Introduction
Mesenchymal stem cells (MSCs) can be derived from
various tissues, including fat, muscle and the umbilical cord. MSCs
are capable of self-renewing and differentiating into distinct cell
types, such as chondrocytes, osteoblasts and adipocytes, and thus
hold promise for regenerative medicine (1). MSCs express several surface proteins,
including CD29, CD59, CD90, CD105 and CD44, but low levels of
undetectable hematopoietic markers, such as CD14, CD34, CD31 and
HLA class I molecules (2). MSCs
also lack expression of co-stimulatory CD80, CD86 or CD40, even
following IFN-γ stimulation (3).
Another important feature of MSCs is that they may inhibit
activation, proliferation and function of immune cells, including T
cells, B cells, NK cells and antigen-presenting cells (4–6). Due
to these features, MSCs hold great promise for treating various
diseases. MSCs had now been used in many clinical trials to treat
many diseases, such as graft vs. host disease, live fibrosis and
cardiovascular diseases (5–7).
However, accumulated evidence indicates that engrafted MSCs could
stimulate the immune responses and, finally, result in the
rejection of MSCs (8). In murine
bone marrow transplantation models, memory T cells may reject the
engrafted MSCs (9) and it was also
demonstrated that NK cell ligands, which expressed on MSC, mediated
the rejection of MSC by NK cells (10).
The Toll-like receptor (TLR) family involves types
of molecules that are expressed in immune and non-immune cells and
act as important sensors for the detection of heterogeneous
pathogens and endogenous danger signals (11). Moreover, generally TLRs activate a
rapid effector response, which implies intrinsic and adaptive
immune responses (12).
Stimulation of cells with a TLR ligand recruits intracellular
adaptor proteins, including myeloid-differentiation
primary-response protein 88 and adaptor-inducing interferon
(IFN)-β, triggering downstream signaling cascades and productions
of pro-inflammatory cytokines and chemokines (13). A previous study indicated that MSCs
are activated by TLR ligands leading to modulation of the
differentiation, migration, proliferation, survival and
immunosuppression capacities (14). TLR3 in MSCs has been reported to
significantly prolong the survival and function of neutrophils
(14). It was also reported that
TLR8 and TLR9 activation increased the migration of MSCs, but
activation of the TLR8 pathway had no significant effects on
immunogenicity, immunosuppression and survival of MSCs when
isolated from bone marrow and adipose (15–17).
In the present study, MSCs were isolated from the
umbilical cord and stimulated with the TLR8 agonist, R848. The aim
of the current study was to evaluate the role of TLR8 in mediating
the changing of immune status of UCMSCs.
Materials and methods
Isolation and culture of UCMSCs
The UCMSCs were provided by the Sichuan Cord Blood
Bank (Chengdu, China) and maintained in Dulbecco's modified Eagle's
medium (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) containing 10% fetal bovine serum, 2 mM glutamine, 1 mM sodium
pyruvate, 100 U/ml penicillin and 100 g/ml streptomycin (all from
Invitrogen; Thermo Fisher Scientific, Inc.), and subcultured every
3–4 days by using fresh media. UCMSCs were incubated at 37°C in a
5% CO2 humidified atmosphere and used in the experiments
only after 2 to 3 expansion passages to ensure depletion of
monocytes/macrophages.
TLR8 agonists that stimulate MSCs
TLR8 reagent R848 was from Enzo Life Sciences, Inc.
(ALX-420-040; Farmingdale, NY, USA) and dissolved in DMSO as a
storage concentration of 25 mg/ml. UCMSCs were seeded into six-well
plate at a concentration of 1.5×105 in 2 ml medium and the TLR8
agonist was added into the UCMSC culture medium at a final
stimulation concentration of 5 µg/ml. PBMCs were marked with
carboxyfluorescein succinimidyl ester.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated from confluent MSCs using the
RNeasy mini kit (74104; Qiagen GmbH, Hilden, Germany). cDNA
synthesis was performed using ReverTra Ace qPCR RT kit (FSQ-101;
Toyobo Co., Ltd., Osaka, Japan) under the conditions: 65°C for 5
min, followed by 37°C for 15 min and 98°C for 5 min. The levels of
mRNA of genes of interest were measured by RT-qPCR
(iCycleriQ™ Optical Module, Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) using RealMaster Mix (SYBR-Green; FP202, Tiangen
Biotech Co., Ltd., Beijing, China), under the following conditions:
One cycle at 95°C for 30 sec, 40 cycles at 95°C for 30 sec, 58°C
for 30 sec and 72°C for 30 sec, followed by a melt curve from 55 to
95°C in 0.5°C increments and 10 sec intervals.
Total amount of mRNA was normalized to endogenous
GAPDH mRNA (18). Sequences of PCR
primer pairs are presented in Table
I. All experiments were performed three times.
 | Table I.List of oligonucleotides used for
reverse transcription-quantitative polymerase chain reaction
analysis. |
Table I.
List of oligonucleotides used for
reverse transcription-quantitative polymerase chain reaction
analysis.
