Introduction
Malignant melanoma is the most common type of skin
cancer that begins in the melanocytes (1,2). The
incidence of this disease is increasing yearly around the world
(3). Malignant melanoma is
characterized by a complex and heterogeneous etiology (4), which is still largely unknown.
Surgical therapy is effective for localized disease, however, the
median survival of patients with metastatic malignant melanoma is
only 6–9 months after treatment with radiation and conventional
chemotherapy drugs (5). In
addition, the prognosis for metastatic melanoma is extremely poor
due to highly resistance to radiation and chemotherapy drugs
(6). Therefore, a better
understanding of key mechanism underlying malignant melanoma
facilitates to the development of therapeutic strategies.
MicroRNAs (miRNAs), small non-coding RNA molecules,
are known to play a crucial role in regulating carcinogenesis in
various cancers, including malignant melanoma (7,8).
Accumulating evidences have confirmed a complex crosstalk between
apoptosis and autophagy in cancer development (9,10).
miRNAs are found to regulate autophagy and exhibit important roles
in the crosstalk between apoptosis and autophagy in disease
progression (11). For instance,
upregulation of microRNA-21 can influence the apoptosis of
melanocytic cells in malignant melanoma (12). Depletion of miR-638 induces cell
apoptosis and autophagy in melanoma (13). Thus, identification of key miRNAs
associated with apoptosis and autophagy of melanoma cells will have
great significance in the therapy of melanoma. Recently,
miR-24-1-5p (also known as miR-24-1*) is found to regulate high
molecular weight Hyaluronan (hmw-Ha)-mediated enhancement of human
pulmonary endothelial barrier and vascular integrity (14,15).
Importantly, miR-24-1-5p is reported to be downregulated in
cutaneous malignant melanoma by means of microarray analysis of
microRNA expression profiles (16). However, whether miR-24-1-5p
contributes to the development of malignant melanoma via regulating
cell apoptosis and autophagy is largely unknown.
In this study, the miR-24-1-5p expression in
malignant melanoma tissues was investigated. The effects of
miR-24-1-5p overexpression of on the expression levels of cell
autophagy- and apoptosis-related proteins were investigated. In
addition, whether ubiquitin D (UBD) was a target of miR-24-1-5p was
explored. Besides, the expression levels of janus kinase (JNK)
pathway-related proteins were determined after overexpression of
miR-24-1-5p and UBD. The objective of our study was to investigate
the potential roles of miR-24-1-5p in regulating apoptosis and
autophagy of malignant melanoma cells, thus to elucidate the
possible regulatory mechanism of miR-24-1-5p in malignant
melanoma.
Materials and methods
Specimen collection
A total of 77 malignant melanoma specimens stored in
our hospital were enrolled in this study. None of the patients
received preoperative anticancer treatment. Thereinto, 29 specimens
were primary malignant melanoma, 31 specimens were metastatic
malignant melanoma, and 17 specimens were malignant melanoma
associated with lymph node metastasis. Specimens that collected
from the patients, was diagnosed by pathological analysis, and none
of the patients received preoperative anticancer treatment. Tumor
tissues and their adjacent normal tissues were collected from
clinically ongoing surgical specimens. These samples were then
snap-frozen with liquid nitrogen, and stored at −80°C. This study
was approved by our hospital's ethics committee and each patient
provided their written informed consent.
Cell culture
Human melanoma cell line A375 was purchased from
American Type Culture Colleciton (ATCC; Manassas, VA, USA). Then
A375 cells were cultured in RPMI-1640 medium (Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany) supplemented with 10% fetal bovine serum
(FBS; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) at
37°C in a humidified incubator with 5% CO2. The medium
was replaced every 2 days during subculture.
Cell transfection
A375 cells were digested and plated in 6-well plates
and incubated at 37°C in 5% CO2 for 24 h. After 80–90%
confluence, cells were transfected with miR-24-1-5p mimics, mimic
control, si-UBD, si-control, pcDNA3.1 vector, and pcDNA3.1-UBD
using Lipofectamine RNAiMAX (Life Technologies; Thermo Fisher
Scientific, Inc.) following the instructions of manufacturer.
Detection of apoptosis
Cell apoptosis was detected using flow cytometry
after staining with Annexin V-FITC and propidium iodide (PI;
Sigma-Aldrich; Merck KGaA). Briefly, cells were plated in dish and
allowed to settle for 48 h. Cells were then harvested, washed with
ice-cold PBS and then double labelled with Annexin V-FITC and PI
according to the protocol recommended by the manufacturer. The
mixtures were then analyzed using the FACSCalibur flow cytometer
(BD Biosciences, San Jose, CA, USA) equipped with FACStation
running CellQuest 3.0 software (BD Biosciences). Annexin V-FITC was
used to determine the percentage of apoptotic, while PI was used to
stain the dead cells.
Bioinformatic method and luciferase
reporter assay
The target of miR-24-1-5p was predicted according to
the information of TargetScanHuman database and UBD was predicted
as a target of miR-24-1-5p. To further validate the predicted
results, the wild type and mutant 3′UTR of UBD were cloned into the
pGL3 Luciferase assays vector (Promega Corporation, Madison, WI,
USA). The wild-type miR-24-1-5p contained binding sites of UBD
3′UTR with miR-24-1-5p. For luciferase assays, cells were plated in
a 24-well plate and continued to incubate for another 24 h. Cells
were then co-transfected with Firefly luciferase constructs
containing the 3′UTR wild-type or 3′UTR mutant of UBD, pRL-TK
Renilla luciferase normalization control, miR-24-1-5p mimics or
mimic control using Lipofectamine 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.). At 48 h after transfection, lysates were
collected and measured with a Dual-Luciferase Reporter system
(Promega Corporation) according to the protocols provided by the
manufacturer. Renilla luciferase activity was used as an internal
control.
