Introduction
Atherosclerosis (AS) is the leading cause of
cardiovascular disease and is characterized by structural
alterations in the vascular walls of medium and large arteries. A
large number of studies (1,2) have
indicated that AS is caused by several factors; however, its exact
pathogenesis remains unclear. Inflammatory reaction and lipid
metabolism abnormality are two theories of AS (3). Although the development of AS is
caused by the dysfunction of a number of pathways, one of the
important risk factors is oxidized low-density lipoprotein (ox-LDL)
causing endothelial dysfunction (4). In the development of AS, apoptosis of
the endothelial cells is able to alter endothelial integrity and
increase permeability, thus promoting damage of the blood vessels
and the formation of a plaque. Among the numerous risk factors of
AS, ox-LDL is the primary factor in the promotion of apoptosis in
endothelial cells (5). Ox-LDL is
able to accelerate the proliferation and migration of smooth muscle
cells, monocytes, macrophages and fibroblasts, in addition to the
oxidative stress response and cell damage of endothelial cells
(6,7), eventually leading to AS plaque
formation.
Proprotein convertase subtilisin/kexin type 9
(PCSK9) is a member of the proprotein convertase subtilisin/kexin
family, encoding neural apoptosis-regulated convertase 1 protein.
PCSK9 is a newly identified gene, associated with familial
autosomal dominant hypercholesterolemia (8). The inhibition of PCSK9 is able to
significantly reduce the plasma low-density lipoprotein cholesterol
(LDL-C) levels in the normal population and patients with high
cholesterol taking statins (9,10),
reducing the incidence and mortality of cardiovascular disease
(11). In the present study, the
association between PCSK9 and hyperlipidemia was investigated. That
is, whether PCSK9 can increase the levels of LDL-C in plasma by
degrading the low-density lipoprotein receptor (LDLR) of the
hepatocyte surface (12).
Following this, PCSK9 regulates the metabolism of cholesterol and
promotes the development of AS (13). However, there is has been no
advancement in identifying the direct association between PCSK9 and
AS, providing the context for the present study, which aims to
investigate the direct association between PCSK9 and AS and its
possible mechanism.
Materials and methods
Materials
The human umbilical vein endothelial cell (HUVEC)
line EAhy926 was obtained from the Institute of Pharmacology,
Medical University of Tianjin (Tianjin, China). Ox-LDL was
purchased from Xinyuan Jiahe Biotechnology (Beijing, China). Basic
Dulbecco's modified Eagle's medium (DMEM) and fetal bovine serum
were purchased from Invitrogen (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). RNase inhibitor and Moloney murine leukemia
virus reverse transcriptase was purchased from Takara Biotechnology
Co., Ltd. (Dalian, China). Monoclonal antibodies against PCSK9,
B-cell lymphoma 2 (Bcl-2), bcl-2-like protein 4 (Bax), caspase-3,
p38, phosphorylated (p)-p38, extracellular signal-regulated kinases
(ERK), p-ERK, c-Jun N-terminal kinases (JNK) and p-JNK were
purchased from Abcam (Cambridge, MA, USA). SYBR Green PCR Premix
was purchased from Biocentury TransGene (Beijing, China). Annexin V
fluorescein isothiocyanate (FITC) apoptosis kit was purchased from
BD Biosciences (Franklin Lakes, NJ, USA). The flow cytometer
(version, BDFACS Verse) was purchased from BD Biosciences and
FlowJo software version 7.6 (FlowJo, LLC, Ashland, OR, USA) was
used. The lentiviral packaging system, containing helper plasmids
and target plasmids, was purchased from CWBIO (Beijing, China).
Animal AS model
A total of 12 Apolipoprotein E (ApoE)−/− mice on a
C57 black 6 background were purchased from Beijing University
(Beijing, China). Mice used in this study were male, 6–8 weeks old,
weighed 20–25 g, and were housed in the Second Hospital of Tianjin
Medical University Animal Care Facility under pathogen-free
conditions, according to institutional guidelines (temperature,
22±2°C; relative humidity, 55±15%; noise, <60dB; light:dark
cycle, 12/12 h). All animal study protocols were approved by the
Animal Care and Utilization Committee of Tianjin Medical
University. At 8 weeks of age, ApoE−/− mice were randomly divided
into two groups (n=6 for each). Mice in the control group received
a standard diet, while mice in the AS model group received a
high-fat diet (0.25% cholesterol and 15% cocoa butter) to induce
atherosclerotic plaques. After 20 weeks, the animals were
sacrificed to obtain the aorta. The aorta was fixed in 4%
paraformaldehyde overnight for immunohistochemical analysis and
hematoxylin and eosin staining.
Cell culture
EAhy926 cells were grown in a monolayer and
maintained in DMEM containing 10% fetal bovine serum, 100 U/ml
penicillin G and 100 µg/ml streptomycin and cultured at 37°C in a
humidified incubator with 5% CO2. When the cells reached
the platform stage, cell culture, cell cryopreservation and
subsequent experiments were performed.
Detection of apoptosis rate
EAhy926 cells were seeded in a 24-well plate
(2×105/well) and cultured with ox-LDL. The cells were collected at
the end of each time point and stained by FITC Annexin V and
propidium iodide (PI). Apoptosis rates were detected by flow
cytometry in accordance with the manufacturer's protocols.
Lentiviral transfection
The small hairpin (sh)RNA sequence of PCSK9 was
identified on the Sigma-Aldrich website (www.sigmaaldrich.com/life-science/functional-genomics-and-rnai/shrna/individual-genes.html)
and synthesized by GENEWIZ (Suzhou, China). The recombinant plasmid
was added to the Escherichia coli DH5α cells (CWBIO), according to
the molecular cloning manual. The mixed liquid was evenly coated on
the solid lysogeny broth (LB) medium plate and cultured 12–16 h at
37°C in a humidified incubator. The discrete white colonies were
inoculated into 5 ml LB liquid culture medium containing
4-(aminomethyl) piperidine (100 g/ml) then placed in a constant
temperature oscillator overnight. The plasmid was extracted using
the PurePlasmid Mini kit (CWBIO; Beijing, China). The lentiviral
packaging system containing three helper plasmids (Rev 2.5 µg, VSVG
3 µg and pMDL 5 µg) and 279-target plasmid (279-vector 12 µg,
279-iPCSK9-1 12 µg and 279-iPCSK9-2 12 µg) were co-transfected into
293T cells using polyethylenimine. The virus was collected and
transfected into EAhy926 cells. shRNA-PCSK9 sequences are presented
in Table I.
 | Table I.Primers used in the present
study. |
Table I.