| Gene number | Forward primer | Reverse primer | GenBank |
|---|
| CDC2 |
CAGGTTATATCTCATCTTTGAG |
GTTGAGTAACGAGCTGACCCC | AM393287 |
| PTEN |
ACCATAACCCACCACAGC |
CAGTTCGTCCCTTTCCAG | NM_058074 |
| PCNA |
ATTCCAGAACAGGAGTACAGCTGT |
CAGATGTACCCCTTGTTGTAGAGT | NM_002592 |
| VEGF |
GACTTGAGTTGGGAGGGGAA |
GAGGCTCAGCGCCAGGGCTGGG | AF024710 |
| CD80 |
TGGCAACGCTGTCCTGTG |
CCTTTTGCCAGTAGATGCGAG | M27533 |
| CD86 |
GGGCCGCACAAGTTTTGA |
GCCCTTGTCCTTGATCTGAAGA | L25259 |
| IL-1β |
ACGAATCTCCGACCACCACT |
CCATGGCCACAACAACTGAC | M15330 |
| IL-6 |
GACCCAACCACAAATGCCA |
GTCATGTCCTGCAGCCACTG | M14584 |
| IL-8 |
CTGGCCGTGGCTCTCTTG |
CCTTGGCAAAACTGCACCTT | NM_000584 |
| IL-9 |
CTCTGTTTGGGCATTCCCTCT |
GGGTATCTTGTTTGCATGGTGG | M30134 |
| IL-10 |
GGTGATGCCCCAAGCTGA |
TCCCCCAGGGAGTTCACA | U16720 |
| IL-11 |
CGAGCGGACCTACTGTCCTA |
GCCCAGTCAAGTGTCAGGT | NM_000641 |
| IL-12 |
CGGTCATCTGCCGCAAA |
CAAGATGAGCTATAGTAGCGGTCCT | M65272 |
| IL-15 |
TTTCTAACTGAAGCTGGCATTCAT |
CCAGTTGGCTTCTGTTTTAGGA | U14407 |
| I-309 |
GCAGATCATCACCACAGCC |
GTCCACATCTTCCGGCCA | NM_002981 |
| EOTA-3 |
CCAAGACCTGCTGCTTCCAA |
GAATTCATAGCTTCGCACCCA | NM_006072 |
| MCP-4 |
CAGTGCTTCTGTGCCTGCTG |
TGCATCTGGCTGAGCAAGTC | NM_005408 |
| MIP-1β |
CTGCTCTCCAGCGCTCTCA |
GTAAGAAAAGCAGCAGGCGG | NM_002984 |
| GAPDH |
GAAGGTGAAGGTCGGAGTC |
GAAGATGGTGATGGGATTTC | J04038 |
Antibody chip array
Analyzed secretion of protein of supernatant from
two groups TLR8-treated and untreated UCMSCs were collected 4 h
post stimulation using the RayBio Human Antibody Array C Series
1000 (RayBiotech, Inc., Norcross, GA, USA) according to the
manufacturer's protocols. Blots were analyzed with ImageJ software
version 2.0 (National Institutes of Health, Bethesda, MD, USA).
Flow cytometry analysis
For flow cytometry analysis, the following
monoclonal antibodies for detection of co-stimulator and surface
markers were used: CD80, CD86, HLA-E, CD90, CD59, CD29, CD80, CD86
and HLA-1 were used for co-stimulator detection while CD90, CD59
and CD29 were surface markers of MSC (Table II). TLR8-treated and untreated
UCSMCs were harvested 72 h post stimulation and incubated with the
monoclonal antibodies for 30 min then washed twice with phosphate
buffered saline, and appropriate isotopic controls were included.
Flow cytometry was performed using a FACScan flow cytometer (BD
Biosciences, Franklin Lakes, NJ, USA). The acquisition and analysis
gates were chosen based on the high fluorescence intensity of
control group.
 | Table II.Monoclonal antibodies for
fluorescence activated cell sorting analysis. |
Table II.
Monoclonal antibodies for
fluorescence activated cell sorting analysis.
| Name | Company | Catalog number |
|---|
| CD80 | eBioscience | 11–0809 |
| CD86 | eBioscience | 12–0869 |
| HLA-E | eBioscience | 17–9953 |
| CD90 | eBioscience | 45–0909 |
| CD59 | eBioscience | 11–0596 |
| CD29 | eBioscience | 17–0299 |
UCMSC differentiation assay
To assay UCMSC differentiation, conditioned medium
specific was added to chondrogenesis (A10071-01), osteogenesis
(A10072-01) and adipogenesis (A10070-01) differentiation kits (all
from Gibco; Thermo Fisher Scientific, Inc.). UCMSCs were inoculated
into a six-well plate (1.5×105) containing 2 ml differentiation
medium, TLR8 agonist was added at a concentration of 5 µg/ml. The
conditioned mediums were replaced every 3 days. UCMSCs both treated
and untreated by TLR8 were stained for 5, 10 and 15 days for
different cell types. In this assay, Oil Red O was used for
staining of adipocytes, Alizarin Red for osteocytes and Safranin
for chondrocytes.
Statistical analysis
Data are presented as mean ± standard deviation and
performed by SPSS software version 16.0 (SPSS Inc., Chicago, IL,
USA). Statistical significance was assessed by unpaired two-tailed
Student's t-test and differences were P<0.05 was considered to
indicate a statistically significance difference. GraphPad Prism
software version 5 (GraphPad Software, Inc., La Jolla, CA, USA) was
used for diagrams.