Protein extraction and western
blotting
Cells were lysed in whole cell lysis buffer,
including 0.5 M Tris-HCl (pH 6.8), 1% b-mercaptoethanol, 0.02%
bromophenol blue, 10% glycerol and 2% SDS. Then protein
concentrations were detected using BCA protein assay kit (Pierce;
Thermo Fisher Scientific, Inc.). Equal amount of protein sample was
subjected to a 12% SDS-PAGE and then transferred to Hybond ECL
membranes (Amersham Biosciences; GE Healthcare Life Sciences,
Little Chalfont, UK). After being blocked with 5% non-fat milk for
1 h, the membranes were incubated with primary antibodies against
UBD, JNK, p-JNK, c-Jun, p-c-Jun, light chain 3 (LC3), Beclin-1,
Bcl-2, Bcl-xL and GAPDH (1:1,000 dilution; Cell Signaling
Technology, Inc., Danvers, MA, USA) overnight at 4°C. After that,
the membranes were probed with appropriate horseradish-peroxidase
conjugated secondary antibodies (1:2,000 dilution; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) for 2 h. After incubation
with a chromogenic substrate, antibodyreactive proteins were
visualized using the enhanced chemiluminescence (ECL) detection
system (Thermo Fisher Scientific, Inc.). GAPDH was used as the
internal control.
Quantitative real time polymerase
chain reaction (qRT-PCR)
Total RNA was extracted from cultured cells by a
modified method with TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions.
After detecting the quality of isolated RNA using a
NanoDrop® ND-1000 UV-Vis spectrophotometer (Thermo
Fisher Scientific, Inc.), reverse transcription into cDNA was then
conducting using the PrimerScript First Strand cDNA Synthesis kit
(Invitrogen; Thermo Fisher Scientific, Inc.). With a standard
protocol recommended by the manufacturer, qRT-PCR was carried out
using the SYBR ExScript qRT-PCR kit (Takara Biotechnology Co.,
Ltd., Dalian, China). Each reaction was performed in triplicate.
The expressions of targets relative to β-actin was calculated using
the comparative threshold (Ct) cycle (2−ΔΔCt)
method.
Statistical analysis
Data from three independent experiments were
expressed as mean ± standard error of mean (SEM). Statistical
significant differences were assessed between groups using the
Student's t-test or one-way analysis of variance (ANOVA). A value
of P<0.05 was accepted as statistically significant. All
statistical analyses were conducted with the GraphPad Prism 5.0
software (GraphPad Software, Inc., La Jolla, CA, USA).
Results
miR-24-1-5p is downregulated in
malignant melanoma tissues
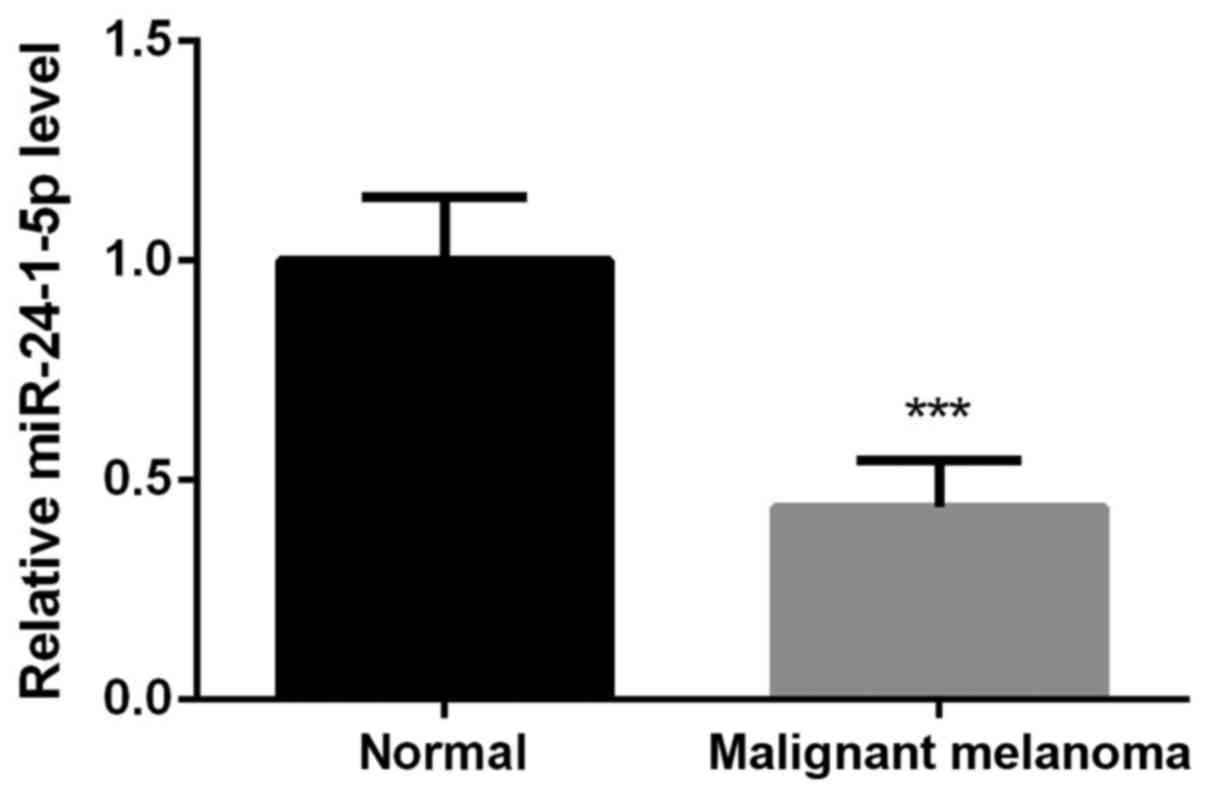
We first detected the expression levels of
miR-24-1-5p in malignant melanoma tissues and their adjacent normal
tissues. The results showed that the expression levels of
miR-24-1-5p in malignant melanoma tissues, including the primary
malignant melanoma, metastatic malignant melanoma, and malignant
melanoma specimens associated with lymph node metastasis were
significantly downregulated in comparison with their adjacent
normal tissues (Fig. 1,
P<0.001). We then selected malignant melanoma A375 cells for the
further analysis.
