely damaged and the chondrocytes had been lost.
Numerous cluster-forming chondrocytes were detected below the
eroded surface (Fig. 4E). ChM-I
protein expression was reduced in the ECM in all zones of the OA
cartilage (Fig. 4E and H). ChM-I
expression was detected primarily in the cytoplasm of the
cluster-forming chondrocytes (Fig.
4E).
Angiogenesis at the osteochondral
junction
The number of vascular channels terminating in the
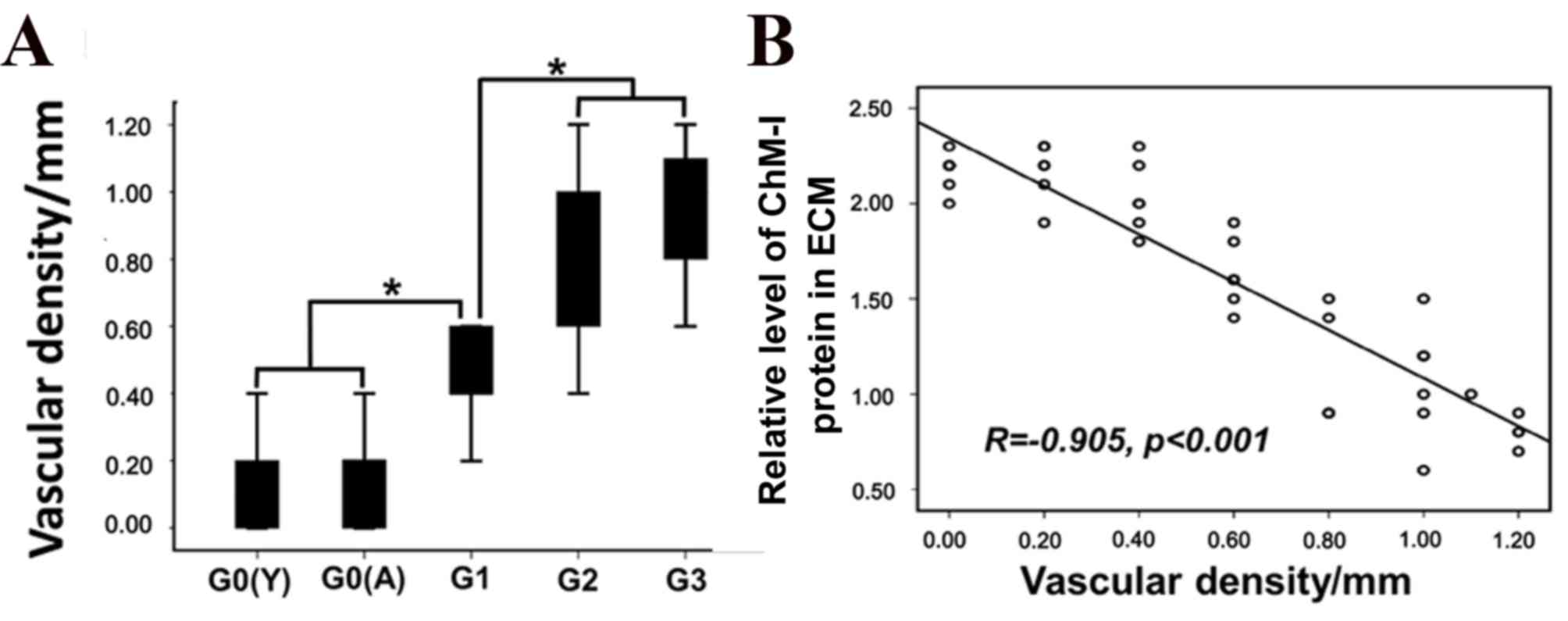
non-calcified cartilage was significantly greater in the OA
cartilage samples compared with normal human cartilage (Fig. 5A; P<0.05). The density of
vascular channels appeared to be correlated with the immunostaining
intensity of ChM-I in ECM (Fig.
5B; r=0.905; P<0.001); however no correlations were
identified between vascular channel density and cytoplasmic ChM-I
protein expression or mRNA levels.
Discussion
It has previously been demonstrated that ChM-I is
expressed in the proliferative and hypertrophic zones of rabbit
condylar cartilage; however, is not expressed in subchondral bone
(17). Increased expression levels
of ChM-I have also been reported in the articular cartilage of
growing and normal adult rat joints (11). The present study demonstrated that
ChM-I was expressed in non-calcified zones of cartilage of young
and aged donors and were without vasculature. These results
indicated that there may be a regulatory role for ChM-I in vascular
invasion during endochondral bone formation (5).