Primers used in the present
study.
| A, Primers used for
plasmid construction |
|---|
|
|---|
| Primers | Sequence |
|---|
| shRNA-PCSK9-1 |
|
Forward | 5GAT
CCC CTC CAC TTC TCT GCC AAA GAT TCA AGA GAT CTT TGG CAG AGA AGT GGA
TTT TTA3′ |
|
Reverse | 5AGC
TTA AAA ATC CAC TTC TCT GCC AAA GAT CTC TTG AAT CTT TGG CAG AGA AGT
GGA GGG3′ |
| shRNA-PCSK9-2 |
|
Forward | 5′GAT
CCC CCA GAG TGA CCA CCG GGA AAT TCA AGA GAT TTC CCG GTG GTC ACT CTG
TTT TTA3′ |
|
Reverse | 5′AGC
TTA AAA ACA GAG TGA CCA CCG GGA AAT CTC TTG AAT TTC CCG GTG GTC ACT
CTG GGG3′ |
|
| B, Primers used for
RT-qPCR |
|
| GAPDH |
|
Forward |
5-CACATGGCCTCCAAGGAGTA-3′ |
|
Reverse |
5-TCCCCTCTTCAAGGGGTCTA −3′ |
| PCSK9 |
|
Forward |
5-TGGAACTCACTCACTCTGGG-3′ |
|
Reverse |
5-AAGAATCCTGCCTCCTTGGT-3′ |
| Bax |
|
Forward |
5-TGATCAGAACCATCATGGGC-3′ |
|
Reverse |
5-GGACATCAGTCGCTTCAGTG-3′ |
| Caspase 3 |
|
Forward |
5-GAGGCCGACTTCTTGTATGC-3′ |
|
Reverse |
5-GTTTCAGCATGGCACAAAGC-3′ |
| Bcl-2 |
|
Forward |
5′-TGATGGGATCGTTGCCTTATG-3′ |
|
Reverse |
5′-CAGTCTACTTCCTCTGTGATGTTG-3′ |
Isolation of RNA and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from cultured cells using
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.).
Reverse transcription was performed using the M-MLV Reverse
Transcription system (Takara Biotechnology Co., Ltd). RT-qPCR was
performed using the SYBR-Green PCR kit as described by the
manufacturer (Biocentury TransGen). Reference gene for the RT-qPCR
was GAPDH. The thermocycling conditions were as follows:
Denaturation (95°C; 30 sec), annealing (58°C; 30 sec), extension
(72°C; 30 sec). The qPCR reaction mix contained: 10 µl 2X
SYBR-Green PCR Premix, 1.5 µl cDNA (dilution, 1:20), 1.5 µl primer
(forward and reverse) and 7 µl ddH2O. PCR primers are
listed in Table I. The qPCR
reaction was carried out using an Applied Biosystems 7500 Fast
Real-Time PCR System (Applied Biosystems; Thermo Fisher Scientific,
Inc.). The relative amount of all mRNAs was calculated using the
2−ΔΔCq method (14).
Western blot analysis
The cells were collected at the end of each
experiment, then washed three times with pre-cooled PBS. Proteins
were extracted from the cells using radioimmunoprecipitation assay
buffer (Beyotime Institute of Biotechnology, Haimen, China). The
protein concentration was determined using the 2-D Quant kit (GE
Healthcare Life Sciences, Chalfont, UK) method. The samples (30
µg/lane) were separated on a 10% SDS-PAGE, and then transferred
onto a PVDF membrane. Subsequently, the membrane was blocked with
5% non-fat milk for 1 h at room temperature. The membrane was
incubated with antibodies against β-actin, PCSK9, Bax, caspase-3,
Bcl-2, p38, p-p38, ERK, p-ERK, JNK and p-JNK at 4°C overnight
(Table II). Following the
overnight incubation, the membrane was washed three times with
TBS-Tween-20 (TBST) for 30 min, and the membrane was incubated with
the secondary antibodies (Abcam) for an additional 1 h at room
temperature. The membrane was washed three times with TBST for 30
min and visualized using the enhanced chemiluminescence Western
blot detection system (EMD Millipore, Billerica, MA, USA).
Reference gene for the western blot analysis was β-actin. All
antibody details are listed in Table
II.
 | Table II.Antibodies used in the present
study. |
Table II.
Antibodies used in the present
study.
|
|
| Primary
antibody | Secondary
antibody |
|
|---|
|
|
|
|
|
|
|---|
| Antibody | Supplier | Catalog number | Dilution | Species raised | Conjugation | Catalog number | Dilution | Molecular weight
(kD) |
|---|
| PCSK9 | Sigma; Merck
KGaA | SAB1302902 | 1:500 | Rabbit | HRP | ab191866 | 1:2,000 | 72 |
| Caspase-3 | Abcam | ab32042 | 1:100 | Rabbit | HRP | ab191866 | 1:5,000 | 32 |
| Bax | Abcam | ab32503 | 1:5,000 | Rabbit | HRP | ab191866 | 1:10,000 | 21 |
| Bcl-2 | Abcam | ab32124 | 1:1,000 | Rabbit | HRP | ab191866 | 1:5,000 | 26 |
| p38 | Abcam | ab27986 | 1:1,000 | Rabbit | HRP | ab191866 | 1:10,000 | 38 |
| p-p38 | Abcam | ab4822 | 1:1,000 | Rabbit | HRP | ab191866 | 1:10,000 | 38 |
| ERK | Abcam | ab17942 | 1:1,000 | Rabbit | HRP | ab191866 | 1:5,000 | 42–44 |
| p-ERK | Abcam | ab214362 | 1:1,000 | Rabbit | HRP | ab191866 | 1:5,000 | 42–44 |
| JNK | Abcam | ab179461 | 1:1,000 | Rabbit | HRP | ab191866 | 1:5,000 | 46 |
| p-JNK | Abcam | ab124956 | 1:1,000 | Rabbit | HRP | ab191866 | 1:5,000 | 46 |
| β-actin | Abcam | ab8226 | 1:10,000 | Mouse | HRP | ab131368 | 1:10,000 | 43 |
Immunohistochemical analysis
Dissected mouse aortae were fixed in 4%
polyformaldehyde (room temperature for 24 h), embedded in paraffin
wax and cut into sections of 3–5 µm. A total of 5 sections were
placed on each slide and the slides placed in an oven at 60°C for 2
h. Following dewaxing by xylene, the sections were passed through
antigen hot fix (121°C for 10 min), DAB staining and hematoxylin
staining. The anti-PCSK9 antibody was used at a dilution of 1:200
(4°C overnight). Finally, the sections were mounted with neutral
gum. The expression of PCSK9 in the tissues was observed under an
optical microscope.
Statistical analysis
All the data are expressed as the mean ± standard
error of the mean. The statistical difference between two
experimental groups was determined using the Student's t-test and
SPSS software (version 19.0; IBM SPSS, Armonk, NY, USA). P<0.05
was considered to indicate a statistically significant
difference.