Overexpression of miR-24-1-5p promotes
autophagy and apoptosis of melanoma A375 cells
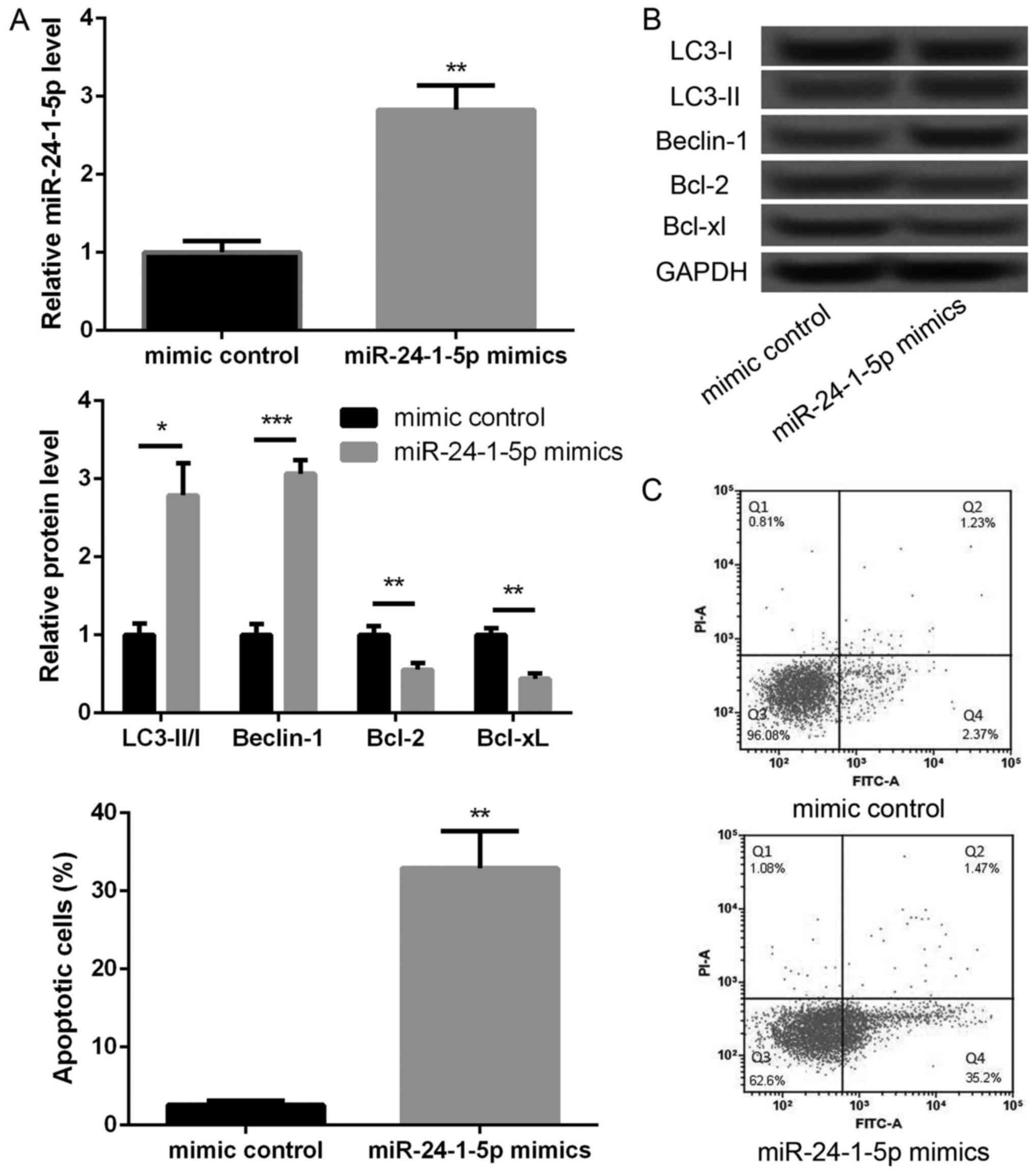
To further investigate the effects of miR-24-1-5p,
we overexpressed miR-24-1-5p in melanoma A375 cells. As shown in
Fig. 2A, the expression of
miR-24-1-5p was significantly increased in miR-24-1-5p mimic
transfected cells compared with that in mimic control transfected
cells (P<0.01), indicating that miR-24-1-5p was successfully
overexpressed in melanoma A375 cell. We then further explore the
effects of miR-24-1-5p overexpression on cell autophagy- and
apoptosis-related proteins. In compared with mimic control group,
the levels of LC3-II/I ratio and Beclin-1 expression were
significantly increased in miR-24-1-5p mimic transfected cells
(Fig. 2B, P<0.05), indicating
that miR-24-1-5p promoted melanoma cell autophagy. In addition, the
protein expression levels of Bcl-2 and Bcl-xL were significantly
decreased in miR-24-1-5p mimic transfected cells compared with
mimic control transfected cells (Fig.
