Introduction
Osteoclasts are large multinuclear cells, formed by
fusion of macrophages derived from hematopoietic stem cells
(1). Osteoclasts are involved in
bone remodeling under normal conditions; osteoblasts build the bone
matrix, while osteoclasts absorb the matrix. Dysfunction of
osteoblasts or over-activity of osteoclasts leads to a breakdown in
the balance of bone remodeling. In inflammation (2), osteoclasts are overactive and
upregulated, which leads to an imbalance between bone formation and
resorption, causing bone loss (3).
Diseases associated with osteoclasts frequently arise in the fields
of orthopedics (4), stomatology
(5), otorhinolaryngology (5) and rheumatology (6).
In order to investigate diseases associated with
osteoclasts, it is necessary to culture osteoclasts in
vitro. Osteoclasts are terminally-differentiated cells which
lack the ability to proliferate; in addition, it is difficult to
separate osteoclasts from the surface of cortical bone in
vitro. Therefore, it is not possible to separate osteoclasts
from cortical bone to culture for further experiments. At present,
there are three kinds of cells commonly used for osteoclast
modeling: Murine bone marrow cells (BMCs) (7,8);
murine RAW 264.7 cells (9); and,
human peripheral blood mononuclear cells (PBMCs) (10). Various stimulators have been used,
including receptor activator of nuclear factor κ-B ligand (RANKL),
macrophage colony-stimulating factor (MCSF), tumor necrosis
factor-α, 1α, 25-dihydroxyvitamin D3, lipopolysaccharide and
mercaptopurine (11); only RANKL
and MCSF have been identified to be effective.
RAW 264.7 murine monocyte-macrophage cells
differentiate into osteoclasts following stimulation with RANKL and
MCSF (9); this model is the most
commonly used to date.
A combination of tartrate-resistant acid phosphatase
(TRAP) staining and a bone resorption test are required to identify
osteoclasts, as other osteoclast-like cells may be TRAP-positive
(11).
There are few studies using a functional osteoclast
model induced from a human cell line (7–10).
THP-1 cells are a monocyte cell line derived from a patient with
acute leukemia. It remains unknown whether THP-1 cells are able to
differentiate into osteoclasts following stimulation with RANKL and
MCSF. Suspended THP-1 monocytes will differentiate into adherent
macrophages with the stimulation of phorbol-12 myristate-13 acetate
(PMA), an activator of the protein kinase C (PKC) signaling pathway
(12); macrophages are the
intermediate stage between monocytes and osteoclasts.
In order to investigate human osteoclasts, a human
osteoclast model is required. The present study aimed to construct
a human osteoclast model from THP-1 cells, using stimulation with
PMA, RANKL and MCSF.
Materials and methods
Materials
THP-1 cells were obtained from Zhongshan School of
Medicine, Sun Yat-sen University (Guangzhou, China). PMA, RANKL and
MCSF were purchased from PeproTech (Rocky Hill, NJ, USA). RPMI 1640
medium, fetal bovine serum (FBS) and penicillin/streptomycin were
purchased from Gibco (Thermo Fisher Scientific, Inc., Waltham, MA,
USA). TRAP staining was performed using a leukocyte acid
phosphatase kit from Sigma-Aldrich (Merck KGaA, Darmstadt,
Germany). APC-conjugated anti-cluster of differentiation (CD)14
antibody (CD14-APC; 9017-0149; 1:40) and PE-conjugated anti-CD11b
antibody (CD11b-PE; 9012-0118; 1:40) were purchased from
eBioscience, Inc. (Thermo Fisher Scientific, Inc.). Sclerites were
purchased from Southwest Hospital, Third Military Medical
University (Chongqing, China).
Cell adherence
THP-1 cells (1×106 cells/well) were
cultured in RPMI 1640 with 10% FBS, 100 IU/ml penicillin and 100
µg/ml streptomycin in 6-well plates in 5% carbon dioxide at 37°C.
The THP-1 cells were incubated with 100 ng/ml PMA for 0, 1, 2 and 3
days respectively, and unattached cells in the supernatant were
counted. The cell adherence rate was calculated according to the
cell number of THP-1 cells without stimulation.