Results
PCSK9 is highly expressed in
atherosclerotic plaques
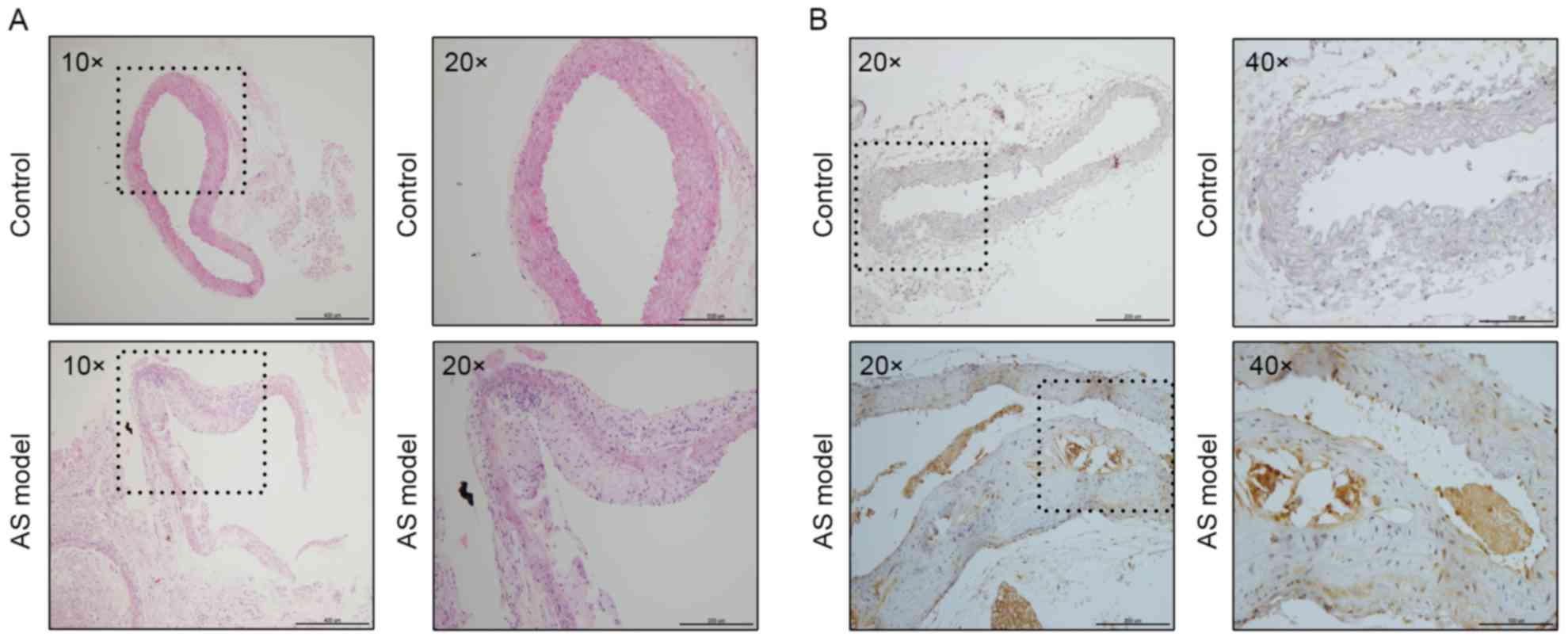
As demonstrated in Fig.
1A, the aortic structure and the vascular endothelium of the
control group was complete and the elastic plate was evident.
Notably, there were no plaques in the arteries. However, the aorta
of the AS model group exhibited marked plaque formation and lipid
deposition. In addition, the vascular endothelial was incomplete,
the smooth muscle layer was broken and there was an infiltration of
numerous inflammatory cells.
To determine the association between PCSK9 and AS,
the aortae of the mice were analyzed by immunohistochemistry. The
nuclei of aortae in the control group were stained clearly;
however, there were no brown-yellow particles around the blue
nucleus. This indicated that PCSK9 is barely expressed in normal
blood vessels. By contrast, there were numerous PCSK9 positive
expression particles in the aortic plaque of the high-fat group. It
demonstrated that PCSK9 exhibited an increased expression level in
atherosclerotic plaques (Fig. 1B).
The results suggest that the expression level of PCSK9 was closely
associated with the presence of AS.
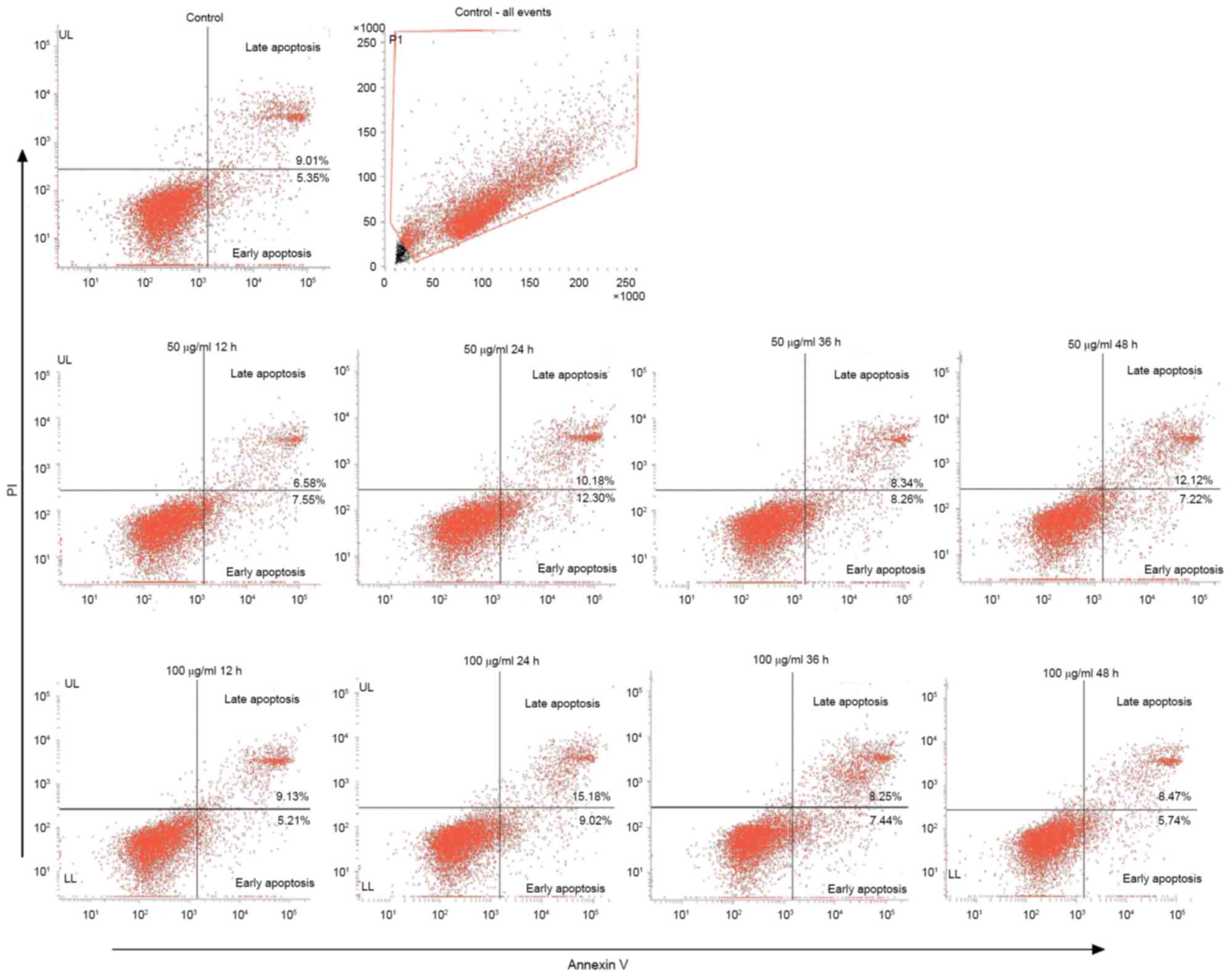
Ox-LDL induces the apoptosis of
EAhy926 cells