Results
Activation of TLR8 significantly
increases the proliferation of peripheral blood mononuclear cells
(PBMCs) and leakage of LDH
To assess whether TLR8 agonist R848 increased the
proliferation of PBMCs, PBMCs were marked with carboxyfluorescein
succinimidyl ester and co-cultured with UCMSCs with or without TLR8
activation. The PBMCs were isolated from healthy human volunteers
and then co-cultured with UCMSCs in the presence of R848. The
results indicated that PBMCs proliferated dramatically in the
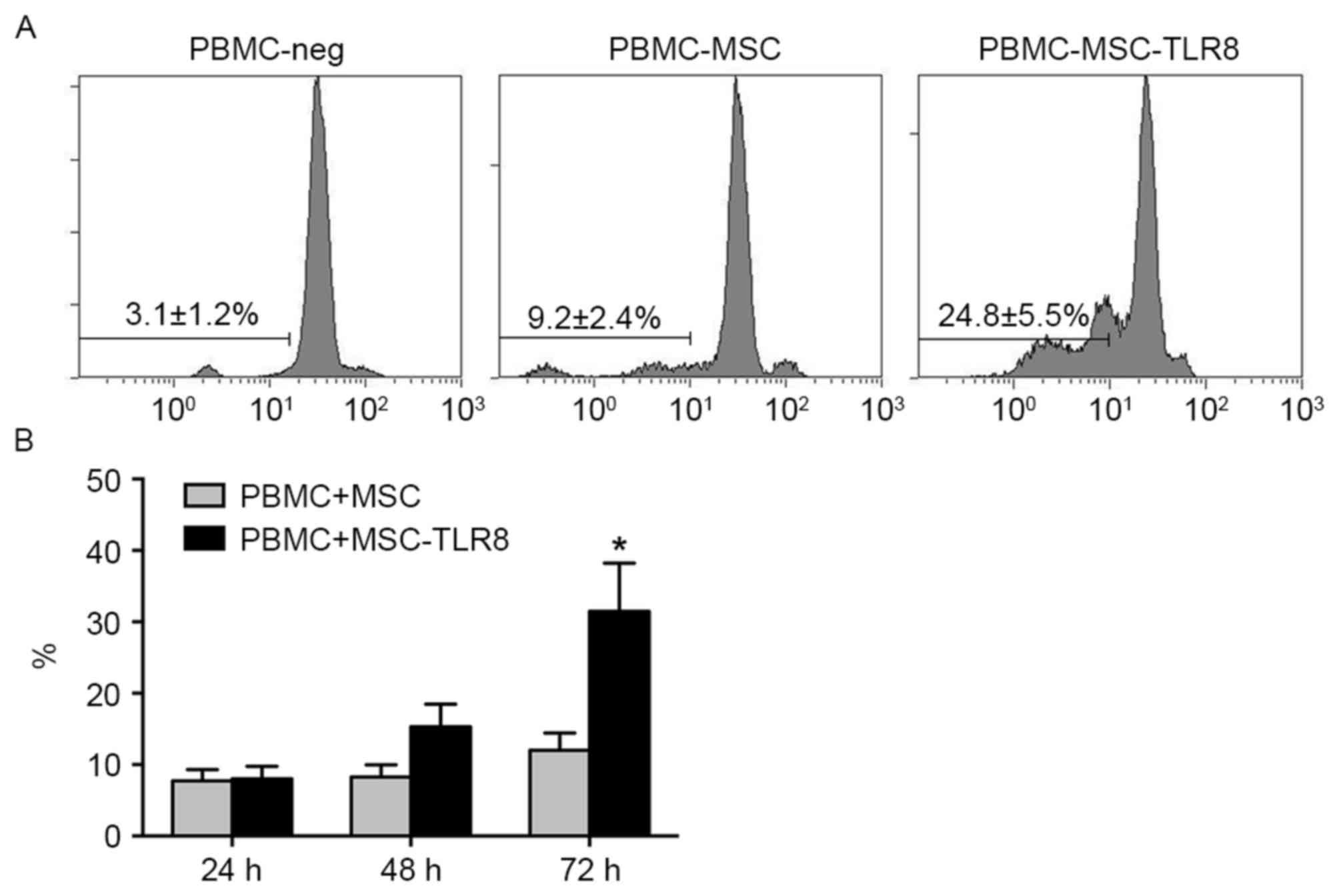
PBMCs-UCMSCs-R848 group (24.8%) following 72 h co-culture compared
with control groups (PBMC-MSC, 9.2%, PBM-Neg, 3.1; Fig. 1A). The result confirmed the induced
immune response upon TLR8 activation.
The next detection was performed to detect
leukocyte-mediated cytotoxicity by measuring the LDH levels in
supernatant released by damaged cells. In the assay, the
supernatant from the PBMC-MSC co-culture was collected for
detection of LDH levels. The LDH levels were demonstrated as not
obviously different at 24 and 48 h co-culture in the presence of
the TLR8 agonist. However, the LDH level was significantly
increased in the TLR8 treatment group when compared with
non-treatment group (P<0.05; Fig.
1B). The LDH detection indicated that activation of TLR8
increased the damage of UCMSCs.
TLR8 ligand dramatically increases the
expression of pro-inflammatory cytokines
UCMSCs were stimulated by specific ligand R848 and
then the expression variation of tumor-related genes (CDC2, PTEN,
PCNA and VEGF), co-stimulators (CD80, CD86), cytokines (IL-1β, −8,
−9, −10, −11, −15) and chemokines (I309, EOTA-3, MCP-4 and MIP-1β)
were measured. The TLR8-treated UCMSC mRNAs were isolated and
detected on different time points (4, 12, 24, 72 and 120 h) and
assayed by RT-qPCR.