Flow cytometry
The THP-1 cells were incubated with 100 ng/ml PMA
for 0, 1, 2 and 3 days respectively. The cells were incubated with
APC conjugated anti-CD14 (CD14-APC) (13) antibody as well as PE conjugated
anti-CD11b (CD11b-PE) (14)
antibody for 30 min at room temperature. Following washing with
PBS, cells were acquired and analyzed on a BD Jazz flow cytometer
(BD Biosciences, San Diego, CA, USA). The obtained data were
analyzed using FlowJo software version 7.6.1 (Tree Star, Inc.,
Ashland, OR, USA).
Osteoclast formation
THP-1 cells (1×105 cells/well) were
cultured in RPMI 1640 with 10% FBS, 100 IU/ml penicillin and 100
µg/ml streptomycin in 48-well plates in 3 groups, as follows: In
1.5 ng/ml group (low-dose, group 1a) or 100 ng/ml (high-dose, group
1b) PMA (12) was added to the
medium and the supernatant was removed 48 h afterwards.
Subsequently, 50 ng/ml RANKL and MCSF (PeproTech) (8) were added to the medium following
observation of cellular morphology (whether the suspending cells
were adherent and fused to be multinuclear cells) under a Zeiss
Axioplan 2 (Zeiss AG, Oberkochen, Germany) light microscope
(magnification, ×200). The culture medium was replaced with fresh
medium and cellular morphology was observed every 2 days for a
total 14 days (15). In group 2,
100 ng/ml PMA was added to the culture medium for 14 days. The
medium was replaced and cellular morphology was observed every 2
days. In group 3, 50 ng/ml RANKL and MCSF were added to the culture
medium for 14 days. The medium was replaced and cellular morphology
was observed every 2 days.
TRAP staining
Beginning on day 10, TRAP staining was performed on
the cells in each group according to the manufacturer's protocol
every 2 days, until the appearance of TRAP-positive cells was
noted. The cells were fixed in 4% paraformaldehyde for 30 sec at
room temperature and washed 3 times in PBS. The mixture of reagents
from the TRAP staining kit was added to each plate in a 37°C
aqueous bath for 45 min.
Bone resorption test
Sclerites were soaked in 75% ethanol overnight, and
sterilized in a benchtop autoclave for 30 min before use.
Sterilized tweezers were used to place the sclerites into 48-well
plates, to which 1×105 THP1 cells were added; other
treatment steps were the same as for group 1.
Following the appearance of TRAP-positive cells, the
sclerites were removed from the 48-well plates, and washed 3 times
with PBS and ultrasound. Following gradient elution using 75, 85
and 95% ethanol, the sclerites were stained with 0.5% toluidine
blue for 2 min at room temperature and washed 3 times in PBS
(16) to observe resorption
lacunae under the Zeiss Axioplan 2 (Zeiss AG) light microscope
(magnification, ×100).
Results
THP-1 cells differentiate into
macrophages following stimulation with PMA
Suspended THP-1 cells without stimulation exhibited
an adherence rate of 0%. The adherence rates of THP-1 cells
following stimulation with 100 ng/ml PMA for 1, 2 and 3 days were
70.1, 94.3 and 97.1% respectively. Therefore, >90% cells were
adherent following 2 days of stimulation with 100 ng/ml PMA
(Fig. 1A).
