Introduction
Inflammatory bowel disease (IBD) is a group of
immunologically and genetically mediated chronic inflammatory
conditions of gastrointestinal (GI) tract, including ulcerative
colitis (UC) and Crohn's disease (CD) (1,2). The
incidence of IBD was emerged and dramatically increased in the last
century, with its cause remained regarded by the mainstream as
unknown (3,4). Up to date, all the treatments are
mainly targeting the inflammation, using corticosteroids,
immunosuppressive agents such as azathioprine or 6-mercaptopurine,
anti-inflammatory agents such as 5-aminosalicates, or biologics
such as anti-TNF-α antibodies (5–7).
Multiple studies showed that patients or animals
with IBD have increased fecal digestive proteases such as trypsin
and chymotrypsin (8–10). Furthermore, the serine proteases
inhibitors (e.g., Bowman-Birk protease inhibitor, BBI) are
important anti-inflammatory agents for various inflammations (e.g.,
skin rosacea, multiple sclerosis) and autoimmune diseases (11–13).
Especially, the therapeutic effect of BBI on IBD patients or
experimental animal colitis was confirmed (14,15).
The digestive enzymes located in the GI tract are the potential and
vital therapeutic targets for IBD treatment accordingly (16,17).
As an important endogenous substance largely
distributed in GI tract, the unconjugated bilirubin (UCB) from heme
metabolism by the heme oxygenase-1 (HO-1) is an effective
antioxidant (18). Our recent
studies using bile duct ligated rats confirmed the critical role of
unconjugated bilirubin in inactivation of digestive proteases and
gut protection (19,20). Whereas, the specific effects of UCB
on the inflammation in colitis, and the modifications of digestive
proteases levels are still unrevealed. Therefore, we designed a UCB
treatment study on an experimental colitis rats model to confirm
the effect of UCB on colitis management and the levels of digestive
proteases in feces.
Materials and methods
Animals
Male Sprague-Dawley (SD) rats (weight ~180 g) were
purchased from the Experimental Animal Center of the Second
Affiliated Hospital of Harbin Medical University. The study was
approved by the Animal Care and Use Committee of the Harbin Medical
University.
Drugs and reagents
Trinitrobenzenesulfonic acid (TNBS) and unconjugated
bilirubin (UCB) were purchased from Sigma-Aldrich (St. Louis, USA).
ELISA kits for trypsin, chymotrypsin, TNF-α, IL-1β and MPO were
obtained from Beijing Propbs Biotechnology Co., Ltd. (Beijing,
China).
Induction of colitis and treatment
with UCB
TNBS-induced colitis was established as described
previously (21). SD rats were
randomly divided into three groups: The normal control group
(Control group), the TNBS model group (TNBS group) and TNBS model
rats treated with UCB group (TNBS + UCB group). After a 24 h
fasting, the animals were slightly anesthetized with amobarbital
sodium (25 mg/K, i.p.), and then a medical-grade polyurethane
cannula was inserted into the anus with the tip positioned about 8
cm proximally to the anus. TNBS group received colonic instillation
of 1 ml of 50% ethanol in saline containing 25 mg TNBS, while the
control group received 1 ml saline (22,23).
After colonic instillation, the UCB treatment group received an
intra-gastric gavages of 3.5 ml UCB (40 µM, UCB is dissolved in
0.4% dimethyl sulfoxide at concentrations up to 40 µM) (19,23),
while the Control and TNBS groups received equal volume of saline
solution. All animals were recorded daily for body weight and total
feces were collected daily and stored at −4°C (24). On day 1, 3 and 7 after UCB
treatment, rats were sacrificed and colon about 8 cm above the anus
was harvested and stored for further analysis (19,22–24).
Assessment of colonic damage
Colonic damage was assessed by both Macroscopic
Damage Scores (MDS) as shown in Table
I (25,26), and histological inflammation scores
using Hematoxylin and eosin (H&E) staining (19), based on the following parameters
(Table II): Inflammatory cell
infiltrate, loss of mucosal architecture, gut wall layers
infiltrated, and edema (19,27).
 | Table I.Criteria for the assessment of
macroscopic colonic damage scores. |
Table I.
Criteria for the assessment of
macroscopic colonic damage scores.
| Criteria | Score | Appearance |
|---|
| Ulceration and
inflammation | 0 | Normal, no
damage |
|
| 1 | Focal hyperemia, no
ulcers |
|
| 2 | Ulcer without
significant inflammation |
|
| 3 | Ulcer with
significant inflammation at one site |
|
| 4–5 | Two or more major
sites of ulceration/inflammation, or major sites extending >1 cm
along the length of colon |
|
| 6–10 | Major damage
extending >2 cm along the length of colon, and the score is
increased by one point for each additional centimeter of
damage |
| Adhesions | 0 | Absence |
|
| 1 | Minor adhesions,
easily separable from other tissues |
|
| 2 | Sever
adhesions |
 | Table II.Criteria for the assessment of
microscopic colonic inflammation scores. |
Table II.