2B, P<0.05). The results of flow cytometry also showed that
the percentage of apoptotic cells was significantly increased after
overexpression of miR-24-1-5p (Fig.
2C, P<0.01).
UBD is a direct target of
miR-24-1-5p
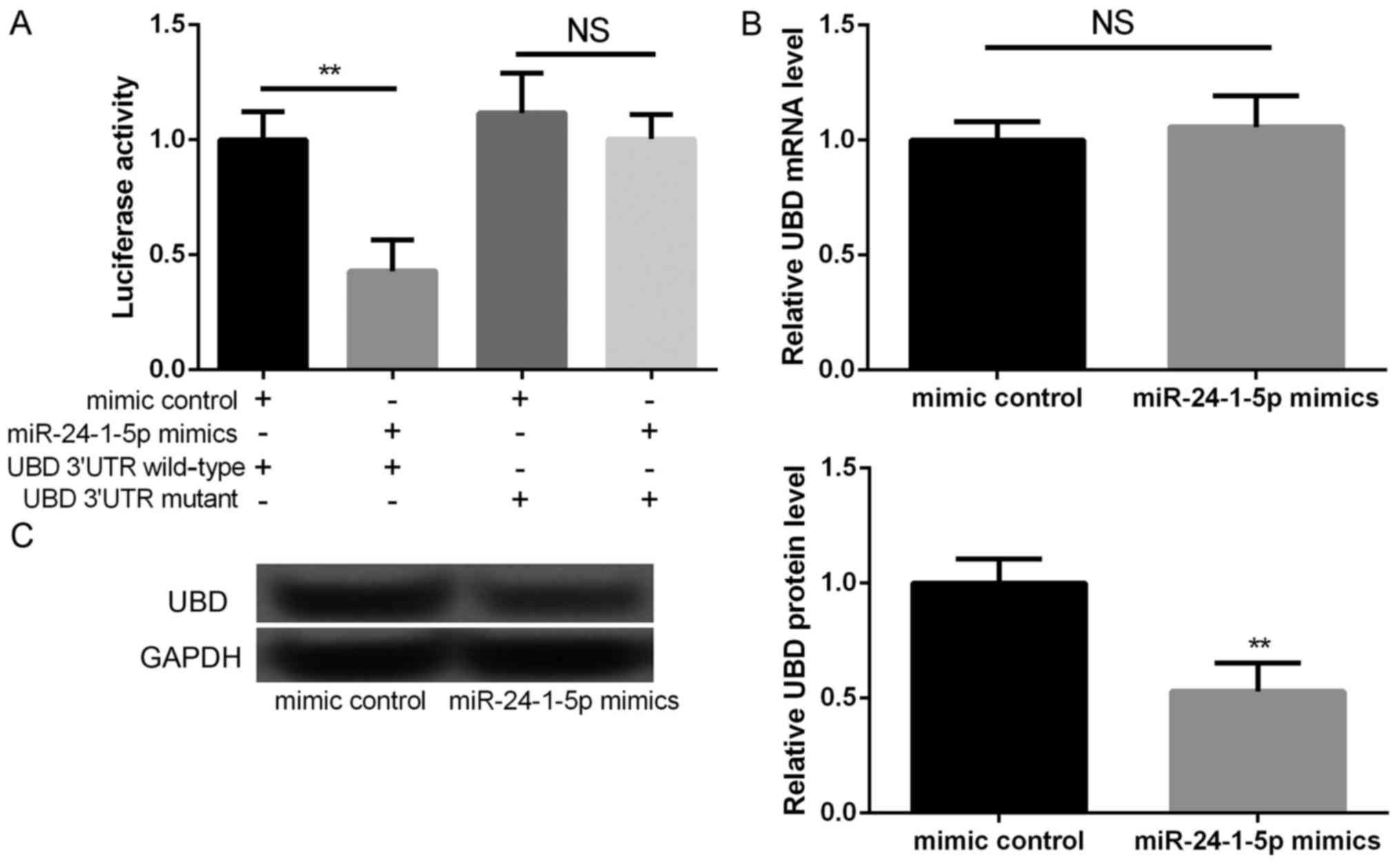
According to the information of TargetScanHuman
database, UBD was predicted as a potential target of miR-24-1-5p,
which was further verified by luciferase reporter analysis. We
found that the relative luciferase activities containing the
wild-type 3′UTR of UBD were significantly decreased in miR-24-1-5p
mimic transfected cells compared to that in mimic control
transfected cells (Fig. 3A,
P<0.01), but not mutant 3′UTR of UBD. Moreover, although there
is no significant different in the mRNA expression of UBD between
miR-24-1-5p mimic transfection group and mimic control group
(Fig. 3B), the protein expression
of UBD was significantly decreased miR-24-1-5p mimic transfection
group compared with mimic control group (Fig. 3C, P<0.01). These findings
indicated that UBD was the direct target of miR-24-1-5p.