Damage to endothelial cells is considered to be an
important basis of the occurrence of AS. Endothelial cell apoptosis
is able to alter the integrity of the endothelium, promote plaque
formation and increase instability of plaques. Ox-LDL is a strong
apoptosis-inducing factor in plaque lesions, and is able to induce
apoptosis in a number of ways. EAhy926 cells were treated with
ox-LDL (50 and 100 µg/ml) at different time points. Cells were
stained with an Annexin V FITC apoptosis kit and the apoptotic rate
was detected by flow cytometry. The results demonstrated that
ox-LDL is able to induce apoptosis in endothelial cells. The
apoptotic rates for 50 µg/ml at 0, 12, 24, 36 and 48 h were 14.36,
14.13, 22.48, 16.6 and 19.34%, respectively. The apoptotic rate of
the 100 µg/ml group were 14.36, 14.33, 24.19, 15.69 and 14.21%.
They demonstrated the highest apoptotic rate resulting from 24 h
treatment. However, no significant difference between the two
groups was observed (Fig. 2).
Therefore, 50 µg/ml ox-LDL was used in the subsequent
experiments.
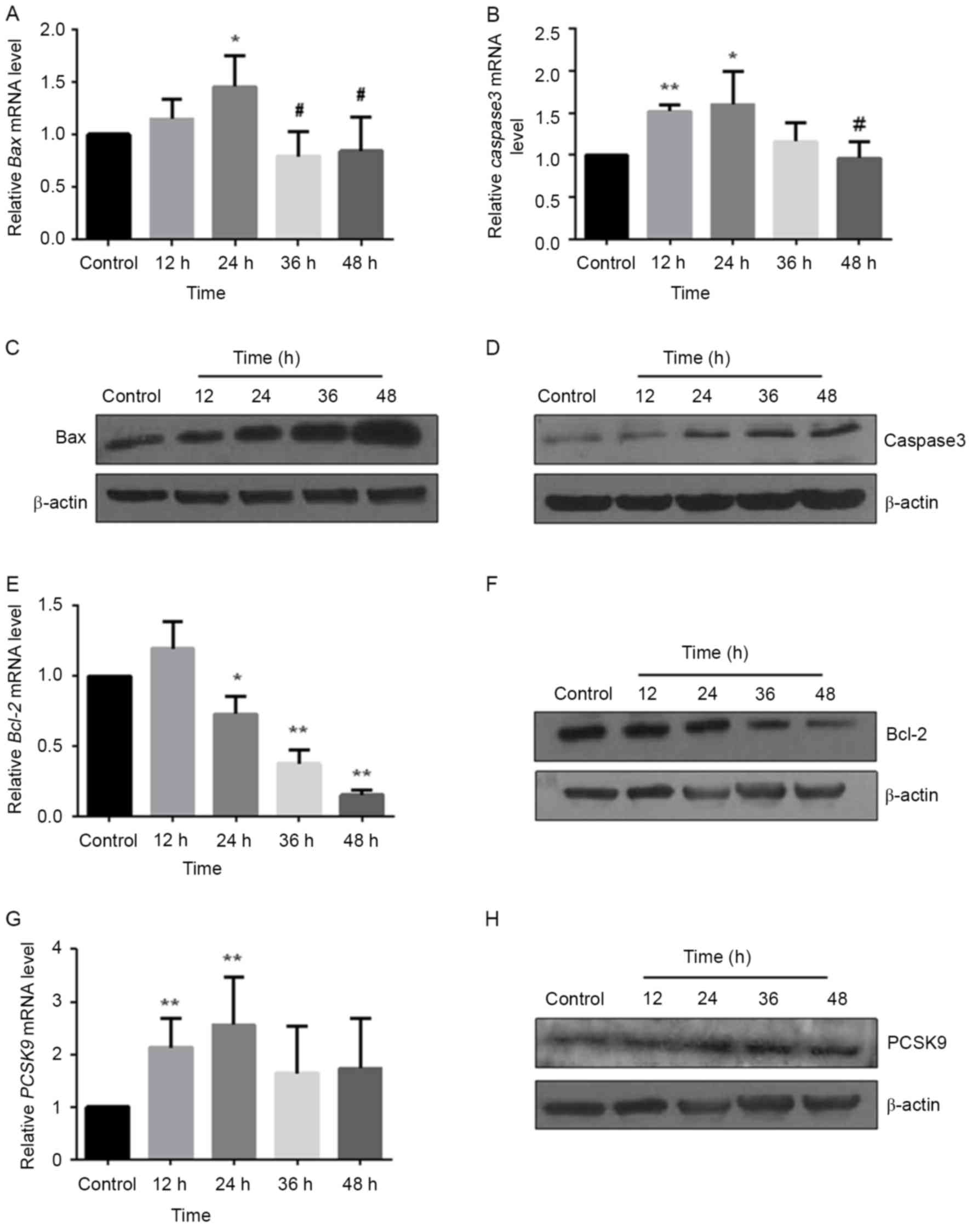
Ox-LDL upregulates pro-apoptotic
factors Bax and caspase-3, and downregulates anti-apoptotic factor
Bcl-2 expression
Bax in the Bcl-2 family and caspase-3 in the
caspase-family serve a key role in the promotion of apoptosis.