Criteria for the assessment of
microscopic colonic inflammation scores.
| Criteria | Score |
|---|
| Inflammatory cell
infiltrate | 0–3 |
| Gut wall layers
infiltrated | 0–3 |
| Loss of mucosal
architecture | 0–3 |
| Edema | 0–1 |
| Max score | 10 |
Assay of trypsin and chymotrypsin in
feces, and TNF-α, IL-1β and myeloperoxidase (MPO) in colonic
tissue
The concentrations of trypsin and chymotrypsin in
feces, TNF-α, IL-1β and myeloperoxidase (MPO) in colon tissue, were
assessed by ELISA kits, based on the manufacturer's instructions.
Results of trypsin, TNF-α, IL-1β were expressed as pg/mg, and
chymotrypsin and MPO were expressed as U/g.
Statistical analysis
Results were expressed as mean ± SEM. Differences
between groups were determined by one-way ANOVA with LSD or Tamhane
multiple comparisons post hoc tests, using SPSS version 19.0 (IBM
SPSS, Armonk, NY, USA). The correlations were assessed using linear
fit in Origin-Pro8 (OriginLab Corporation, Northampton, MA, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
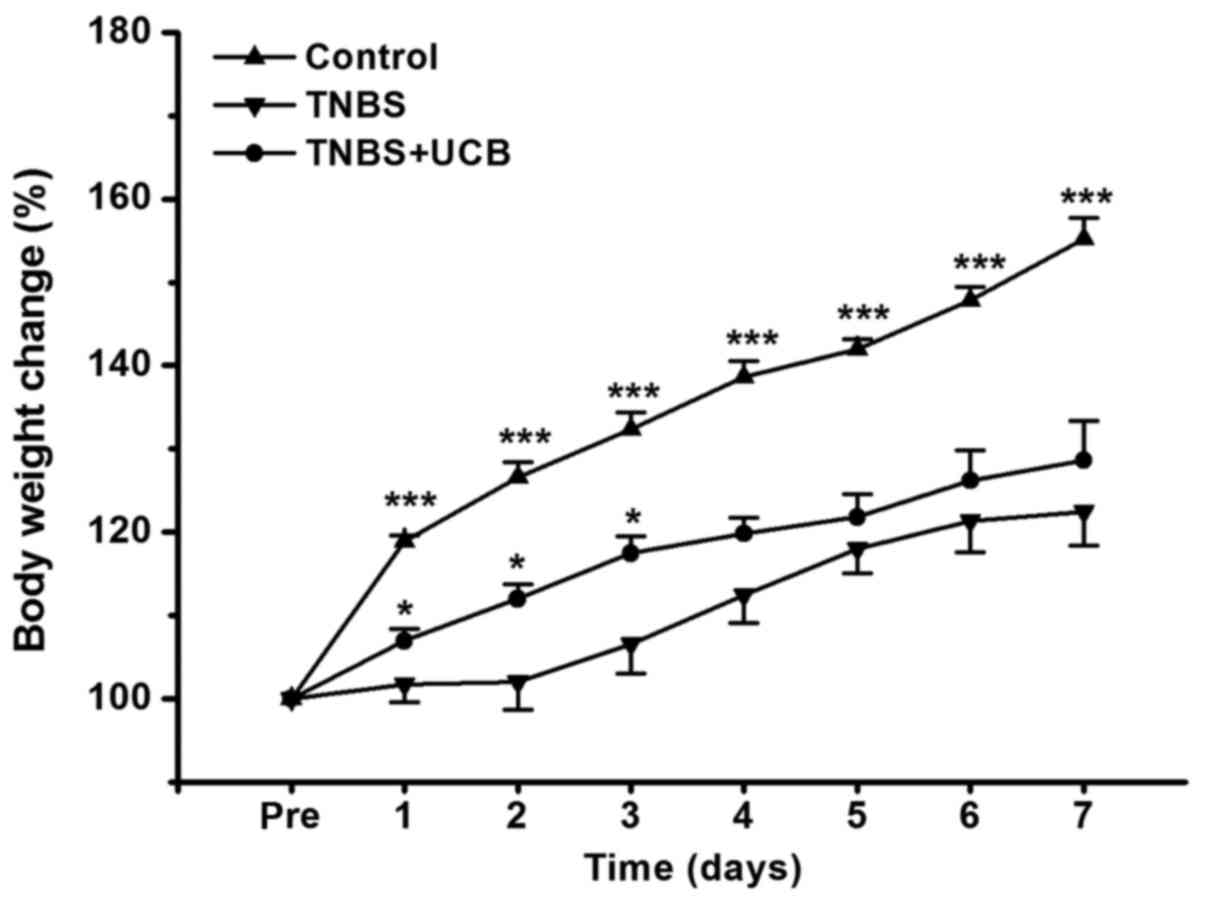
UCB alleviates loss of body weight in
TNBS-treated rats
The body weight of rats was measured once daily for
7 days, and the body weight change relative to pre-treatment of
rats was calculated. From our data (Fig. 1), TNBS caused dramatic reduction in
body weight gain, while UCB treatment significantly alleviated this
body weight loss from day 1 to day 3 (P<0.05).