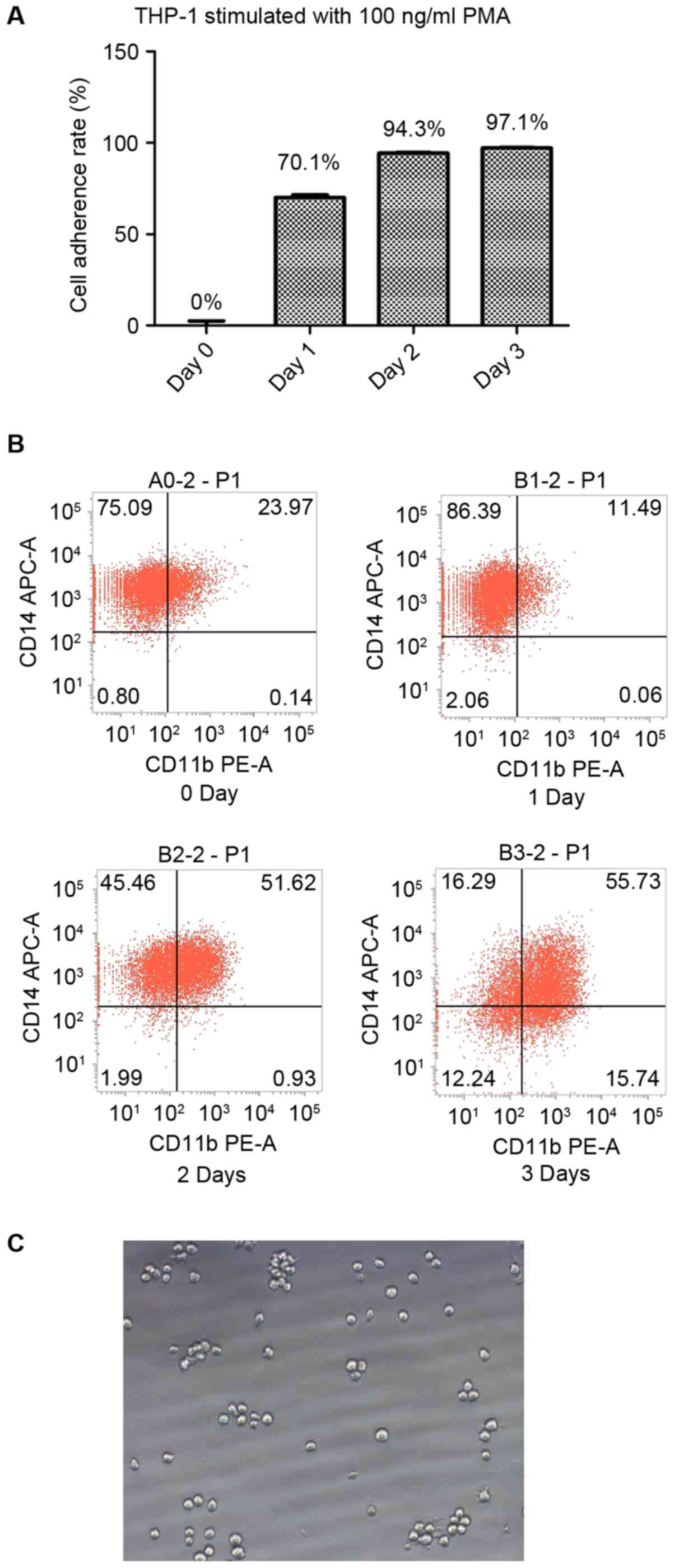 | Figure 1.THP-1 cells differentiate into
macrophages under stimulation with PMA. (A) The cell adherence
rates of THP-1 cells following stimulation with 100 ng/ml PMA for
0, 1, 2 and 3 days were 0, 70.1, 94.3 and 97.1% respectively. (B)
Without stimulation, 75.09% of the THP-1 cells were identified to
be CD14+CD11b−. Following stimulation with
100 ng/ml PMA for 1 day, 86.39% of the THP-1 cells were identified
to be CD14+CD11b−; following 2 days of
stimulation, 45.46% of the cells were identified to be
CD14+CD11b− and 51.62% were identified to be
CD14+CD11b+; and following 3 days of
stimulation, 55.73% cells were identified to be
CD14+CD11b+. (C) The cellular morphology
(magnification, ×200) of THP-1 cells in the absence of stimulation.
CD, cluster of differentiation; PMA, phorbol-12 myristate-13
acetate; APC, allophycocyanin; PE, phycoerythrin. |
Flow cytometric analysis demonstrated that the
majority of the THP-1 cells were CD14+/CD11b−
prior to stimulation. Following 1 day of stimulation with 100 ng/ml
PMA, the percentage of CD14+/CD11b− cells was
upregulated; however, one-half of the cells were
CD14+/CD11b+ following 2 days of stimulation
and the majority were CD14+/CD11b+ following
3 days of stimulation. The results of the present study suggest
that 2 days of stimulation with 100 ng/ml PMA is sufficient to
induce THP-1 cell differentiation into CD11b+ (14) macrophages (Fig. 1B).