In addition, it was observed that vascular density
in OA cartilage gradually increased with the level of cartilage
degeneration, whereas ChM-I protein expression levels in the ECM
decreased. This suggested that decreased ChM-I expression may lead
to a decreased ability to inhibit angiogenesis during OA
progression. Besides ChM-I, there are other factors that inhibit
angiogenesis, such as thrombospondin-1, type XVIII-derived
endostatin, secreted protein acidic and rich in cysteine and the
type II collagen-derived N-terminal propeptide (18). However, the mechanism that results
in ChM-I reduction in OA cartilage remains to be elucidated. The
results of the present study indicated that the reasons for reduced
ChM-I expression differed at various stages of OA progression. In
early OA, the degeneration of cartilage is mild; a decrease of
ChM-I gene expression in cytoplasm may be associated with cartilage
fibrosis. Fibrillation on the surface of mildly degenerated
cartilage has been observed in a rat OA model and in human OA
samples (11,13), and ChM-I expression was
significantly decreased in the superficial zone of mildly and
moderately degenerated cartilage (19). The expression of certain factors
that promote cartilage fibrosis, such as transforming growth
factor-β and basic fibroblast growth factor, have been demonstrated
to be increased in the superficial zone of OA cartilage and have
been reported to inhibit ChM-I expression (20–24).
The present study also observed more chondrocyte
clusters in moderately and severely degenerated cartilage compared
with mildly degenerated cartilage. ChM-I protein expression was
increased in the cytoplasm of chondrocytes in the middle zone of
moderate OA cartilage. Similarly, ChM-I mRNA levels were increased
in moderately degenerated cartilage compared with mildly
degenerated cartilage. These results appear to be consistent with
the theory that ChM-I promotes chondrocyte proliferation (25). It has been suggested that the
increase in ChM-I protein expression in the cytoplasm may be
associated with the anoxia of OA cartilage (26,27).
Fibrosis of the cartilage surface disrupts oxygen diffusion from
the synovial membrane to the cartilage, and cartilage hypoxia
further promotes ChM-I expression via hypoxia inducible factor 2α
(28). Therefore, chondrocyte
proliferation and increased ChM-I protein expression in cytoplasm
may be upregulated through a defense mechanism against the hypoxia
during the degenerative processes of cartilage (29).
In the middle zone of moderately and severely
degenerated cartilage, ChM-I protein expression in the cytoplasm
was increased; however in the ECM of the middle zone and the deep
zone, the overall level of ChM-I significantly decreased.
Therefore, in the late stages of OA when the cartilage is more
damaged, cartilage matrix loss is very serious, which leads to
decreased ChM-I in the cartilage ECM. A previous study revealed
that ChM-I precursor proteins are cleaved intracellularly and the
mature glycopeptide is rapidly secreted (30). This observation suggested that
newly secreted ChM-I may not be retained in the degenerated
matrices owing to its high solubility in aqueous fluid.
Alternatively, the decrease of ChM-I expression may indicate an
increase of proteolytic enzymes in OA cartilage (30,31).
Notably, the alterations in ChM-I mRNA and protein
expression levels in the rat OA model differ from that in human OA
cartilage. This may be due to the fact that the rats used in the
studies aforementioned were still growing (11). Chondrocytes are proliferative
during the growing stages and may be able to rapidly respond to
cartilage damage. However, OA primarily occurs in the aged, where
the vitality of chondrocytes in OA cartilage is weak and cell
response to cartilage damage is slow (32). Therefore, the use of adult or aged
animal models to study OA may be able to mimic human OA more
successfully.
Several limitations existed in the present study:
First, due to traditional practices in China, few elderly patients
are willing to undergo amputation, causing difficulty in obtaining
knee specimens form elderly patients. The specimens of aged
patients were obtained from the femoral heads, where the
biomechanics are different from that of knee; however, the obtained
specimens were from a weight-bearing area and are close to the
mechanical environment. Second, vascular channels were not
identified with a specific antibody, however the morphology was
observed to be different from other tissues at the chondro-osseous
junction (33).
In conclusion, with the degeneration of cartilage,
the expression of ChM-I in the cytoplasm decreased in mild OA
cartilage and then increased in the moderate OA cartilage. The
ChM-I in ECM of cartilage decreased gradually that was correlated
with the angiogenesis in cartilage. These results suggest that
maintaining ChM-I levels in the ECM may help to improve the ability
to resist vascular ingrowth to the cartilage, especially in mild
osteoarthritis.