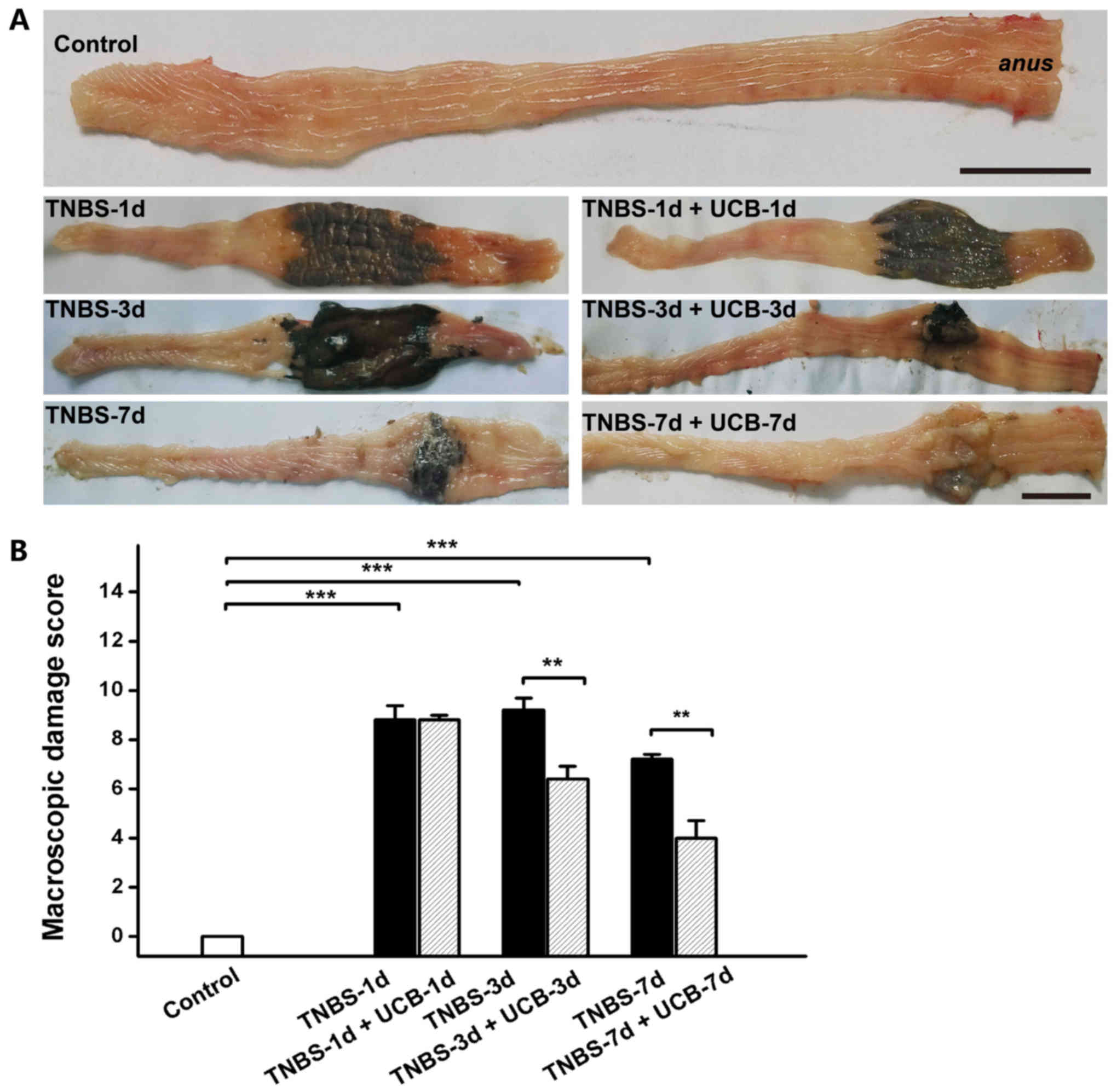
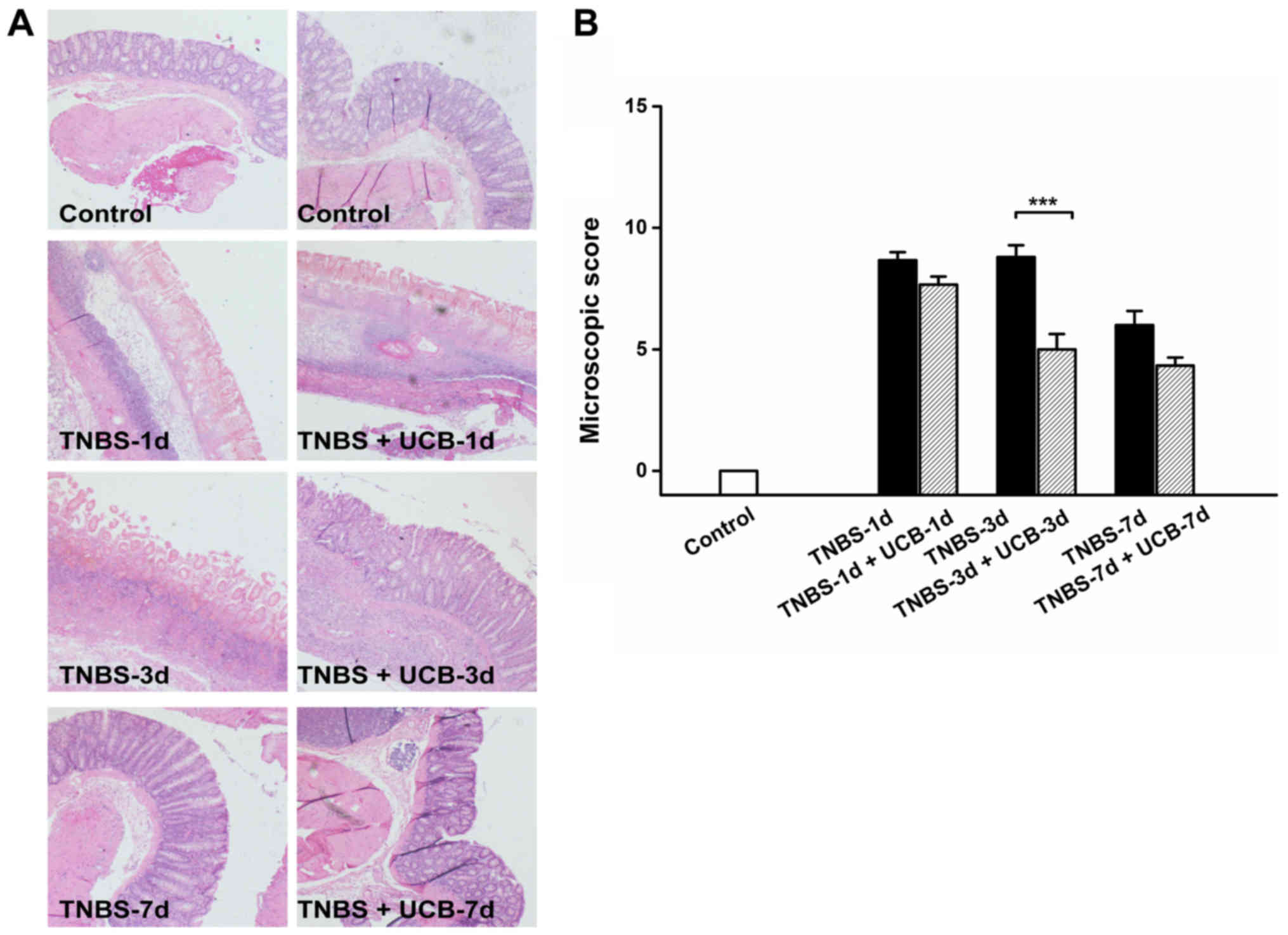
Effects of UCB on macroscopic and
histological pathological changes of rats with TNBS-induced
colitis
Meanwhile, TNBS caused momentous damage of colonic
tissues (Fig. 2A). Furthermore,
the MDS of TNBS-treated rats were significant higher than control
rats (P<0.001 from day 1 to day 7), whereas the MDS of UCB
treated rats was significantly lower compared to TNBS alone
(P<0.01 at day 3 and 7) (Fig.
2B and Table I). While there
was no significant difference at day 1 and day 7, our data
demonstrated significant ameliorating effect by UCB treatment at
day 3 with histological staining (Fig.
3A) and microscopic colonic inflammation scores (Fig. 3B, P<0.001).