High-dose PMA plus RANKL and MCSF
induce THP-1 differentiation into human functional osteoclasts
Cellular morphology
In the absence of stimulation, the THP-1 cells were
small round cells, suspended in the medium or deposited on the
bottom of the plates (Fig. 1C).
Following 48 h stimulation with PMA, THP-1 cells differentiated
into macrophages; certain cells were adherent with long spindles,
while others were large round cells. Cellular morphology was
unaltered in the high-dose PMA group compared with the low-dose PMA
group (Fig. 2A). Following
stimulation with RANKL and MCSF, the cells were fused further into
large multinuclear cells, which were irregular in shape. However,
it is difficult to identify osteoclasts solely by cellular
morphology (Fig. 2A). Under
stimulation with PMA alone, the THP-1 cells became adherent;
however, they did not exhibit further fusion. Under stimulation
with RANKL and MSCF only, there was no clear alteration observed in
the THP-1 cells; the majority of the cells were suspended or
deposited on the bottom of the plates.
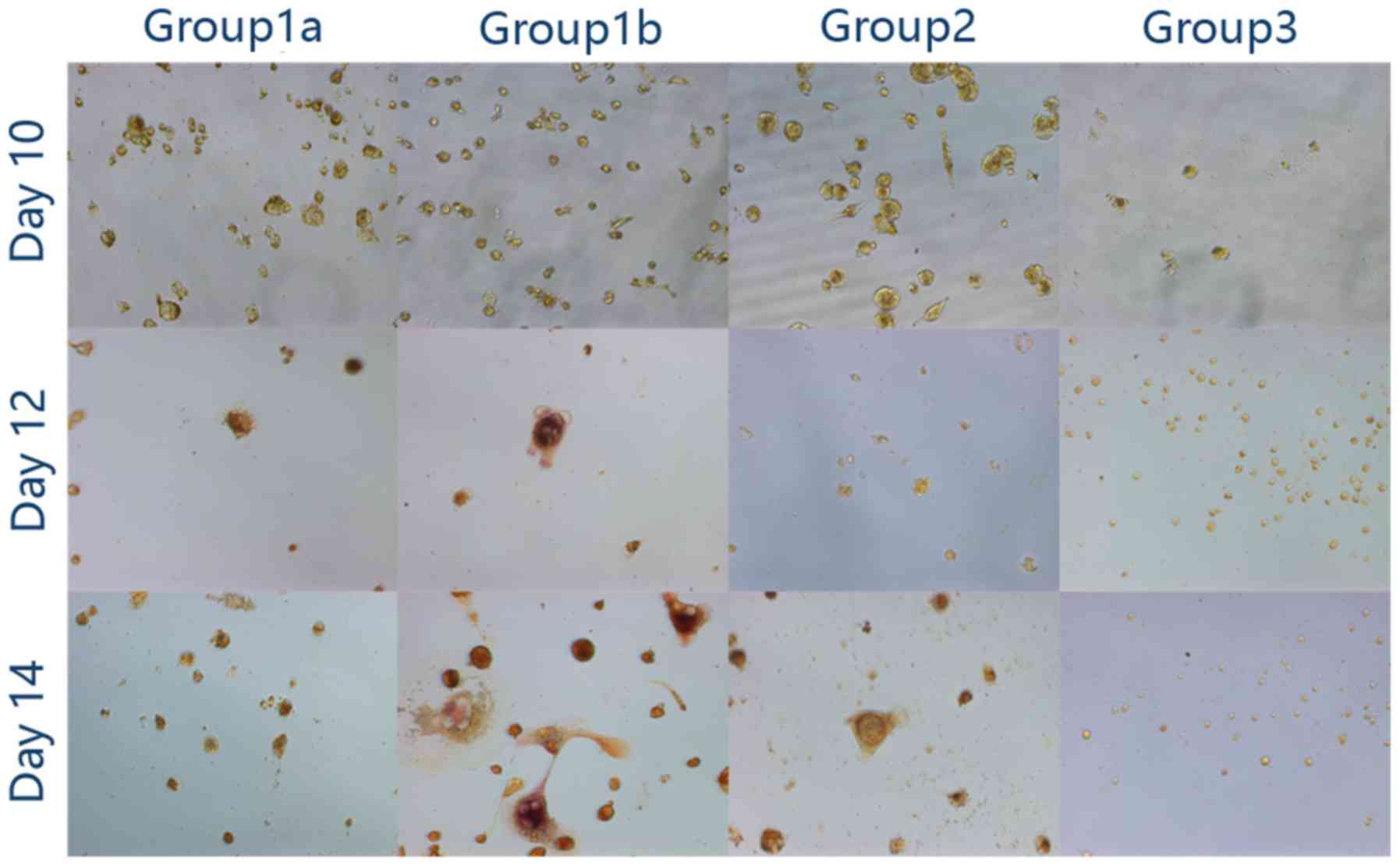
 | Figure 2.Two-step stimulation using PMA with
RANKL and MCSF. (A) Cellular morphology (magnification, ×200): In
group 1a and group 1b, following 48 h of stimulation with PMA, the
cells became adherent. RANKL and MCSF promoted further fusion in
group 1a and group 1b; however, little distinction was observed
between the two groups. In group 2, the cells became adherent