Silencing of UBD promotes autophagy
and apoptosis of melanoma A375 cells
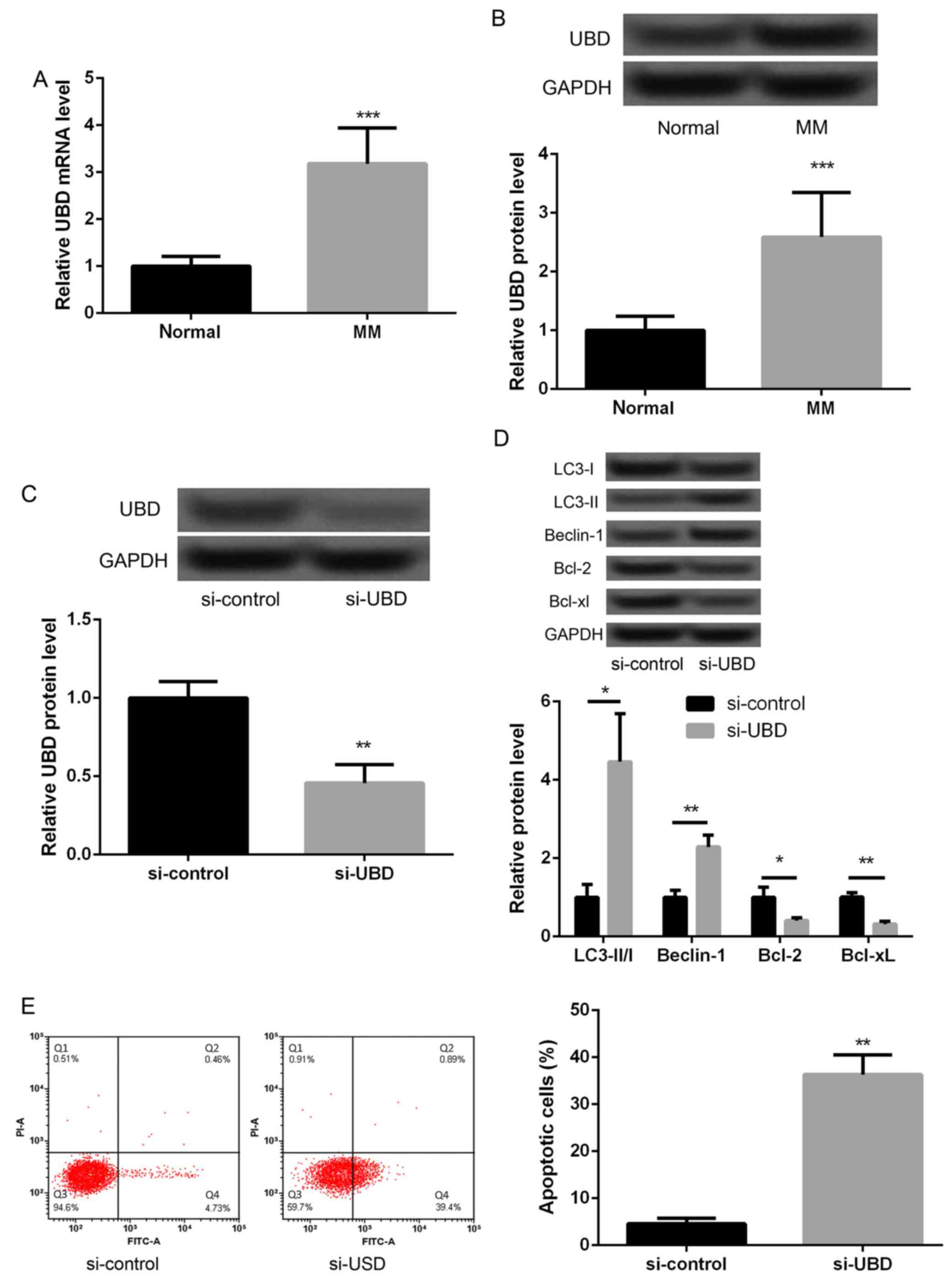
We further detected the expression of UBD in
malignant melanoma tissues. The results showed that the expression
of UBD in malignant melanoma tissues was significantly higher than
that in their adjacent normal tissues (Fig. 4A and B, P<0.05). In addition,
UBD was silenced to investigate whether UBD could regulate melanoma
cell autophagy and apoptosis. As displayed in Fig. 4C, the protein expression of UBD in
si-UBD group was significantly decreased compared with that in
si-control (Fig. 4C, P<0.05),
suggesting that UBD was successfully silenced inmalignant melanoma
cells. In addition, we found that after silencing of UBD, the
levels of LC3-II/I ratio and Beclin-1 expression were significantly
increased and the expression of Bcl-2 and Bcl-xL were markedly
decreased (Fig. 4D, P<0.05).
The results of flow cytometry also displayed an obviously increased
apoptotic cells after silencing of UBD (Fig. 4E, P<0.05). These data indicated
that silencing of UBD promoted melanoma cell autophagy and
apoptosis significantly.