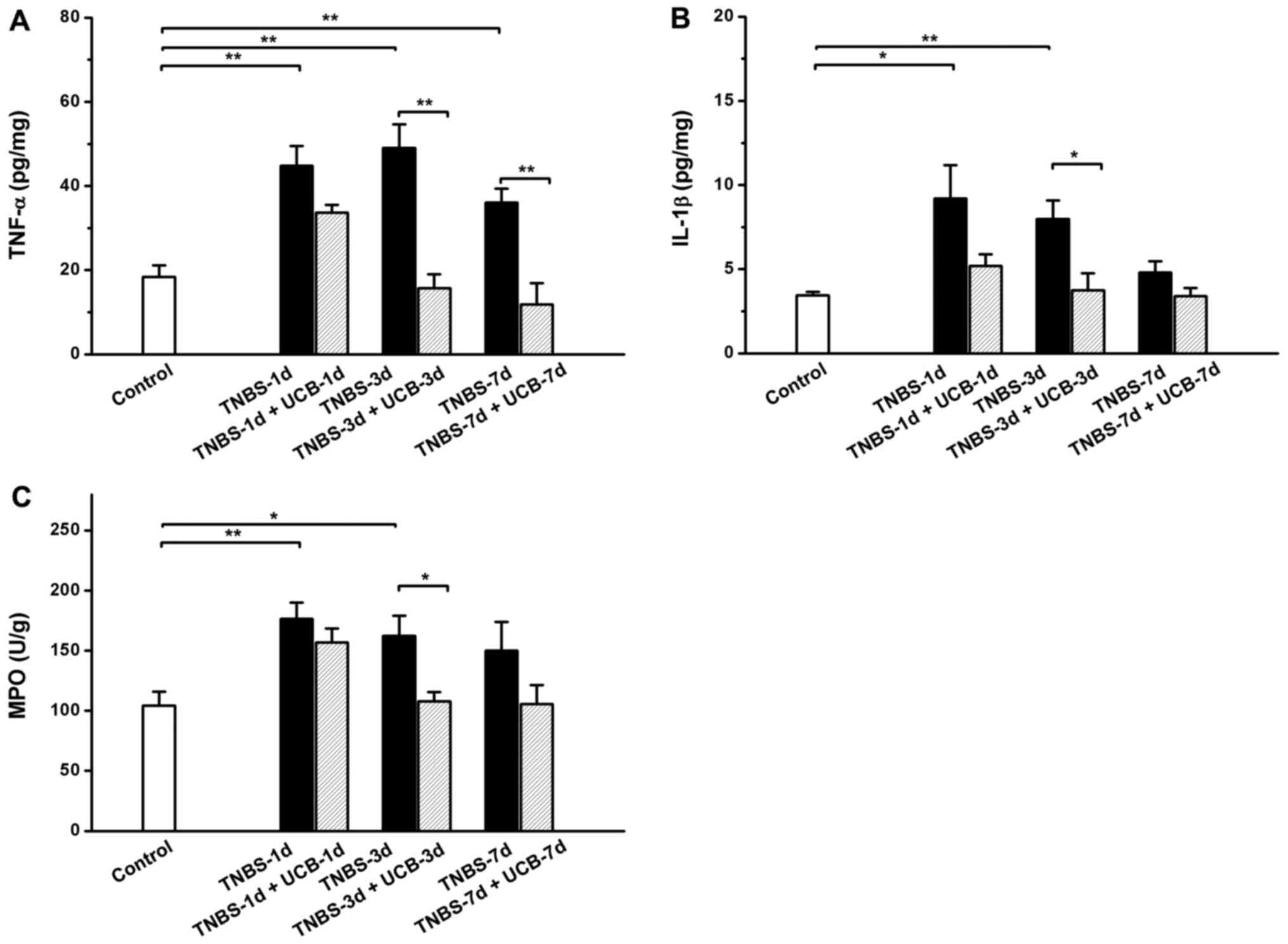
Effects of UCB on TNBS-induced
increases of pro-inflammatory cytokines in the colon tissue
Same as reported by others (28), the results of our experiment showed
TNBS caused significant increases in TNF-α, IL-1β and MPO in the
colon, while treatment with UCB alleviates these changes (Fig. 4A-C).