Bcl-2 is also the main factor in the anti-apoptosis process. In the
present study, HUVECs were treated with 50 µg/ml ox-LDL at
different time points. The levels of Bax, Bcl-2 and caspase-3 mRNA
and protein were measured using RT-qPCR and western blot analysis,
respectively. With the increase of processing time, the expression
of Bax (Fig. 3A) and caspase-3
(Fig. 3B) mRNA presented a trend
of first increase and then decrease, with the highest expression of
resulting from 24 h treatments (Bax: P<0.05; caspase-3:
P<0.05). The expression levels of Bax and caspase-3 protein were
upregulated in a time-dependent manner (Fig. 3C and D). By contrast, Bcl-2 was
decreased in mRNA (P<0.05; Fig.
3E) and protein (Fig. 3F)
levels induced by ox-LDL.
Ox-LDL upregulates PCSK9 expression in
EAhy926 cells
Although PCSK9 is highly expressed in the AS
plaques, the present study investigated whether it is also
expressed in endothelial cells and whether it is involved in the
apoptosis of endothelial cells. EAhy926 cells were treated with 50
µg/ml ox-LDL at different time periods and the levels of PCSK9 mRNA
were measured using RT-qPCR. As demonstrated in Fig. 3G, the amounts of PCSK9 mRNA
exhibited a gradually increased trend, and reached a peak at 24 h
(P<0.001). The amounts of PCSK9 protein were determined by
western blot analysis. Ox-LDL increased the amounts of PCSK9 in a
time-dependent manner (Fig. 3H).
These results suggested that ox-LDL upregulated the mRNA expression
and protein level of PCSK9 during endothelial cell apoptosis,
implying that PCSK9 may be involved in the development of AS by
participating in the apoptosis of endothelial cells.
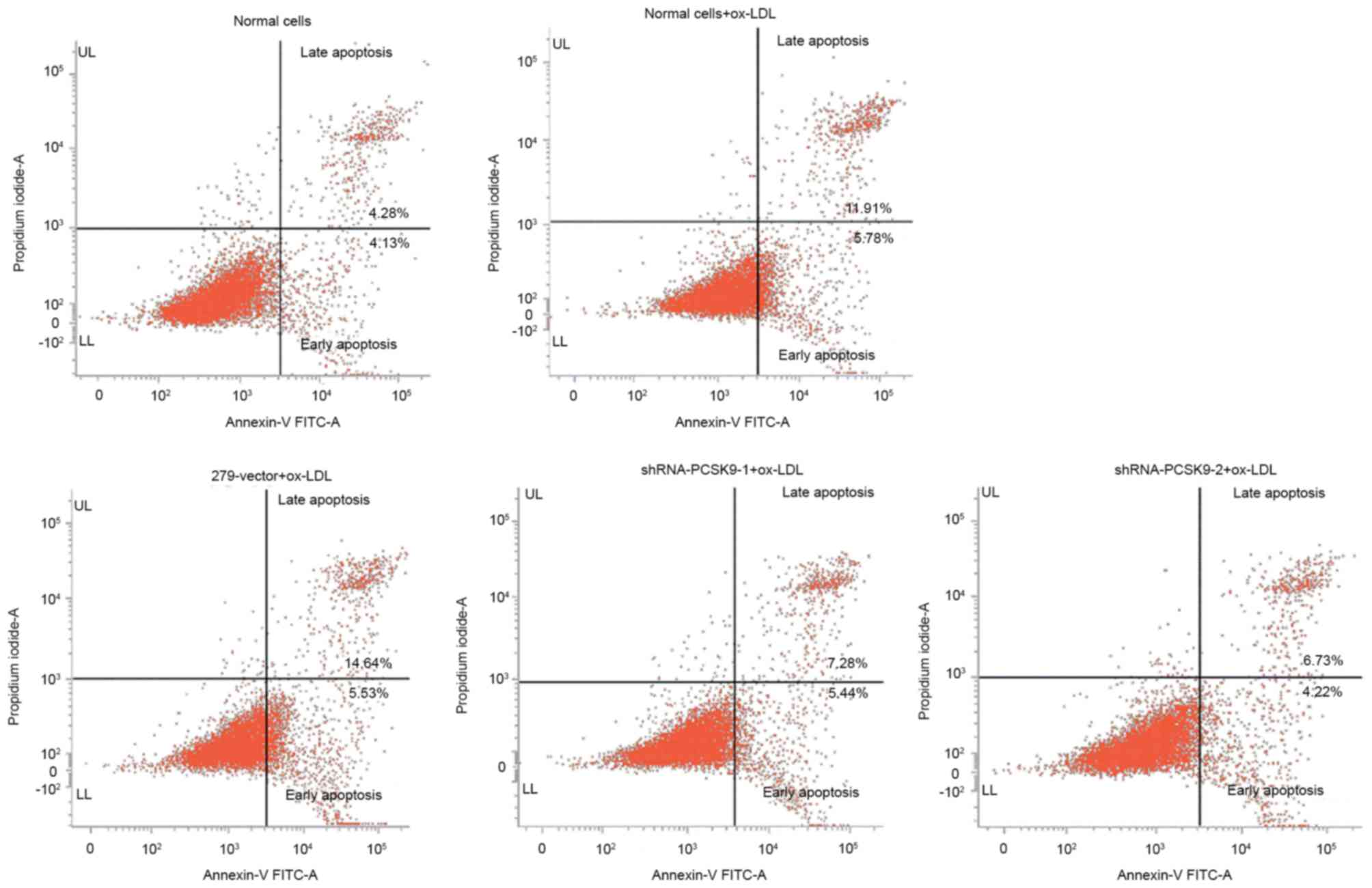
Downregulation of PCSK9 by shRNA-PCSK9
reduces the apoptotic rate of EAhy926 cells
Following transfected by shRNA, the cells were
treated with 50 µg/ml ox-LDL for 24 h and the apoptotic rate was
detected. The apoptotic rate of 279 vector, shRNA-PCSK9-1 and
shRNA-PCSK9-2 were 20.17, 12.72 and 10.95%, respectively (Fig. 4). The results demonstrated that the
apoptosis of EAhy926 cells is reduced with downregulation of
PCSK9.
Detection of transfection
efficiency
To downregulate PCSK9, EAhy926 cells were
transfected with shRNA-PCSK9 packaged by lentivirus. PCSK9 mRNA and
protein levels were detected in the ox-LDL treated endothelial
cells. As demonstrated in Fig. 5A,
compared with the slow virus without the target plasmid
(279-vector), the PCSK9 level was significantly reduced by
transfected shRNA-PCSK9-1 and shRNA-PCSK9-2 (P<0.001; Fig. 5A).