JNK pathway may be a potential
mechanism involved in miR-24-1-5p-mediated autophagy and apoptosis
in melanoma A375 cells
In previous study, activation of JNK pathway is
shown to be involved in the induction of tumor cell autophagy and
Apoptosis (17), thus we detected
the expression changes of phosphorylated JNK and c-Jun after
overexpression of miR-24-1-5p and UBD. As shown in Fig. 5, the levels of p-JNK/JNK ratio and
p-c-Jun/Jun ratio were significantly increased after miR-24-1-5p
overexpression alone (P<0.05), indicating that overexpression of
miR-24-1-5p could promote the activation of JNK pathway. However,
the increased levels of p-JNK/JNK ratio and p-c-Jun/Jun ratio were
significantly reversed after overexpression of miR-24-1-5p and UBD
(P<0.05). Moreover, overexpression of UBD could also
significantly reverse the increased levels of LC3-II/I ratio and
Beclin-1 expression, as well as the decreased expression of Bcl-2
and Bcl-xL (P<0.05). These data imply that miR-24-1-5p may
regulate the balance of apoptosis and autophagy via targeting UBD
and involving in JNK pathway.
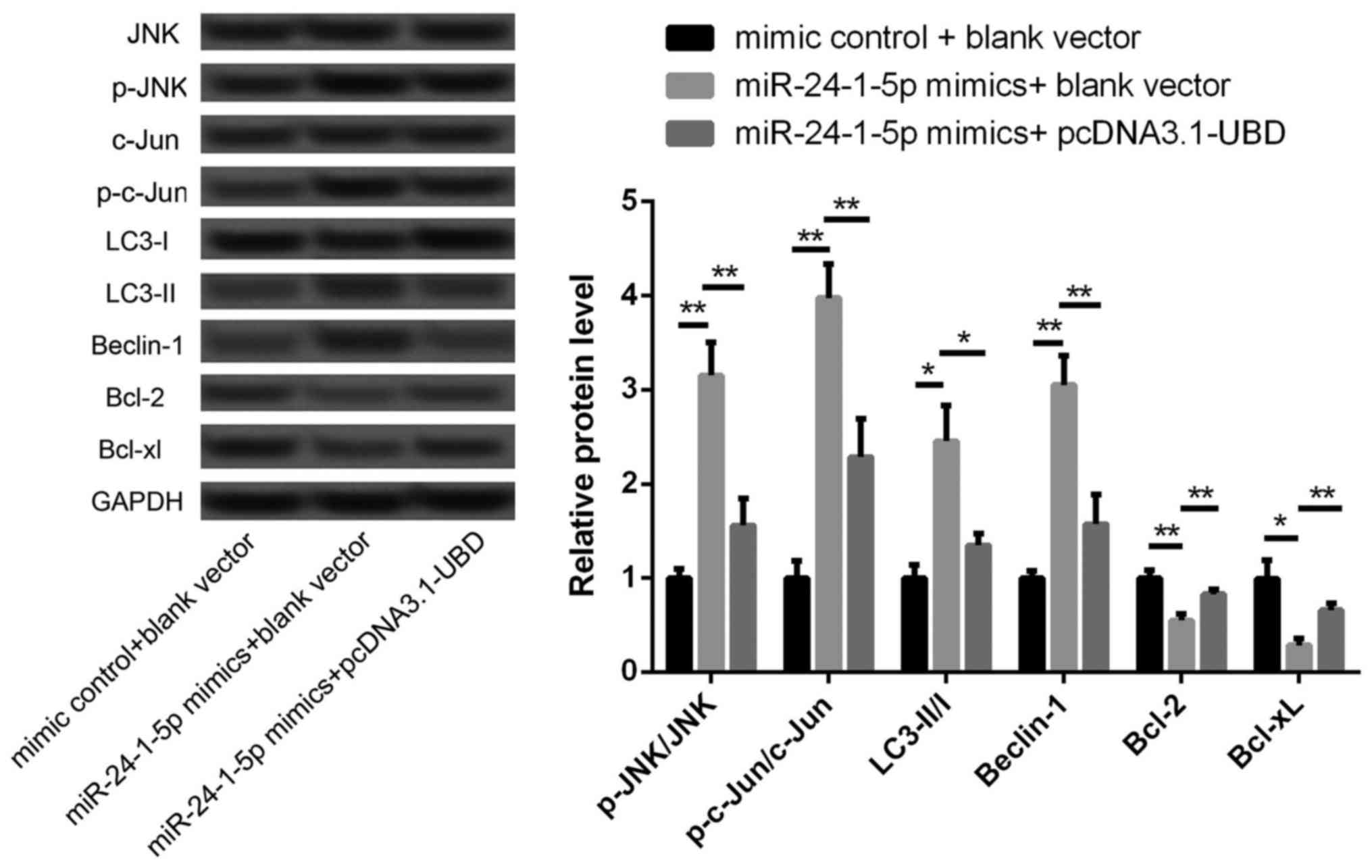 | Figure 5.The expression levels of JNK, P-JNK,
c-Jun, p-c-Jun, LC3-I, LC3-II, Beclin-1, Bcl-2 and Bcl-xL in
different transfected cells. *P<0.05, **P<0.01. JNK, janus
kinase; P-JNK, phosphorylated -JNK; LC3, light chain 3; UBD,
ubiquitin D. |
Discussion
In the present study, we found that miR-24-1-5p was
significantly downregulated in malignant melanoma tissues, which
was consistent with previous findings that miR-24-1 was a tumor
suppressor and might serve as a disease progression marker in
prostate cancer (16). Moreover,
overexpression of miR-24-1-5p significantly increased the levels of
LC3-II/I ratio and Beclin-1 expression, and decreased the
expression levels of Bcl-2 and Bcl-xL. UBD was confirmed as a
direct target of miR-24-1-5p. Silencing of UBD promoted melanoma
cell autophagy and apoptosis via regulating the expressions of
their related proteins. Besides, the levels of p-JNK/JNK ratio and
p-c-Jun/Jun ratio were significantly increased after miR-24-1-5p
overexpression alone, which were reversed after overexpression of
miR-24-1-5p and UBD.