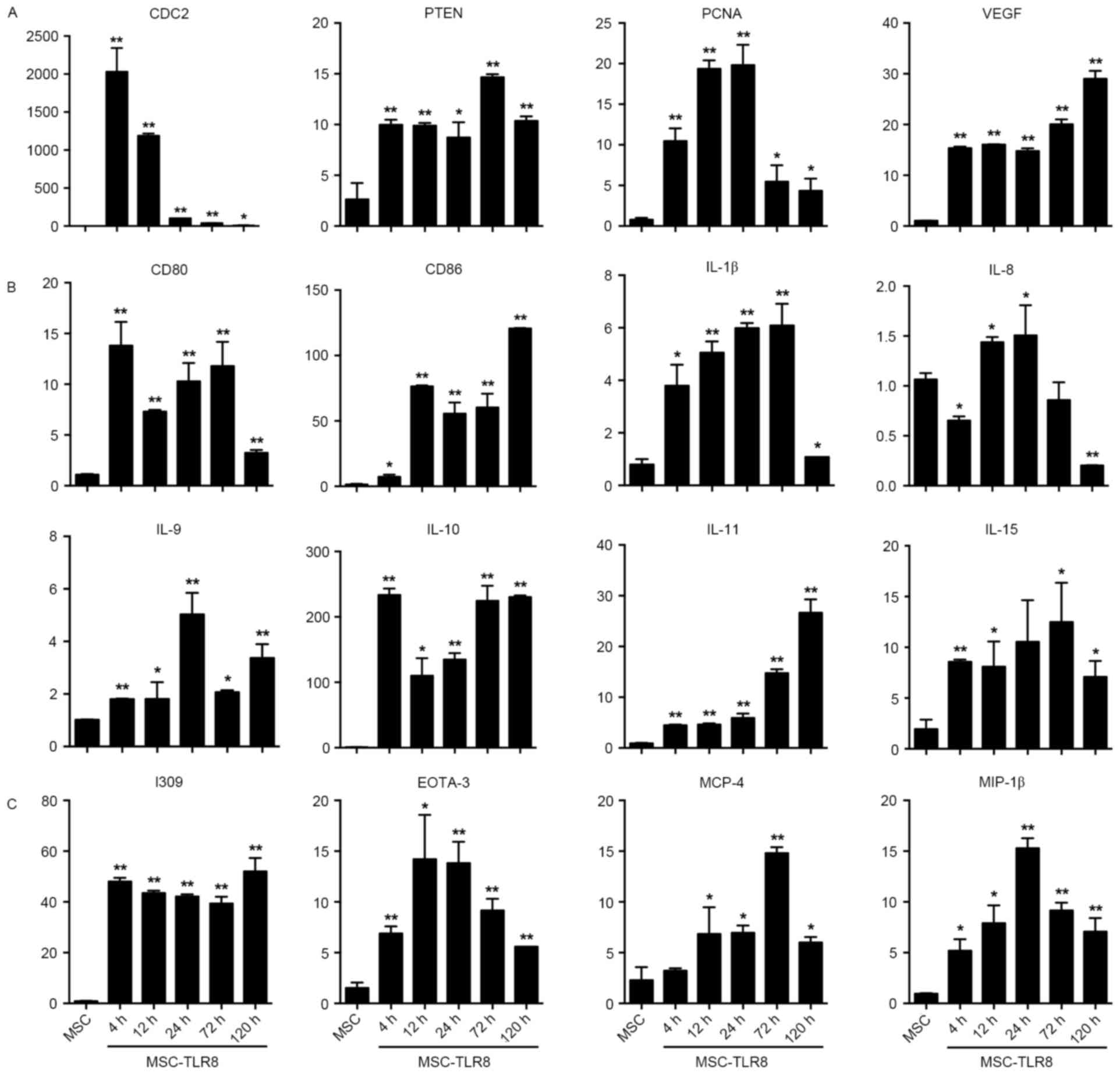
The fold induction of CDC2 production in response to
R848 increased greatly and reached the peak at 4 h then gradually
decreased. The response study indicated that TLR8 ligand activation
induced production of PTEN, PCNA and VEGF. While expression of PCNA
increased 4-, 12- and 24 h post stimulation but decreased from 72-
and 120 h (Fig. 2A).
Co-stimulators CD80 and CD86 presented a noticeable rise following
stimulation by TLR8 ligand (Fig.
2B). Interleukins IL-β, −9, −10, −11 and −15 expression
increased upon TLR8 agonist stimulation, but IL-8 decreased, even
under the level of the negative control at 120 h (Fig. 2B). With chemokines I309, EOTA-3,
MCP-4 and MIP-1β, the data demonstrated that the fold induction of
chemokines production in response to R848 increased greatly and
EOTA-3, MIP-1β reached a peak at 24 h, whilst MCP-4 reached a peak
at 72 h (Fig. 2C).
TLR8 agonist induces the expression of
co-stimulatory molecules and inhibited the expression of stem
makers in UCMSCs
Gene expression and UCMSCs-PBMC co-culture