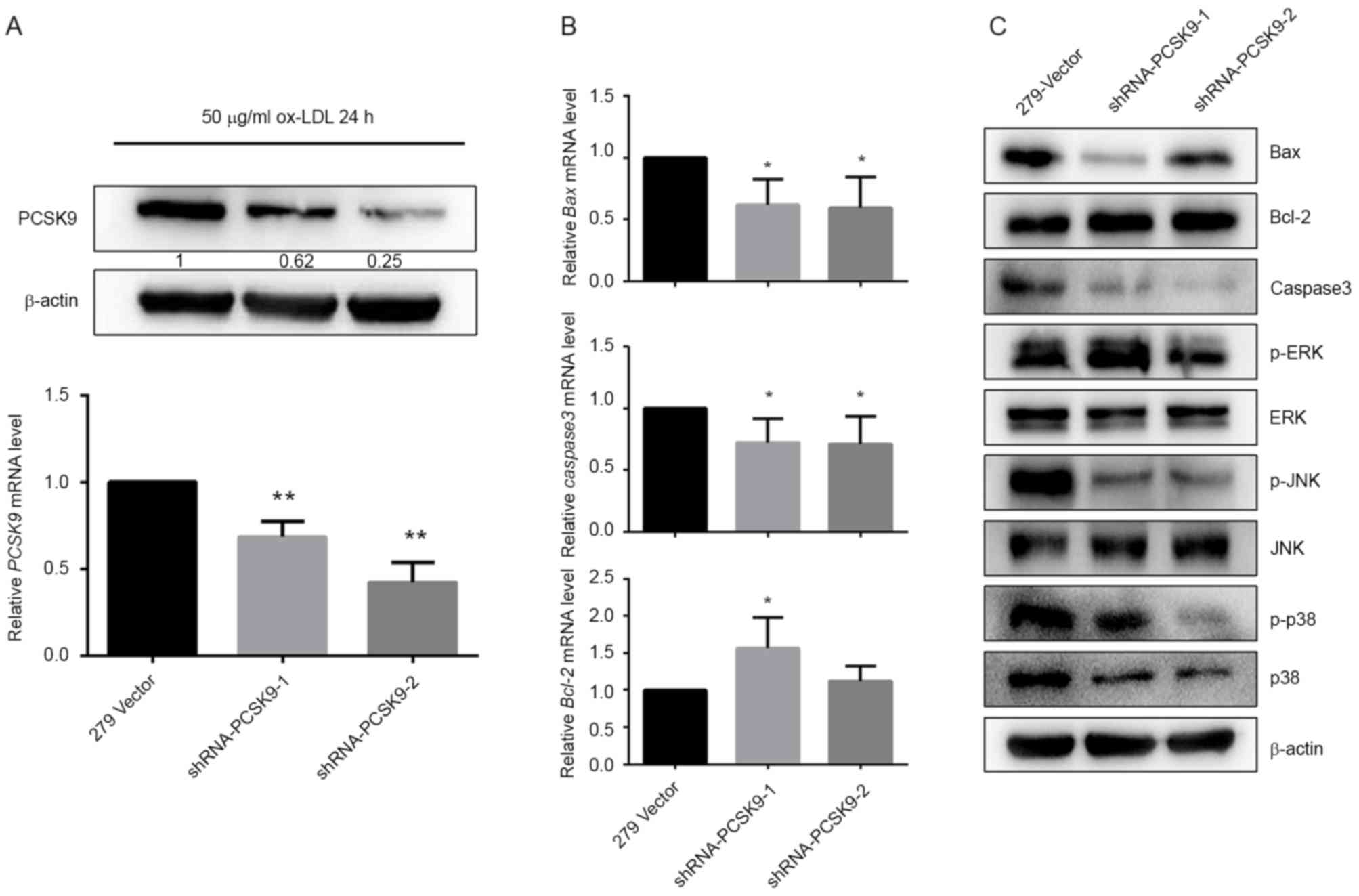 | Figure 5.Effects of shRNA-PCSK9 on expression
of apoptosis-associated and MAPK pathway proteins. EAhy926 cells
were transfected with PCSK9 shRNA and induced by 50 µg/ml ox-LDL
over 24 h. (A) Effects of shRNA-PCSK9 on PCSK9 mRNA and protein
levels. (B) Effects of PCSK9 deficiency on Bax, Bcl-2 and caspase-3
mRNA expression levels. (C) Effects of PCSK9 deficiency on Bax,
Bcl-2, caspase-3, p38, ERK and JNK protein expression levels and
phosphorylation in the ox-LDL treated EAhy926 cells. *P<0.05 and
**P<0.01 vs. 279-vector. All results are expressed as the mean ±
standard error of the mean. shRNA, short hairpin RNA; PCSK9,
proprotein convertase subtilisin/kexin type 9; MAPK,
mitogen-activated protein kinase; ox-LDL, oxidized low-density
lipoprotein; Bax, bcl-2-like protein 4; Bcl-2, B-cell lymphoma 2;
ERK, extracellular signal-regulated kinases; JNK, c-Jun N-terminal
kinases; p-, phosphorylated. |
Downregulation of PCSK9 by shRNA-PCSK9
affects apoptosis related proteins and mitogen-activated protein
kinase (MAPK) pathway proteins expression induced by ox-LDL
Compared with the control group (279-vector), levels
of pro-apoptotic proteins, including Bax and caspase-3, were
suppressed in EAhy926 cells harboring shRNA-PCSK9, while
anti-apoptosis protein Bcl-2 was increased in mRNA and protein
levels (Fig. 5B and C).
Considering that MAPK signaling has been implicated
in cells apoptosis, it was subsequently assessed whether the
pathway was involved in the PCSK9-induced endothelial apoptotic
response in AS. No significant effects of shRNA-PCSK9 on protein
expression levels of JNK and ERK were determined. However, p38,
phosphorylation of p38 and phosphorylation of JNK were inhibited
(Fig. 5C).
Discussion
AS is the primary cause of mortality in developed
countries and a number of developing countries, and is
characterized by plaque formation (15). The rupture of an AS plaque may
cause acute coronary syndrome, a threat to human health (16). Although there are a number of
factors in association with AS, including age, sex, smoking,
diabetes, hypertension and hyperlipidemia, the initiating factors
remain to be completely elucidated (17). Substantial evidence suggests that
AS is a chronic inflammatory and multifactorial disease, in which
vascular endothelial cell dysfunction serves an important role
(18). Vascular endothelial cells
are located on the inner surface of the vessel wall, which acts as
an important permeability barrier between circulating blood and
tissue. These cells are also involved in cellular cholesterol,
lipid homeostasis, signal transduction, inflammation and immunity
(19). Excessive apoptosis of
endothelial cells promotes the formation and rupture of unstable
plaques, which cause arterial wall abnormalities in morphogenesis,
stable structure and metabolism. Therefore, the role of cell
apoptosis cannot be ignored (20).