Acknowledgements
The present study was supported by The National
Natural Science Foundation of China (grant nos. 31130021 and
31070865), the chief project of The Military Medical Research in
the 12th Five-Year Plan of China (grant no. BWS11C040) and The
National High Technology Research and Development Program of China
(grant no. 2012AA020504).
References
|
1
|
Ashraf S and Walsh DA: Angiogenesis in
osteoarthritis. Curr Opin Rheumatol. 20:573–580. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Suri S and Walsh DA: Osteochondral
alterations in osteoarthritis. Bone. 51:204–211. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Walsh DA, McWilliams DF, Turley MJ, Dixon
MR, Fransès RE, Mapp PI and Wilson D: Angiogenesis and nerve growth
factor at the osteochondral junction in rheumatoid arthritis and
osteoarthritis. Rheumatology (Oxford). 49:1852–1861. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hiraki Y, Tanaka H, Inoue H, Kondo J,
Kamizono A and Suzuki F: Molecular cloning of a new class of
cartilage-specific matrix, chondromodulin-I, which stimulates
growth of cultured chondrocytes. Biochem Biophys Res Commun.
175:971–977. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hiraki Y, Inoue H, Iyama K, Kamizono A,
Ochiai M, Shukunami C, Iijima S, Suzuki F and Kondo J:
Identification of chondromodulin I as a novel endothelial cell
growth inhibitor. Purification and its localization in the
avascular zone of epiphyseal cartilage. J Biol Chem.
272:32419–32426. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shukunami C, Iyama K, Inoue H and Hiraki
Y: Spatiotemporal pattern of the mouse chondromodulin-I gene
expression and its regulatory role in vascular invasion into
cartilage during endochondral bone formation. Int J Dev Biol.
43:39–49. 1999.PubMed/NCBI
|
|
7
|
Kusafuka K, Hiraki Y, Shukunami C,
Yamaguchi A, Kayano T and Takemura T: Cartilage-specific matrix
protein chondromodulin-I is associated with chondroid formation in
salivary pleomorphic adenomas: Immunohistochemical analysis. Am J
Pathol. 158:1465–1472. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Miura S, Mitsui K, Heishi T, Shukunami C,
Sekiguchi K, Kondo J, Sato Y and Hiraki Y: Impairment of
VEGF-A-stimulated lamellipodial extensions and motility of vascular
endothelial cells by chondromodulin-I, a cartilage-derived
angiogenesis inhibitor. Exp Cell Res. 316:775–788. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shukunami C and Hiraki Y: Role of
cartilage-derived anti-angiogenic factor, chondromodulin-I, during
endochondral bone formation. Osteoarthritis Cartilage. 9 Suppl
A:S91–S101. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kusafuka K, Hiraki Y, Shukunami C, Kayano
T and Takemura T: Cartilage-specific matrix protein,
chondromodulin-I (ChM-I), is a strong angio-inhibitor in
endochondral ossification of human neonatal vertebral tissues in
vivo: Relationship with angiogenic factors in the cartilage. Acta
Histochem. 104:167–175. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hayami T, Funaki H, Yaoeda K, Mitui K,
Yamagiwa H, Tokunaga K, Hatano H, Kondo J, Hiraki Y, Yamamoto T, et
al: Expression of the cartilage derived anti-angiogenic factor
chondromodulin-I decreases in the early stage of experimental
osteoarthritis. J Rheumatol. 30:2207–2217. 2003.PubMed/NCBI
|
|
12
|
Sakamoto J, Origuchi T, Okita M, Nakano J,
Kato K, Yoshimura T, Izumi S, Komori T, Nakamura H, Ida H, et al:
Immobilization-induced cartilage degeneration mediated through
expression of hypoxia-inducible factor-1alpha, vascular endothelial
growth factor, and chondromodulin-I. Connect Tissue Res. 50:37–45.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang QY, Dai J, Kuang B, Zhang J, Yu SB,
Duan YZ and Wang MQ: Osteochondral angiogenesis in rat mandibular
condyles with osteoarthritis-like changes. Arch Oral Biol.
57:620–629. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pritzker KP, Gay S, Jimenez SA, Ostergaard
K, Pelletier JP, Revell PA, Salter D and van den Berg WB:
Osteoarthritis cartilage histopathology: Grading and staging.