observations suggested that the TLR8 agonist greatly increased the
immunogenic response. It was hypothesized that TLR8 may upregulate
the expression of co-stimulation molecules, which finally mediated
the TLR8-induced immune response. To analyze co-stimulation protein
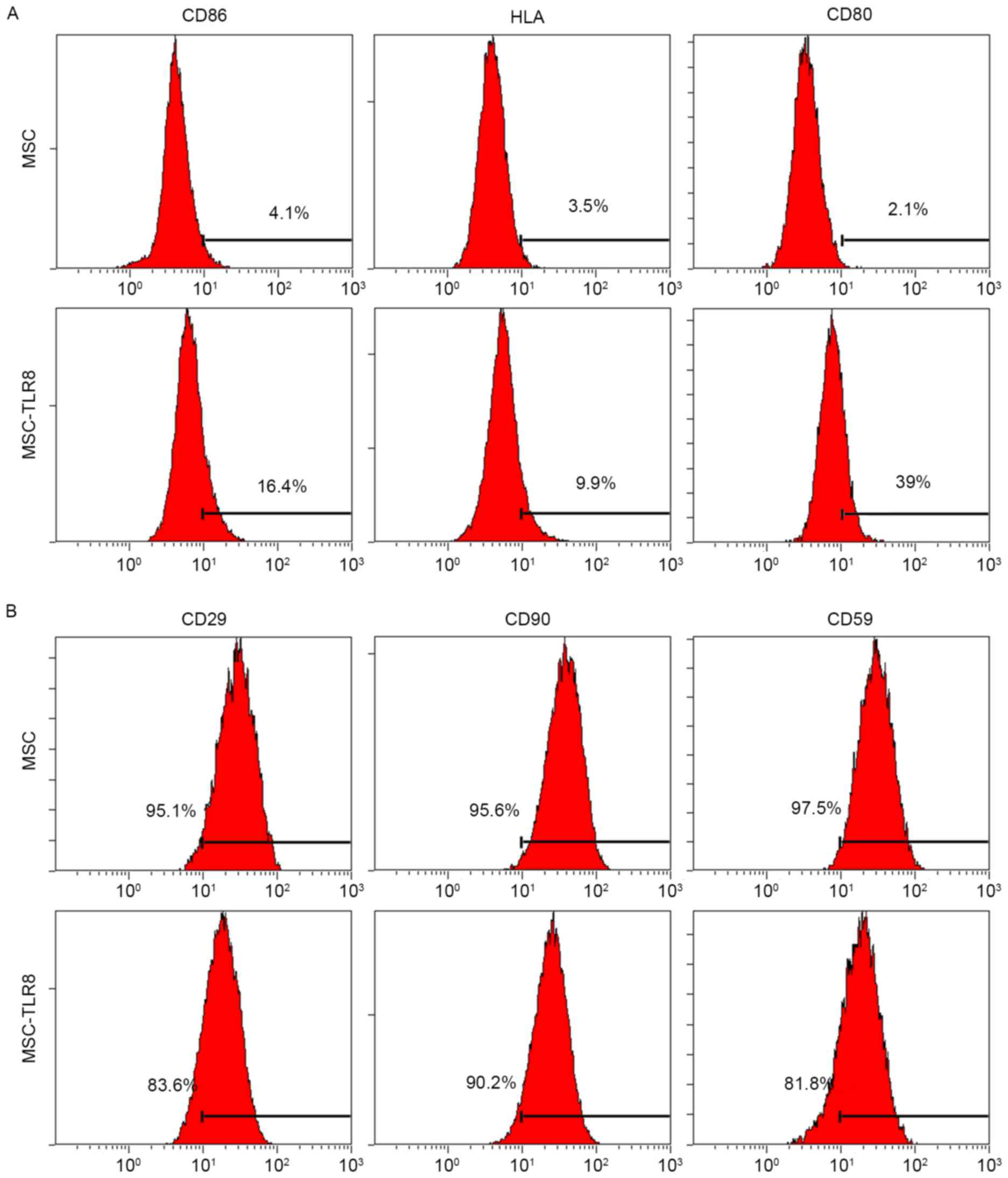
expression in UCMSCs, flow cytometry was used with specific
antibodies to human CD86, CD80 and HLA-E. Negative control UCMSCs
and TLR8-treated UCMSCs were collected 5 days post-stimulation.
Flow cytometry analysis indicated that three co-stimulation
proteins had increased in various degrees by TLR8 treatment, the
most notable being CD80 (39% vs. 2.1%; Fig. 3A). In addition, specific surface
MSC markers CD29, CD90 and CD59 were used for detection. TLR8
treatment of UCMSCs resulted in the decreased expression of CD29
(83.6% vs. 95.1%) and CD59 (81.8% vs. 97.5%), which suggested that
stemness of UCMSCs was diminished upon TLR8 agonist treatment
(Fig. 3B).
TLR8 stimulation in UCMSCs induces the
secretion of cytokines
To assess whether TLR8 can enhance the cytokine
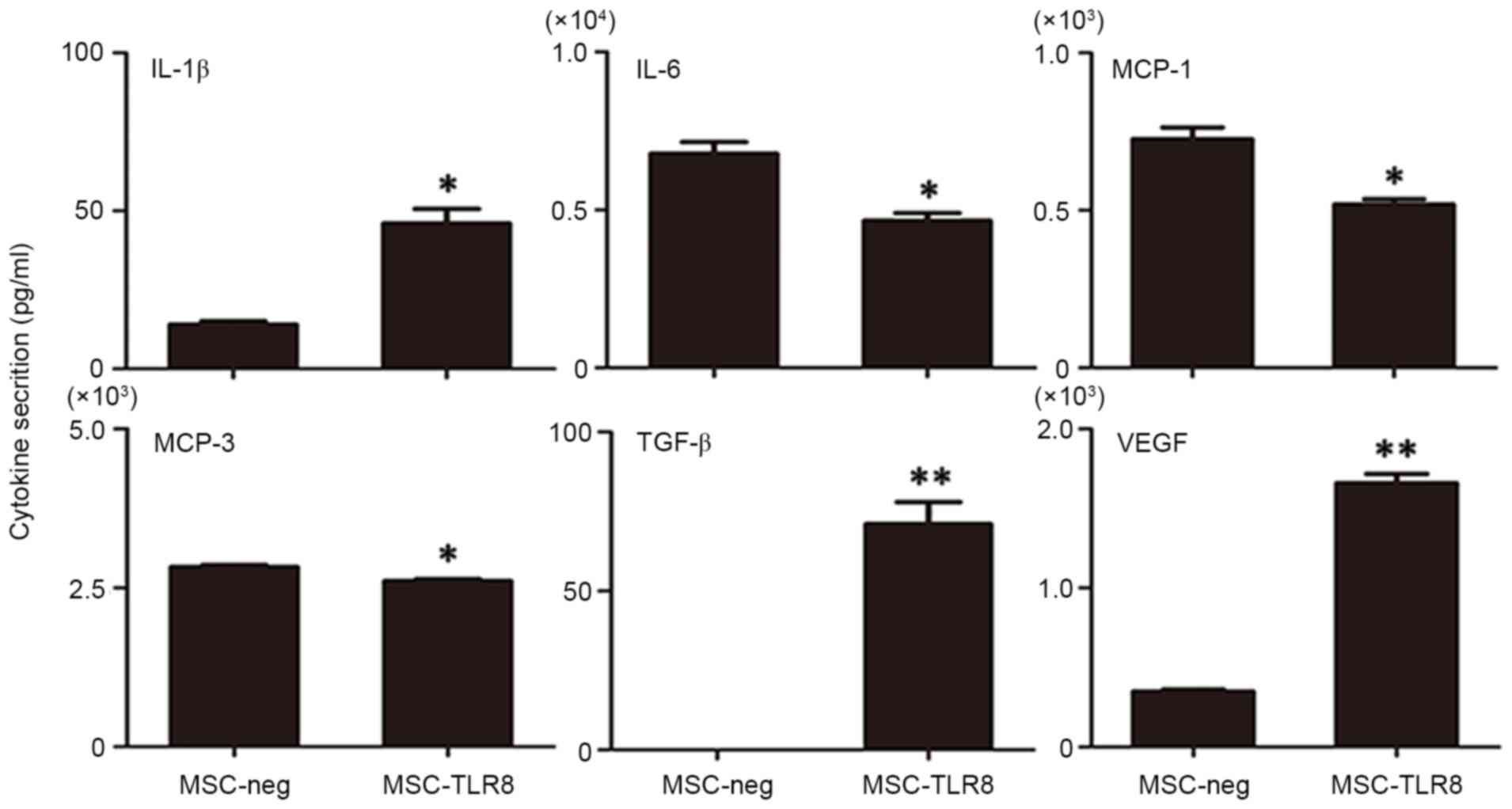