following 48 h of stimulation with PMA and the cells remained
adherent, although with no apparent fusion, at the end of the
observation. In group 3, following 48 h of stimulation with RANKL
and MCSF, no clear alteration was observed. (B) At day 12, bone
lacunae were observed solely in group 1b (magnification, ×200).
PMA, phorbol-12 myristate-13 acetate; RANKL, receptor activator of
nuclear factor k-B ligand; MCSF, macrophage colony stimulating
factor. |
Bone resorption test
A total of 2 days of low-dose PMA stimulation
followed by RANKL and MCSF did not lead to clear bone resorption.
However, high-dose PMA followed by RANKL and MCSF led to clear
lacuna formation (Fig. 2B).
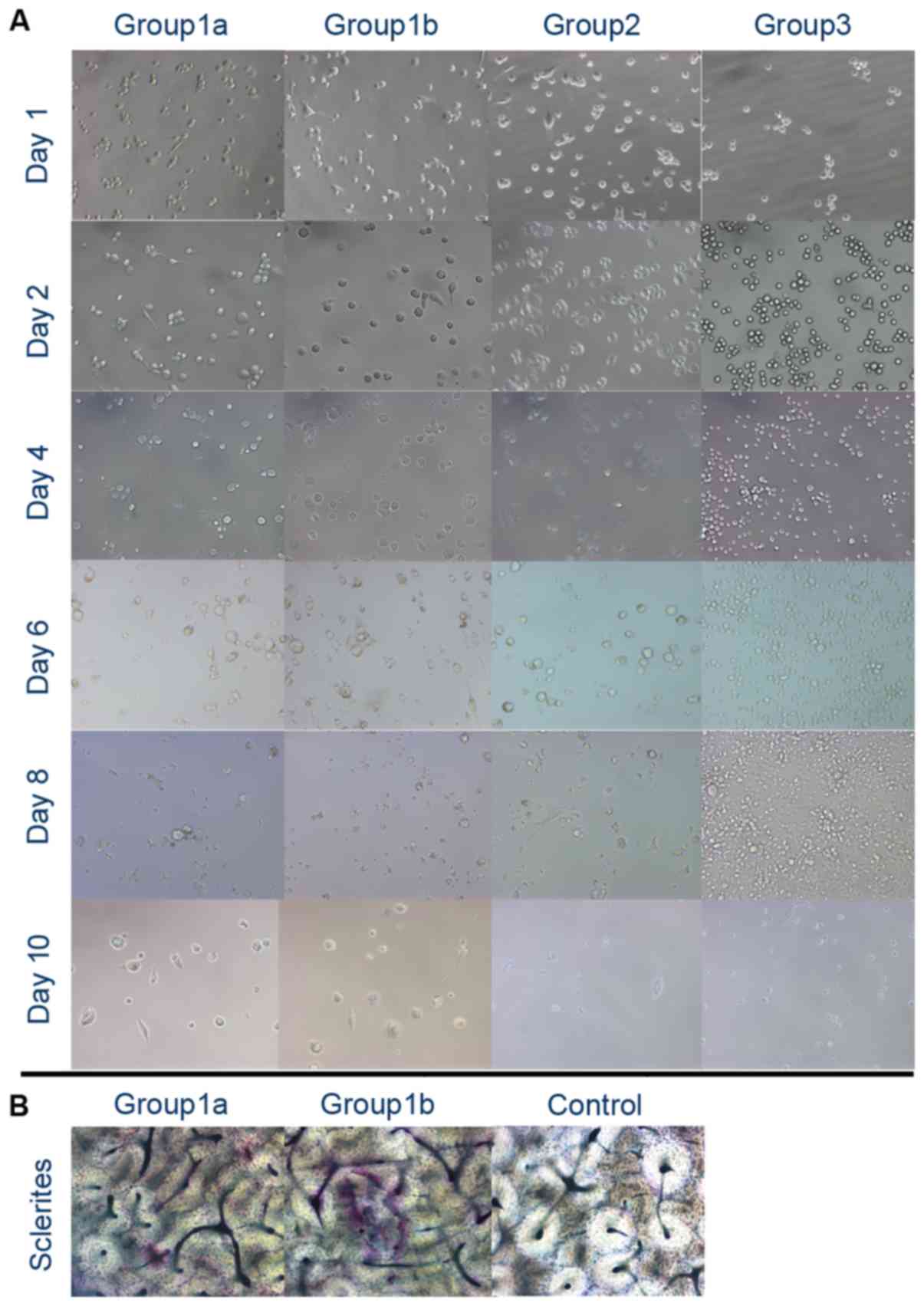
Trap staining
In group 1, following 2 days of stimulation with
high-dose or low-dose PMA, and 8 days stimulation with 50 ng/ml
RANKL and MCSF, no TRAP-positive cells were identified. Following 2
days of stimulation with high-dose PMA, and 10 days stimulation
with 50 ng/ml RANKL and MCSF, the cells appeared to contain
wine-red particles (TRAP-positive) and were multinuclear. However,
wine-red particles were not apparent in the low-dose PMA group
(group 1a). At day 14, the cells under stimulation with high-dose
PMA, RANKL and MCSF appeared to be TRAP-positive and multinuclear.
Under 14 days of stimulation with 100 ng/ml PMA alone, the THP-1
cells differentiated into large TRAP-negative macrophages. However,
under 14 days of stimulation with 50 ng/ml RANKL and MCSF, the