In previous years, increasing attention has been
focused on PCSK9, a newly discovered gene associated with autosomal
dominant hypercholesterolemia. Similar to LDLR and apolipoprotein
(Apo) B-100, PCSK9 serves an important role in lipid metabolism.
Gene mutations or polymorphisms of PCSK9 are involved in familial
hypercholesterolemia and lead to a high risk of atherosclerotic
cardiovascular disease (21–23).
The removal of LDL-C depends on the binding efficiency of LDL
particles and LDLR. Following the clearing of LDL-C, LDLR is
released and recycled to the liver cell surface, thus LDLR serves a
crucial role in cholesterol homeostasis. A number of studies
(24–26) have confirmed that PCSK9 is able to
reduce the amount of LDLR and interfere with the LDL-C removal
process. PCSK9 is secreted into the blood by hepatocytes and
subsequently binds to LDLR, becoming internalized into the lysosome
for degradation (27). Based on
this, PCSK9 is able to regulate the plasma concentration of LDL-C
beyond the cellular level. However, the effect of PCSK9 on the
degradation of LDLR is independent of its catalytic activity;
however, in its role as a molecular chaperone of LDLR recycling
(28). According to the present
study, PCSK9 is also able to exhibit a direct toxic effect on the
vascular wall.
Apo is a protein component of plasma lipoproteins
and is divided into five groups: A, B, C, D and E. ApoE removes
ligands, participating in a receptor-mediated apo-lipoprotein
cleaning process, primarily formed of chylomicrons and very LDLs
(29). ApoE gene knockout may lead
to upregulation of total plasma cholesterol and LDL-C, thus
inducing arterial intimal injury and lipid deposition, and
ultimately the development of AS (30). Therefore, the present study used
ApoE−/− mice on a high-fat diet, successfully
establishing an AS model. Immunohistochemical analysis of mice
aortae demonstrated that PCSK9 was highly expressed in AS plaques
and the expression levels of PCSK9 are associated with AS.
RNA interference (RNAi) is a highly conserved and
post-transcriptional gene silencing phenomenon, induced by a double
stranded RNA molecule that triggers the highly efficient
degradation of homologous mRNA. It is widespread in the
evolutionary process. At the beginning of the 1990s, the botanist
first identified this phenomenon and designated it gene repression
(31). Fire et al (32) also identified the inhibition of
endogenous gene expression caused by this double stranded RNA in a
nematode and was the first to designate it RNAi. In 2001, RNAi was
observed in mammalian cells (33).
RNAi was gradually demonstrated to exist widely in a number of
eukaryotic organisms.
The apoptosis of vascular endothelial cells is an
important factor in the pathogenesis of AS and is an important
target for preventing and delaying the disease (34). The Bcl protein family is an
important factor in apoptosis regulation, in which Bcl-2 is an
anti-apoptotic protein and Bax is a pro-apoptotic protein. The
shift in the balance between pro-apoptotic and anti-apoptotic
proteins leads to the occurrence of apoptosis (35). In the process of inducing
apoptosis, the activation of the caspase pathway is another
specific marker (36). Caspase is
frequently used as a target for anti-AS drugs in the process of
endothelial cell apoptosis and the development of AS (37). In the present study, apoptosis was
induced in HUVECs by ox-LDL and the expression of pro-apoptotic
protein Bax and caspase-3 was significantly promoted in a
time-dependent manner. Conversely, expression of Bcl-2 was blocked.
At the same time, PCSK9 expression in mRNA and protein levels was
significantly increased in apoptotic HUVECs compared with normal
cells. However, PCSK9 was downregulated during shRNA-PCSK9
transfection. The present study also identified that transfection
of cells with shRNA-PCSK9 inhibited the mRNA and protein expression
of Bax and caspase-3, while Bcl-2 demonstrated the opposite role.
The ratio of Bcl-2/Bax was increased by PCSK9 deficiency in
ox-LDL-induced apoptosis.
The MAPK signaling pathway is one of the most
important in the eukaryotic signaling network, involved in a
variety of cellular functions, particularly cell proliferation,
differentiation and apoptosis (38). It has been reported that JNK and
p38 MAPK serve an important role in inflammation and cell apoptosis
due to activation by TNF-α, IL-1, G protein coupled receptors,
stress and oxidative damage (39).
The ERK signaling pathway serves an important role in the process
of cell proliferation mediated by growth factors (40). It is notable that the sustained
activation of ERK can prevent the occurrence of apoptosis (41). In the present study, shRNA-PCSK9
markedly decreased the phosphorylation of p38 and JNK, suggesting
that the p38/JNK-dependent pathway may be involved in
PCSK9-mediated endothelial cell apoptosis in AS.
In conclusion, the findings of the present study
suggested that PCSK9 promotes the apoptosis of endothelial cells
induced by ox-LDL in AS via the JNK/p38 MAPK pathway. The
comprehensive understanding of PCSK9 provides a new insight into
the mechanism of AS and a new target and direction for its
treatment.
Acknowledgements
The present study was supported by grants to G.L.
from the National Natural Science Foundation of China (grant no.
81370300) and the Specialized Research Fund for the Doctoral
Program of Higher Education (grant no. 20121202110004), and a grant
to Y.W. from the Science and Technology Foundation of Tianjin
Sanitary Bureau (grant no. 2015KZ105).
References
|
1
|
Pescetelli I, Zimarino M, Ghirarduzzi A
and De Caterina R: Localizing factors in atherosclerosis. J
Cardiovasc Med (Hagerstown). 16:824–830. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yusuf S, Hawken S, Ounpuu S, Dans T,
Avezum A, Lanas F, McQueen M, Budaj A, Pais P, Varigos J, et al:
Effect of potentially modifiable risk factors associated with
myocardial infarction in 52 countries (the INTERHEART study):
Case-control study. Lancet. 364:937–952. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Weber C and Noels H: Atherosclerosis:
Current pathogenesis and therapeutic options. Nat Med.
17:1410–1422. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Napoli C: Oxidation of LDL, atherogenesis,
and apoptosis. Ann N Y Acad Sci. 1010:698–709. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lu J, Mitra S, Wang X, Khaidakov M and
Mehta JL: Oxidative stress and lectin-like ox-LDL-receptor LOX-1 in
atherogenesis and tumorigenesis. Antioxid Redox Signal.
15:2301–2333. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Geng YJ: Biologic effect and molecular
regulation of vascular apoptosis in atherosclerosis. Curr
Atheroscler Rep. 3:234–242. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cai H and Harrison DG: Endothelial
dysfunction in cardiovascular diseases: The role of oxidant stress.