secretion of UCMSCs, an antibody chip assay was performed to detect
the dose of cytokines. UCMSCs were stimulated with the TLR8 ligand
for 4 h prior to supernatant collection and 20 cytokines,
chemokines and other important factors were analyzed (AFP, albumin,
E-selectin, ICAM-1, IFN-α, IFN-γ, IL-10, IL-12, IL-18, IL-1β, IL-4,
IL-5, IL-6, IL-8, MCP-1, MCP-3, MIP-1α, Notch-1, TGF-β and VEGF).
In these molecules, secretion of TGF-β and VEGF was induced
markedly upon R848 stimulation (P<0.001) and IL-1β also enhanced
in the treatment group. The marked decreased secretion in the R848
treatment group was IL-6, MCP-1 and MCP-3 (Fig. 4). Nevertheless, other target genes
either did not result in detectable protein levels, or protein
levels remained the same. Overall, the secretion pattern of
R848-treated UCMSCs appeared to favor pro-inflammatory
molecules.
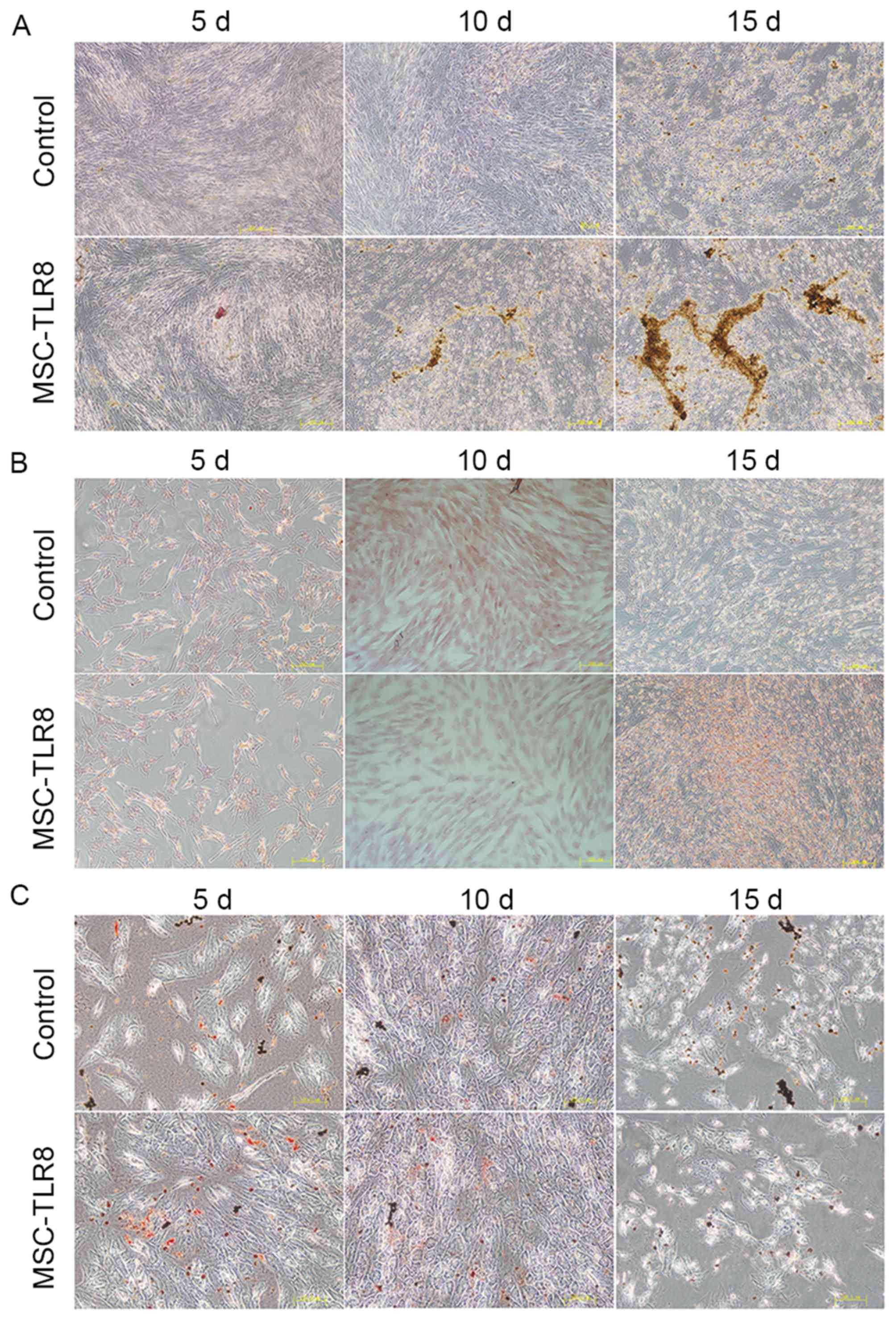
TLR8 enhances the induced osteoblast
differentiation of UCMSCs
The effects of TLR8 on the differentiation of UCMSCs
into osteocytes, adipocytes and chondrocytes was further examined.