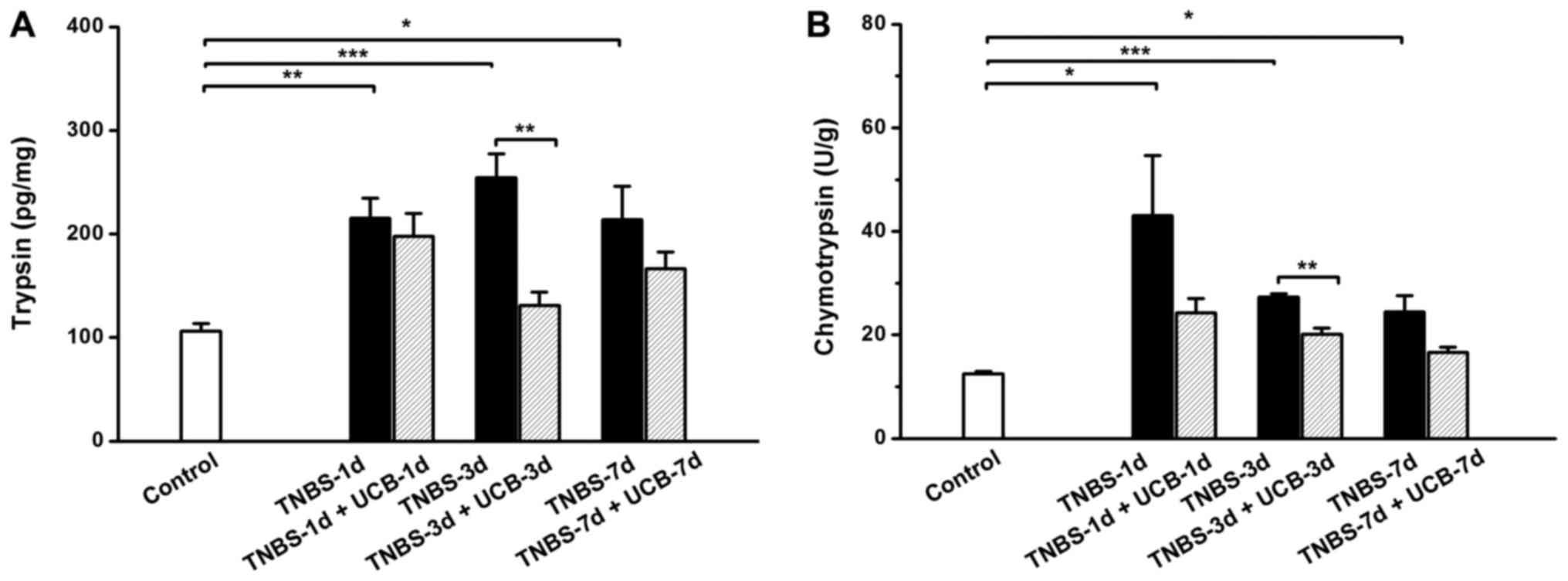
Changes of fecal digestive
proteases
As previous studies showed that the fecal digestive
proteases (trypsin and chymotrypsin) were increased in IBD
(8,10,29).
Therefore, we measured the fecal trypsin and chymotrypsin levels of
the different groups of rats. Our results demonstrated that both
trypsin and chymotrypsin were significant increased from day 1 to 7
after TNBS treatment (Fig. 5),
however, the UCB treatment significantly reduced the levels of
trypsin and chymotrypsin (P<0.01 on day 3). To explore the
relationship among changes of digestive proteases in gut lumen and
pro-inflammatory cytokines in colon tissue, we further conducted a
correlation analysis among these parameters using the data
collected on day 3. It showed positive significant correlations
(Pearson's correlation coefficient, slop >0, P<0.05) among
these parameters (Table
III).
 | Table III.Correlation analysis of digestive
proteases and inflammatory markers. |
Table III.
Correlation analysis of digestive
proteases and inflammatory markers.
|
| Digestive
enzymes |
|---|
|
|
|
|---|
| Statistics of
linear fit | Chymotrypsin
(U/g) | Trypsin
(pg/mg) |
|---|
|
|
|
|---|
| Inflammatory
markers | Slope | R-Square | P-value | Slope | R-Square | P-value |
|---|
| MPO (U/g) | 1.704 | 0.775 | 0.016 | 0.604 | 0.839 |
3.241E-4 |
| TNF-α (pg/mg) | 0.455 | 0.749 | 0.007 | 0.212 | 0.916 |
3.276E-5 |
| IL-1β (pg/mg) | 0.112 | 0.750 | 0.007 | 0.033 | 0.823 | 0.001 |
Discussion
UCB is previously known as a toxic endogenous
substance on nervous system in high concentrations, but also a
pivotal antioxidant in low concentrations (18), that plays an important potential
protection role in vascular endothelial function, experimental
murine colitis (30–32), and other disorders including
non-alcoholic steatohepatitis and advanced fibrosis (30). Moreover, as a key upstream
modulator for endogenous biliverdin generation, HO-1 has been
proved with various protective effects on atherosclerosis (30,33),
immuno-related inflammation (33),
and also experimental murine colitis mediated by UCB (31,34).
However, the anti-inflammatory mechanism of HO-1/UCB axis are still
unclear, and the role of UCB on inflammatory bowel diseases with
anti-inflammatory property is attractive.