Apoptosis is the programmed cell death that
maintains the balance of survival and death in living organisms
(18,19). The expression levels of
antiapoptotic Bcl-2 and Bcl-xL have been confirmed to increase with
progression of malignant melanoma, which can reflect an increased
malignant potential of metastatic melanoma cells after inhibiting
cell apoptosis (20).
Significantly, antisense-mediated inhibition of Bcl-2 is proved to
sensitize malignant melanoma to apoptosis-inducing treatment
modalities, and targeting Bcl-2 may be used as a highly effective
strategy in the targeted therapy of malignant melanoma (21). In addition, autophagy is an
evolutionarily conserved catabolic process which functions a dual
role, playing pro-death or pro-survival role depending on the cell
type and strength of specific stimuli (22,23).
Low levels of autophagy-regulated proteins LC3A and Beclin 1 are
associated with an increased vascular density in cutaneous
malignant melanoma (24).
Autophagy genes Beclin 1 and MAP1LC3 are also confirmed to play a
key role in tumor development in melanoma (25). Moreover, miR-204 can regulate
hypoxia-reoxygenation-induced cardiomyocyte autophagy via
regulating LC3-II (26). In our
study, miR-24-1-5p overexpression significantly increased the
levels of autophagy-related proteins (LC3-II/I ratio and Beclin-1),
and decreased the expression levels of apoptosis-related proteins
(Bcl-2 and Bcl-xL). Although the roles of miR-24-1-5p in malignant
melanoma have been fully explored, our results prompt that
overexpression of miR-24-1-5p may promote cell autophagy and
apoptosis in malignant melanoma.
Furthermore, UBD was confirmed as a direct target of
miR-24-1-5p. In previous studies, UBD is found to play a crucial
role in tumor development. For instance, increased expression of
UBD in hepatic cancer cells is reported to have a growth advantage
than cells without UBD expression (27). Overexpression of UBD is correlated
with lymph node metastasis and TNM staging in gastric cancer, and
its levels could be used as independent prognostic factors
(28). Yan et al also
demonstrated that UBD contributed to colon cancer progression and
might serve as a novel prognostic indicator for predicting
recurrence of stage II–III patients after curative surgery
(29). Remarkably, UBD could
regulates IRE1α/JNK-dependent apoptosis in pancreatic β cells
(30). Activating of JNK and ERK
is key mechanisms involved in Timosaponin AIII-induced apoptosis
and autophagy in human melanoma A375-S2 cells (31). In our study, silencing of UBD
promoted melanoma cell autophagy and apoptosis. Also, the levels of
p-JNK/JNK ratio and p-c-Jun/Jun ratio were significantly increased
after miR-24-1-5p overexpression alone, which were reversed after
overexpression of miR-24-1-5p and UBD. Collectively, our data
implying the regulatory relationship among miR-24-1-5p, UBD and JNK
pathway in melanoma cell autophagy and apoptosis.
In conclusion, overexpression of miR-24-1-5p may
target UBD and subsequently promote melanoma cell autophagy and
apoptosis via activation of JNK pathway. miR-24-1-5p may function
as a diagnostic marker or therapeutic target for malignant
melanoma. However, we did not identify possible mechanisms related
to the evolution of melanoma in different clinical stages, thus
failing to evaluate if these changes were observed in the same
patient or if it is an evolutionary effect of the melanoma.
Exploration of the evolution of melanoma in different clinical
stages is still needed to be performed in further
investigations.
References
|
1
|
Gray-Schopfer V, Wellbrock C and Marais R:
Melanoma biology and new targeted therapy. Nature. 445:851–857.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Luna JM, Hernández Guerrero A, Méndez R
Romero and Ortiz JL Luviano: Solution of the inverse bio-heat
transfer problem for a simplified dermatological application: Case
of skin cancer. Ingenierí Mecáni Tecnolog Desarroll. 4:2014.
|
|
3
|
Little EG and Eide MJ: Update on the
current state of melanoma incidence. Dermatol Clin. 30:355–361.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bloethner S, Scherer D, Drechsel M,
Hemminki K and Kumar R: Malignant melanoma-a genetic overview.
Actas Dermosifiliogr. 100 Suppl 1:S38–S51. 2009. View Article : Google Scholar
|
|
5
|
Gogas HJ, Kirkwood JM and Sondak VK:
Chemotherapy for metastatic melanoma: Time for a change? Cancer.
109:455–464. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu L, Kritsanida M, Magiatis P, Gaboriaud
N, Wang Y, Wu J, Buettner R, Yang F, Nam S, Skaltsounis L and Jove
R: A novel 7-bromoindirubin with potent anticancer activity
suppresses survival of human melanoma cells associated with
inhibition of STAT3 and Akt signaling. Cancer Biol Ther.
13:1255–1261. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Glud M and Gniadecki R: MicroRNAs in the
pathogenesis of malignant melanoma. J Eur Acad Dermatol Venereol.
27:142–150. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Völler D, Ott C and Bosserhoff A:
MicroRNAs in malignant melanoma. Clin Biochem. 46:909–917. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Maiuri MC, Zalckvar E, Kimchi A and
Kroemer G: Self-eating and self-killing: Crosstalk between
autophagy and apoptosis. Nat Rev Mol Cell Biol. 8:741–752. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xu J, Wang Y, Tan X and Jing H: MicroRNAs
in autophagy and their emerging roles in crosstalk with apoptosis.