Circ Res. 87:840–844. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Abifadel M, Guerin M, Benjannet S, Rabès
JP, Le Goff W, Julia Z, Hamelin J, Carreau V, Varret M, Bruckert E,
et al: Identification and characterization of new gain-of-function
mutations in the PCSK9 gene responsible for autosomal dominant
hypercholesterolemia. Atherosclerosis. 223:394–400. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Roth EM, McKenney JM, Hanotin C, Asset G
and Stein EA: Atorvastatin with or without an antibody to PCSK9 in
primary hypercholesterolemia. N Engl J Med. 367:1891–1900. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee P and Hegele RA: Current Phase II
proprotein convertase subtilisin/kexin 9 inhibitor therapies for
dyslipidemia. Expert Opin Investig Drugs. 22:1411–1423. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Poirier S and Mayer G: The biology of
PCSK9 from the endoplasmic reticulum to lysosomes: New and emerging
therapeutics to control low-density lipoprotein cholesterol. Drug
Des Devel Ther. 7:1135–1148. 2013.PubMed/NCBI
|
|
12
|
Leren TP: Sorting an LDL receptor with
bound PCSK9 to intracellular degradation. Atherosclerosis.
237:76–81. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Constantinides A, Kappelle PJ, Lambert G
and Dullaart RP: Plasma lipoprotein-associated phospholipase A2 is
inversely correlated with proprotein convertase subtilisin-kexin
type 9. Arch Med Res. 43:11–14. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Libby P, Ridker PM and Maseri A:
Inflammation and atherosclerosis. Circulation. 105:1135–1143. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rioufol G, Finet G, Ginon I, André-Fouët
X, Rossi R, Vialle E, Desjoyaux E, Convert G, Huret JF and Tabib A:
Multiple atherosclerotic plaque rupture in acute coronary syndrome:
A three-vessel intravascular ultrasound study. Circulation.
106:804–808. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gu HF, Tang CK and Yang YZ: Psychological
stress, immune response, and atherosclerosis. Atherosclerosis.
223:69–77. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hansson GK: Inflammation, atherosclerosis,
and coronary artery disease. N Engl J Med. 352:1685–1695. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Simionescu M and Antohe F: Functional
ultrastructure of the vascular endothelium: Changes in various
pathologies. Handb Exp Pharmacol. 41–69. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kwon GP, Schroeder JL, Amar MJ, Remaley AT
and Balaban RS: Contribution of macromolecular structure to the
retention of low-density lipoprotein at arterial branch points.
Circulation. 117:2919–2927. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Benn M, Nordestgaard BG, Grande P, Schnohr
P and Tybjaerg-Hansen A: PCSK9 R46L, low-density lipoprotein
cholesterol levels, and risk of ischemic heart disease: 3
independent studies and meta-analyses. J Am Coll Cardiol.
55:2833–2842. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang L, Yuan F, Liu P, Fei L, Huang Y, Xu
L, Hao L, Qiu X, Le Y, Yang X, et al: Association between PCSK9 and
LDLR gene polymorphisms with coronary heart disease: Case-control
study and meta-analysis. Clin Biochem. 46:727–732. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wu NQ and Li JJ: PCSK9 gene mutations and
low-density lipoprotein cholesterol. Clin Chim Acta. 431:148–153.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang Y, Huang Y, Hobbs HH and Cohen JC:
Molecular characterization of proprotein convertase
subtilisin/kexin type 9-mediated degradation of the LDLR. J Lipid
Res. 53:1932–1943. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tavori H, Fan D, Blakemore JL, Yancey PG,
Ding L, Linton MF and Fazio S: Serum proprotein convertase
subtilisin/kexin type 9 and cell surface low-density lipoprotein
receptor: Evidence for a reciprocal regulation. Circulation.
127:2403–2413. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nguyen MA, Kosenko T and Lagace TA:
Internalized PCSK9 dissociates from recycling LDL receptors in
PCSK9-resistant SV-589 fibroblasts. J Lipid Res. 55:266–275. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang DW, Lagace TA, Garuti R, Zhao Z,
McDonald M, Horton JD, Cohen JC and Hobbs HH: Binding of proprotein
convertase subtilisin/kexin type 9 to epidermal growth factor-like
repeat A of low density lipoprotein receptor decreases receptor
recycling and increases degradation. J Biol Chem. 282:18602–18612.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mousavi SA, Berge KE and Leren TP: The
unique role of proprotein convertase subtilisin/kexin 9 in
cholesterol homeostasis. J Intern Med. 266:507–519. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Plump AS and Breslow JL: Apolipoprotein E
and the apolipoprotein E-deficient mouse. Annu Rev Nutr.
15:495–518. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Getz GS and Reardon CA: Animal models of
atherosclerosis. Arterioscler Thromb Vasc Biol. 32:1104–1115. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jorgensen RA: Cosuppression, flower color
patterns, and metastable gene expression states. Science.
268:686–691. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fire A, Xu S, Montgomery MK, Kostas SA,
Driver SE and Mello CC: Potent and specific genetic interference by
double-stranded RNA in Caenorhabditis elegans. Nature. 391:806–811.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Elbashir SM, Martinez J, Patkaniowska A,
Lendeckel W and Tuschl T: Functional anatomy of siRNAs for
mediating efficient RNAi in Drosophila melanogaster embryo lysate.
EMBO J. 20:6877–6888. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Onat D, Brillon D, Colombo PC and Schmidt
AM: Human vascular endothelial cells: A model system for studying
vascular inflammation in diabetes and atherosclerosis. Curr Diab
Rep. 11:193–202. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Berens HM and Tyler KL: The proapoptotic
Bcl-2 protein Bax plays an important role in the pathogenesis of
reovirus encephalitis. J Virol. 85:3858–3871. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sanz AB, Santamaría B, Ruiz-Ortega M,
Egido J and Ortiz A: Mechanisms of renal apoptosis in health and
disease. J Am Soc Nephrol. 19:1634–1642. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang Y, Chen N, Zhang J and Tong Y:
Hsa-let-7g miRNA targets caspase-3 and inhibits the apoptosis
induced by ox-LDL in endothelial cells. Int J Mol Sci.
14:22708–22720. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Aplin AE, Hogan BP, Tomeu J and Juliano
RL: Cell adhesion differentially regulates the nucleocytoplasmic
distribution of active MAP kinases. J Cell Sci. 115:2781–2790.
2002.PubMed/NCBI
|
|
39
|
Weston CR and Davis RJ: The JNK signal
transduction pathway. Curr Opin Genet Dev. 12:14–21. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Poulikakos PI, Persaud Y, Janakiraman M,
Kong X, Ng C, Moriceau G, Shi H, Atefi M, Titz B, Gabay MT, et al:
RAF inhibitor resistance is mediated by dimerization of aberrantly
spliced BRAF(V600E). Nature. 480:387–390. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Krens SF, Spaink HP and Snaar-Jagalska BE:
Functions of the MAPK family in vertebrate-development. FEBS Lett.
580:4984–4990. 2006. View Article : Google Scholar : PubMed/NCBI
|