In our previous studies, we have observed increased
activities of fecal trypsin and chymotrypsin in animals with bile
duct ligation (BDL) (19,20). From results above, UCB
administrated ameliorates the tissue damage and inflammation in the
gastrointestinal tract of TNBS-induced colitis. Interestingly, the
levels of trypsin and chymotrypsin in feces of TNBS group were both
significantly increased, and ameliorated under the UCB treatment,
while did not change after administration in normal control rats
(data not shown). Moreover, significant positive correlations were
identified by the linear fitting analysis results of digestive
enzymes (trypsin and chymotrypsin) and inflammatory markers levels
(MPO, TNF-α and IL-1β). In addition, proteases are important in
inflammation process via protease activated receptors in various
tissues (11,12,29),
and the anti-inflammatory roles of digestive enzymes inhibitors or
serine protease inhibitors for trypsin and chymotrypsin has been
reported (17). Therefore, the
digestive proteases could be the key mediators and targets for the
anti-inflammatory effects of UCB, and the proteases reduction by
UCB would be one of the important therapeutic pathway for the
treatment of inflammatory diseases like colitis. Notably, the
limitations of this study are that the detail mechanisms for direct
anti-inflammatory effects or digestive proteases inactivation
dependent anti-inflammatory effects of UCB on colitis are still
unrevealed, while our results in this study have demonstrated that
the UCB treatment ameliorates the inflammation and digestive
proteases increase in the gastrointestinal tract of TNBS-induced
colitis.
Acknowledgements
We are grateful to thank Kangkang Zhou and Guojing
Shi for excellent laboratory assistance, and Fu-Lai Chen for the
histological staining of colonic tissues. This study was supported
by the National Natural Science Foundation of China (no.
30973596).
References
|
1
|
Bouma G and Strober W: The immunological
and genetic basis of inflammatory bowel disease. Nat Rev Immunol.
3:521–533. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mokry M, Middendorp S, Wiegerinck CL,
Witte M, Teunissen H, Meddens CA, Cuppen E, Clevers H and
Nieuwenhuis EE: Many inflammatory bowel disease risk loci include
regions that regulate gene expression in immune cells and the
intestinal epithelium. Gastroenterology. 146:1040–1047. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cosnes J, Gower-Rousseau C, Seksik P and
Cortot A: Epidemiology and natural history of inflammatory bowel
diseases. Gastroenterology. 140:1785–1794. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kirsner JB: The historical basis of the
idiopathic inflammatory bowel diseases. Inflammatory Bowel Dis.
1:2–26. 1995. View Article : Google Scholar
|
|
5
|
Pithadia AB and Jain S: Treatment of
inflammatory bowel disease (IBD). Pharmacol Rep. 63:629–642. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rietdijk ST and D'Haens GR: Recent
developments in the treatment of inflammatory bowel disease. J Dig
Dis. 14:282–287. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sohrabpour AA, Malekzadeh R and
Keshavarzian A: Current therapeutic approaches in inflammatory
bowel disease. Curr Pharm Des. 16:3668–3683. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
van de Merwe JP and Mol GJ: Levels of
Trypsin and alpha-chymotrypsin in feces from patients with Crohn's
disease. Digestion. 24:1–4. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Maeda S, Ohno K, Uchida K, Igarashi H,
Goto-Koshino Y, Fujino Y and Tsujimoto H: Intestinal
protease-activated receptor-2 and fecal serine protease activity
are increased in canine inflammatory bowel disease and may
contribute to intestinal cytokine expression. J Vet Med Sci.
76:1119–1127. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Midtvedt T, Zabarovsky E, Norin E, Bark J,
Gizatullin R, Kashuba V, Ljungqvist O, Zabarovska V, Möllby R and
Ernberg I: Increase of faecal tryptic activity relates to changes
in the intestinal microbiome: Analysis of Crohn's disease with a
multidisciplinary platform. PLoS One. 8:e660742013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Safavi F and Rostami A: Role of serine
proteases in inflammation: Bowman-Birk protease inhibitor (BBI) as
a potential therapy for autoimmune diseases. Exp Mol Pathol.
93:428–433. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yamasaki K, Di Nardo A, Bardan A, Murakami
M, Ohtake T, Coda A, Dorschner RA, Bonnart C, Descargues P,
Hovnanian A, et al: Increased serine protease activity and
cathelicidin promotes skin inflammation in rosacea. Nat Med.
13:975–980. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gran B, Tabibzadeh N, Martin A, Ventura
ES, Ware JH, Zhang GX, Parr JL, Kennedy AR and Rostami AM: The
protease inhibitor, Bowman-Birk Inhibitor, suppresses experimental
autoimmune encephalomyelitis: A potential oral therapy for multiple
sclerosis. Mult Scler. 12:688–697. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lichtenstein GR, Deren JJ, Katz S, Lewis
JD, Kennedy AR and Ware JH: Bowman-Birk inhibitor concentrate: A
novel therapeutic agent for patients with active ulcerative
colitis. Dig Dis Sci. 53:175–180. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ware JH, Wan XS, Newberne P and Kennedy
AR: Bowman-Birk inhibitor concentrate reduces colon inflammation in
mice with dextran sulfate sodium-induced ulcerative colitis. Dig
Dis Sci. 44:986–990. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Qin XF: Impaired inactivation of digestive
proteases by deconjugated bilirubin: The possible mechanism for
inflammatory bowel disease. Med Hypotheses. 59:159–163. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bermúdez-Humarán LG, Motta JP, Aubry C,
Kharrat P, Rous-Martin L, Sallenave JM, Deraison C, Vergnolle N and
Langella P: Serine protease inhibitors protect better than IL-10
and TGF-β anti-inflammatory cytokines against mouse colitis when
delivered by recombinant lactococci. Microb Cell Fact. 14:262015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Stocker R, Yamamoto Y, McDonagh AF, Glazer
AN and Ames BN: Bilirubin is an antioxidant of possible
physiological importance. Science. 235:1043–1046. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhou K, Jiang M, Liu Y, Qu Y, Shi G, Yang
X, Qin X and Wang X: Effect of bile pigments on the compromised gut
barrier function in a rat model of bile duct ligation. PLoS One.