UCMSCs were incubated with osteogenic, adipogenic or chondrogenic
induction medium, with or without TLR8, and were then allowed to
differentiate. As presented in Fig.
5A, the presence of TLR8 in the osteogenic medium greatly
enhanced the calcium deposition, which was detected by Alizarin red
staining of cells, compared with the control group without R848,
especially 10 and 15 days post differentiation. The presence of
TLR8 in the adipogenic and chondrogenic medium presented no obvious
differences compared with the control groups, and was detected by
Oil red O staining and Safranin (Fig.
5B and C).
Discussion
MSCs are widely believed to have an immunologically
privileged phenotype and an immunosuppressive capacity and thus
these cells hold potential for clinical applications for minimally
invasive cell therapy to promote the regeneration of damaged
tissue, to treat inflammation and to promote angiogenesis (19). In a series of clinical trials, MSC
administration has been demonstrated to be a clinically feasible
and relatively safe procedure without major adverse effects in
patients with multiple sclerosis, amyotrophic lateral sclerosis and
Graft vs. host disease (9,20). However, it is also commonly
observed, in human and animal studies, that most of the MSCs
disappeared within a few days following infusion, indicating that
these cells are rapidly recognized and rejected by the hosts
through a yet unknown mechanism (21). The latest disappointing results
from MSC clinical trials further support the suspicion that,
immediately following infusion, MSC viability and/or activities are
markedly reduced, leading to failed treatment efficacy (22).
Toll-like receptors (TLR) were first identified in
Drosophila, where they are required both for immunity
against pathogens and for embryonic development (23–27).
Both TLR7 and TLR8 have been identified as natural receptors for
single-stranded RNA, and they are thought to act as potent
activators of innate immune responses upon viral infections
(28). TLRs activation has been
involved in the pathology of a series of inflammatory diseases, as
they can either initiate or perpetuate the chronic inflammation
attributed to the continue exposure to TLR ligands (29). Therefore, MSCs employed in therapy
can be potentially exposed to TLR ligands, which may modulate MSC
therapeutic potential in vivo (17). However, there have been no studies
about the role of TLR8 in regulating immunogenicity of TLRs in
UCMSCs.
In the present study, TLR8was employed to evaluate
whether its activation influenced the immunogenicity of USMSCs,
including cytokine release, multilineage differentiation and
proliferation. In a PBMC-UCMSC co-culture system, it was
demonstrated that activation of TLR8 promoted the proliferation of
human PBMCs, and the increased release of LDH from R848 stimulation
confirmed the occurrence of immune attack; these results clearly
demonstrated that activation of TLR8 pathway increased the immune
responses. Following this, the expression variation of
pro-inflammatory molecules that were important in mediating immune
response was measured. The results confirmed the upregulation of
many pro-inflammatory cytokines in both mRNA expression and protein
secretion, including VEGF, IL6, IL-8, IL-10, CD80 and CD86. Flow
cytometry analysis in the present study also confirmed that
expression of co-stimulatory factors (CD80, CD86 and HLA-E) in
UCMSCs was markedly enhanced in the presence of TLR8 agonist. Huang
et al (30) suggested that
the enhanced differentiation of MSCs into cardiovascular cells
could finally increase the immunogenicity of MSCs. As a result, it
was hypothesized that activation of the TLR8 pathway may influence
the differentiation ability of UCMSCs and it was demonstrated that
the osteoblast differentiation of UCMSCs was significantly enhanced
in the presence of the TLR8 agonist. All these observations
suggested that TLR8 stimulation in UCMSCs was critical in shaping
the immunogenic status of UCMSC.
As a number of endogenous ligands have been
identified, including heparin sulfate, hyaluronan, heat shock
protein 70, intracellular components of fragmented cells and
eosinophil-derived neurotoxin (14), it was important to confirm the role
of TLRs in regulating biological functions, especially
immunogenicity of MSC. To the best of the authors' knowledge, the
current study was the first report concerning the role of TLR8 in
regulating immunogenicity of UCMSCs. The results clearly indicated
that TLR8 activation by agonist has implications for the immune
modulating the function of UCMSCs.
The current study provided the foundation for
further characterization of TLR8-activated UCMSCs using in
vivo experimental systems to establish the physiological role
for TLRs in regulation of UCSMC functions.
Acknowledgements
The present study was supported by a grant from the
National Natural Scientific Foundation of China (grant no.
81202023).