Osteoarthritis Cartilage. 14:13–29. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fransès RE, McWilliams DF, Mapp PI and
Walsh DA: Osteochondral angiogenesis and increased protease
inhibitor expression in OA. Osteoarthritis Cartilage. 18:563–571.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
gene expression data using real-time quantitative PCR and the
2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fang W, Friis TE, Long X and Xiao Y:
Expression of chondromodulin-1 in the temporomandibular joint
condylar cartilage and disc. J Oral Pathol Med. 39:356–360.
2010.PubMed/NCBI
|
|
18
|
Patra D and Sandell LJ: Antiangiogenic and
anticancer molecules in cartilage. Expert Rev Mol Med. 14:e102012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xing S, Wang Z, Xi H, Zhou L, Wang D, Sang
L, Wang X, Qi M and Zhai L: Establishment of rat bone mesenchymal
stem cell lines stably expressing Chondromodulin I. Int J Clin Exp
Med. 5:34–43. 2012.PubMed/NCBI
|
|
20
|
Pauli C, Whiteside R, Heras FL, Nesic D,
Koziol J, Grogan SP, Matyas J, Pritzker KP, D'Lima DD and Lotz MK:
Comparison of cartilage histopathology assessment systems on human
knee joints at all stages of osteoarthritis development.
Osteoarthritis Cartilage. 20:476–485. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mononen ME, Mikkola MT, Julkunen P, Ojala
R, Nieminen MT, Jurvelin JS and Korhonen RK: Effect of superficial
collagen patterns and fibrillation of femoral articular cartilage
on knee joint mechanics-a 3D finite element analysis. J Biomech.
45:579–587. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tardif G, Pelletier JP, Boileau C and
Martel-Pelletier J: The BMP antagonists follistatin and gremlin in
normal and early osteoarthritic cartilage: An immunohistochemical
study. Osteoarthritis Cartilage. 17:263–270. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Guo J, Zhang W, Li Q, Gan H and Wang Z:
Significance of expressions of matrix metalloproteinase 9 mRNA,
transforming growth factor beta1, mRNA and corresponding proteins
in osteoarthritis. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi.
25:992–997. 2011.(In Chinese). PubMed/NCBI
|
|
24
|
Shukunami C and Hiraki Y: Expression of
cartilage-specific functional matrix chondromodulin-I mRNA in
rabbit growth plate chondrocytes and its responsiveness to growth
stimuli in vitro. Biochem Biophys Res Commun. 249:885–890. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hiraki Y, Inoue H, Kondo J, Kamizono A,
Yoshitake Y, Shukunami C and Suzuki F: A novel growth-promoting
factor derived from fetal bovine cartilage, chondromodulin II
Purification and amino acid sequence. J Biol Chem. 271:22657–22662.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Levick JR: Hypoxia and acidosis in chronic
inflammatory arthritis; relation to vascular supply and dynamic
effusion pressure. J Rheumatol. 17:579–582. 1990.PubMed/NCBI
|
|
27
|
Biniecka M, Kennedy A, Fearon U, Ng CT,
Veale DJ and O'Sullivan JN: Oxidative damage in synovial tissue is
associated with in vivo hypoxic status in the arthritic joint. Ann
Rheum Dis. 69:1172–1178. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lafont JE, Talma S, Hopfgarten C and
Murphy CL: Hypoxia promotes the differentiated human articular
chondrocyte phenotype through SOX9-dependent and -independent
pathways. J Biol Chem. 283:4778–4786. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Takao T, Iwaki T, Kondo J and Hiraki Y:
Immunohistochemistry of chondromodulin-I in the human
intervertebral discs with special reference to the degenerative
changes. Histochem J. 32:545–550. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Azizan A, Holaday N and Neame PJ:
Post-translational processing of bovine chondromodulin-I. J Biol
Chem. 276:23632–23638. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Miura S, Kondo J, Takimoto A, Sano-Takai
H, Guo L, Shukunami C, Tanaka H and Hiraki Y: The N-terminal
cleavage of chondromodulin-I in growth-plate cartilage at the
hypertrophic and calcified zones during bone development. PLoS One.
9:e942392014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Barbero A, Grogan S, Schäfer D, Heberer M,
Mainil-Varlet P and Martin I: Age related changes in human
articular chondrocyte yield, proliferation and post-expansion
chondrogenic capacity. Osteoarthritis Cartilage. 12:476–484. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fransès RE, McWilliams DF, Mapp PI and
Walsh DA: Osteochondral angiogenesis and increased protease
inhibitor expression in OA. Osteoarthritis Cartilage. 18:563–571.
2010. View Article : Google Scholar : PubMed/NCBI
|