Autophagy. 8:873–882. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Satzger I, Mattern A, Kuettler U,
Weinspach D, Niebuhr M, Kapp A and Gutzmer R: microRNA-21 is
upregulated in malignant melanoma and influences apoptosis of
melanocytic cells. Exp Dermatol. 21:509–514. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bhattacharya A, Schmitz U, Raatz Y,
Schönherr M, Kottek T, Schauer M, Franz S, Saalbach A, Anderegg U,
Wolkenhauer O, et al: miR-638 promotes melanoma metastasis and
protects melanoma cells from apoptosis and autophagy. Oncotarget.
6:2966–2980. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Singleton PA, Mirzapoiazova T,
Mambetsariev N, Ge L, Mambetsariev B, Lennon FE, Poroyko V, Fang Y,
Mutlu GM and Siddiqui S: miR-24-1-5p regulates high molecular
weight Hyaluronan (hmw-Ha)-mediated enhancement of vascular
Integrity: Role of foxa3. Am J Respir Crit Care Med.
191:A26462015.
|
|
15
|
Singleton PA, Siddiqui SS, Mirzapoiazova
T, Mambetsariev N, Mambetsariev B and Lennon FE: MicroRNA
Hsa-miR-24-1* regulates high molecular weight hyaluronan-mediated
human pulmonary endothelial barrier enhancement. Am J Respir Crit
Care Med. 183:A41732011.
|
|
16
|
Sand M, Skrygan M, Sand D, Georgas D,
Gambichler T, Hahn SA, Altmeyer P and Bechara FG: Comparative
microarray analysis of microRNA expression profiles in primary
cutaneous malignant melanoma, cutaneous malignant melanoma
metastases, and benign melanocytic nevi. Cell Tissue Res.
351:85–98. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hooi WC: The role of ROS-mediated ERK and
JNK activation in the induction of autophagy and apoptosis in
tumour cells by a novel small molecule compound. Ph D2009
|
|
18
|
Hassan M, Watari H, Abualmaaty A, Ohba Y
and Sakuragi N: Apoptosis and molecular targeting therapy in
cancer. Biomed Res Int. 2014:1508452014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sankari SL, Masthan KM, Babu NA,
Bhattacharjee T and Elumalai M: Apoptosis in cancer-an update.
Asian Pac J Cancer Prev. 13:4873–4878. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Leiter U, Schmid RM, Kaskel P, Peter RU
and Krähn G: Antiapoptotic bcl-2 and bcl-xL in advanced malignant
melanoma. Arch Dermatol Res. 292:225–232. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wacheck V, Losert D, Günsberg P,
Vornlocher HP, Hadwiger P, Geick A, Pehamberger H, Müller M and
Jansen B: Small interfering RNA targeting bcl-2 sensitizes
malignant melanoma. Oligonucleotides. 13:393–400. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Maycotte P and Thorburn A: Autophagy and
cancer therapy. Cancer Biol Ther. 11:127–137. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Janku F, Mcconkey DJ, Hong DS and Kurzrock
R: Autophagy as a target for anticancer therapy. Nat Rev Clin
Oncol. 8:528–539. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sivridis E, Koukourakis MI, Mendrinos SE,
Karpouzis A, Fiska A, Kouskoukis C and Giatromanolaki A: Beclin-1
and LC3A expression in cutaneous malignant melanomas: A biphasic
survival pattern for beclin-1. Melanoma Res. 21:188–195. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yuan-Ting SU, Jin HL and Dermatology DO:
The advances in Beclin 1 and MAP1LC3 in malignant melanoma. Chin J
Dermatovenereol. 2014.
|
|
26
|
Jian X, Xiao-yan Z, Bin H, Yu-feng Z, Bo
K, Zhi-nong W and Xin N: miR-204 regulate cardiomyocyte autophagy
induced by hypoxia-reoxygenation through LC3-II. Int J Cardiol.
148:110–112. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Oliva J, Bardaggorce F, French BA, Li J,
McPhaul L, Amidi F, Dedes J, Habibi A, Nguyen S and French SW:
Fat10 is an epigenetic marker for liver preneoplasia in a
drug-primed mouse model of tumorigenesis. Exp Mol Pathol.
84:102–112. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ji F, Jin X, Jiao CH, Xu QW, Wang ZW and
Chen YL: FAT10 level in human gastric cancer and its relation with
mutant p53 level, lymph node metastasis and TNM staging. World J
Gastroenterol. 15:2228–2233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yan DW, Li DW, Yang YX, Xia J, Wang XL,
Zhou CZ, Fan JW, Wen YG, Sun HC, Wang Q, et al: Ubiquitin D is
correlated with colon cancer progression and predicts recurrence
for stage II–III disease after curative surgery. Br J Cancer.
103:961–969. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Brozzi F, Gerlo S, Grieco FA, Juusola M,
Balhuizen A, Lievens S, Gysemans C, Bugliani M, Mathieu C,
Marchetti P, et al: Ubiquitin D regulates IRE1α/c-Jun N-terminal
kinase (JNK) protein-dependent apoptosis in pancreatic beta cells.
J Biol Chem. 291:12040–12056. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang Y, Xu L, Lou LL, Song SJ, Yao GD, Ge
MY, Hayashi T, Tashiro SI, Onodera S and Ikejima T: Timosaponin
AIII induces apoptosis and autophagy in human melanoma A375-S2
cells. Arch Pharm Res. 40:69–78. 2017. View Article : Google Scholar : PubMed/NCBI
|