9:e989052014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhou K, Jiang M, Qin X and Wang X: Role of
bilirubin in digestive proteases inactivation in the lower
intestine. Dig Liver Dis. 47:438–439. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Morris GP, Beck PL, Herridge MS, Depew WT,
Szewczuk MR and Wallace JL: Hapten-induced model of chronic
inflammation and ulceration in the rat colon. Gastroenterology.
96:795–803. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Szalai Z, Szász A, Nagy I, Puskás LG,
Kupai K, Király A, Berkó AM, Pósa A, Strifler G, Baráth Z, et al:
Anti-inflammatory effect of recreational exercise in TNBS-induced
colitis in rats: Role of NOS/HO/MPO system. Oxid Med Cell Longev.
2014:9259812014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhou K, Jiang M, Qin X and Wang X: Role of
bilirubin in digestive proteases inactivation in the lower
intestine. Dig Liver Dis. 47:438–439. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gilani GS and Sepehr E: Protein
digestibility and quality in products containing antinutritional
factors are adversely affected by old age in rats. J Nutr.
133:220–225. 2003.PubMed/NCBI
|
|
25
|
Camuesco D, Peran L, Comalada M, Nieto A,
Di Stasi LC, Rodriguez-Cabezas ME, Concha A, Zarzuelo A and Galvez
J: Preventative effects of lactulose in the
trinitrobenzenesulphonic acid model of rat colitis. Inflamm Bowel
Dis. 11:265–271. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Aube AC, Cherbut C, Barbier M, Xing JH,
Roze C and Galmiche JP: Altered myoelectrical activity in
noninflamed ileum of rats with colitis induced by trinitrobenzene
sulphonic acid. Neurogastroenterol Motil. 11:55–62. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ruyssers NE, de Winter BY, de Man JG,
Loukas A, Pearson MS, Weinstock JV, Van den Bossche RM, Martinet W,
Pelckmans PA and Moreels TG: Therapeutic potential of helminth
soluble proteins in TNBS-induced colitis in mice. Inflamm Bowel
Dis. 15:491–500. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kapitulnik J: Bilirubin: An endogenous
product of heme degradation with both cytotoxic and cytoprotective
properties. Mol Pharmacol. 66:773–779. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Maeda S, Maeda S, Ohno K, Kaji N, Hori M,
Fujino Y and Tsujimoto H: Protease-activated receptor-2 induces
proinflammatory cytokine and chemokine gene expression in canine
keratinocytes. Vet Immunol Immunopathol. 153:17–25. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu J, Wang L, Tian XY, Liu L, Wong WT,
Zhang Y, Han QB, Ho HM, Wang N, Wong SL, et al: Unconjugated
bilirubin mediates heme oxygenase-1-induced vascular benefits in
diabetic mice. Diabetes. 64:1564–1575. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Berberat PO, A-Rahim YI, Yamashita K,
Warny MM, Csizmadia E, Robson SC and Bach FH: Heme
oxygenase-1-generated biliverdin ameliorates experimental murine
colitis. Inflamm Bowel Dis. 11:350–359. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kawamura K, Ishikawa K, Wada Y, Kimura S,
Matsumoto H, Kohro T, Itabe H, Kodama T and Maruyama Y: Bilirubin
from heme oxygenase-1 attenuates vascular endothelial activation
and dysfunction. Arterioscler Thromb Vasc Biol. 25:155–160.
2005.PubMed/NCBI
|
|
33
|
Lee TS and Chau LY: Heme oxygenase-1
mediates the anti-inflammatory effect of interleukin-10 in mice.
Nat Med. 8:240–246. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Varga C, Laszlo F, Fritz P, Cavicchi M,
Lamarque D, Horvath K, Posa A, Berko A and Whittle BJ: Modulation
by heme and zinc protoporphyrin of colonic heme oxygenase-1 and
experimental inflammatory bowel disease in the rat. Eur J
Pharmacol. 561:164–171. 2007. View Article : Google Scholar : PubMed/NCBI
|