References
|
1
|
Colter DC, Sekiya I and Prockop DJ:
Identification of a subpopulation of rapidly self-renewing and
multipotential adult stem cells in colonies of human marrow stromal
cells. Proc Natl Acad Sci USA. 98:7841–7855. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Phinney DG and Prockop DJ: Concise review:
Mesenchymal stem/multipotent stromal cells: The state of
transdifferentiation and modes of tissue repair-current views. Stem
Cells. 25:2896–2902. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bassi E, Aita CA and Câmara NO: Immune
regulatory properties of multipotent mesenchymal stromal cells:
Where do we stand? World J Stem Cells. 3:1–8. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Han KH, Ro H, Hong JH, Lee EM, Cho B, Yeom
HJ, Kim MG, Oh KH, Ahn C and Yang J: Immunosuppressive mechanisms
of embryonic stem cells and mesenchymal stem cells in alloimmune
response. Transpl Immunol. 25:7–15. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Van Poll D, Parekkadan B, Cho CH,
Berthiaume F, Nahmias Y, Tilles AW and Yarmuch ML: Mesenchymal stem
cell-derived molecules directly modulate hepatocellular death and
regeneration in vitro and in vivo. Hepatology. 47:1634–1643. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Le Blanc K, Frassoni F, Ball L, Locatelli
F, Roelofs H, Lewis I, Lanino E, Sundberg B, Bernardo ME, Remberger
M, et al: Mesenchymal stem cells for treatment of
steroid-resistant, severe, acute graft-versus-host disease: A phase
II study. Lancet. 371:1579–1586. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vojtassak J, Danisovic L, Kubes M, Bakos
D, Jarabek L, Ulicná M and Blasko M: Autologous biograft and
mesenchymal stem cells in treatment of the diabetic foot. Neuro
Endocrinol Lett. 27 Suppl 2:134–137. 2006.PubMed/NCBI
|
|
8
|
Ankrum J and Karp JM: Mesenchymal stem
cell therapy: Two steps forward, one step back. Tends Mol Med.
16:203–209. 2010. View Article : Google Scholar
|
|
9
|
Nauta AJ, Westerhuis G, Kruisselbrink AB,
Lurvink EG, Willemze R and Fibbe WE: Donor-derived mesenchymal stem
cells are immunogenic in an allogeneic host and stimulate donor
graft rejection in a nonmyeloablative setting. Blood.
108:2114–2120. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Spaggiari GM, Capobianco A, Becchetti S,
Mingari MC and Moretta L: Mesenchymal stem cell-natural killer cell
interactions: Evidence that activated NK cells are capable of
killing MSCs, whereas MSCs can inhibit IL-2-induced NK-cell
proliferation. Blood. 107:1484–1490. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Akira S, Uematsu S and Takeuchi O:
Pathogen recognition and innate immunity. Cell. 124:783–801. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pasare C and Medzhitov R: Toll-like
receptors: Linking innate and adaptive immunity. Adv Exp Med Biol.
560:11–18. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Beutler B, Hoebe K, Du X and Ulevitch RJ:
How we detect microbes and respond to them: The Toll-like receptors
and their transducers. J Leukoc Biol. 74:479–485. 2004. View Article : Google Scholar
|
|
14
|
DelaRosa O and Lombardo E: Modulation of
adult mesenchymal stem cells activity by toll-like receptors:
Implications on therapeutic potential. Mediators Inflamm.
2010:8656012010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lombardo E, Delarosa O, Mancheño-Corvo P,
Menta R, Ramírez C and Büscher D: Toll-like receptor-mediated
signaling in human adipose-derived stem cells: Implications for
immunogenicity and immunosuppressive potential. Tissue Eng Part A.
15:1579–1589. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liotta F, Angeli R, Cosmi L, Filì L,
Manuelli C, Frosali F, Mazzinghi B, Maggi L, Pasini A, Lisi V, et
al: Toll-like receptors 3 and 4 are expressed by human bone
marrow-derived mesenchymal stem cells and can inhibit their T-cell
modulatory activity by impairing notch signaling. Stem Cells.
26:279–289. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tomchuck SL, Zwezdaryk KJ, Coffelt SB,
Waterman RS, Danka ES and Scandurro AB: Toll-like receptors on
human mesenchymal stem cells drive their migration and
immunomodulating responses. Stem Cells. 26:99–107. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bianco P, Robey PG and Simmons PJ:
Mesenchymal stem cells: Revisiting history, concepts, and assays.
Cell Stem Cell. 2:313–319. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Karussis D, Karageorgiou C,
Vaknin-Dembinsky A, Gowda-Kurkalli B, Gomori JM, Kassis I, Bulte
JW, Petrou P, Ben-Hur T, Abramsky O and Slavin S: Safety and
immunological effects of mesenchymal stem cell transplantation in
patients with multiple sclerosis and amyotrophic lateral sclerosis.
Arch Neurol. 67:1187–1194. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Allison M: Genzyme backs Osiris, despite
prochymal flop. Nat Biotechnol. 27:966–967. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li Y and Lin F: Mesenchymal stem cells are
injured by complement after their contact with serum. Blood.
120:3436–3443. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hoebe K, Janssen E and Beutler B: The
interface between innate and adaptive immunity. Nat Immunol.
5:971–974. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kambris Z, Hoffmann JA, Imler JL and
Capovilla M: Tissue and stage-specific expression of the Tolls in
Drosophila embryos. Gene Expr Patterns. 2:311–317. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Beutler B, Jiang Z, Georgel P, Crozat K,
Croker B, Rutschmann S, Du X and Hoebe K: Genetic analysis of host
resistance: Toll-like receptor signaling and immunity at large.
Annu Rev Immunol. 24:353–389. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ferrandon D, Imler JL and Hoffmann JA:
Sensinginfection in Drosophila: Toll and beyond. Semin Immunol.
16:43–53. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gangloff M, Weber AN, Gibbard RJ and Gay
NJ: Evolutionary relationships, but functional differences, between
the Drosophila and human Toll-like receptor families. Biochem Soc
Trans. 31:659–663. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Schon MP and Schön M: TLR7 and TLR8 as
targets in cancer therapy. Oncogene. 27:190–199. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yamamoto-Furusho JK and Podolsky DK:
Innate immunity in inflammatory bowel disease. World J
Gastroenterol. 13:5577–5580. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Huang XP, Sun Z, Miyagi Y, Kinkaid H
McDonald, Zhang L, Weisel RD and Li RK: Differentiation of
allogeneic mesenchymal stem cells induces immunogenicity and limits
their long-term benefits for myocardial repair. Circulation.
122:2419–2429. 2010. View Article : Google Scholar : PubMed/NCBI
|