THP-1 cells remained as small round cells (Fig. 3).
Discussion
In the present study, a two-step stimulation process
was used in order to construct a human osteoclast model. PMA was
used to stimulate THP1 cell differentiation into macrophages,
followed by MCSF and RANKL to stimulate macrophage differentiation
into osteoclasts. This osteoclast model mimics the recruitment of
monocytes from peripheral blood during inflammation and their
differentiation into osteoclasts, leading to bone resorption.
THP1 cells are globular suspended monocytes. Under
the stimulation of PMA, THP1 cells will differentiate into adherent
macrophages with a round or fusiform morphology. Macrophages are
large compared with monocytes. According to Park et al
(12), there is no difference
between using high-dose or low-dose PMA to stimulate THP1 cell
differentiation into macrophages, although low-dose treatment may
affect cytokine expression. The present study used 50 ng/ml
(8) MCSF and RANKL to stimulate
macrophages. MCSF is able to upregulate the expression of RANK, and
RANKL facilitates the formation of TRAP-positive osteoclasts
(17). In the present study,
high-dose PMA was demonstrated to be the principal factor
influencing the formation of osteoclasts. Following 12 days of
stimulation, the high-dose PMA group exhibited the formation of
TRAP-positive osteoclasts which demonstrated positive results in
the bone resorption assay.
In the control group, using PMA alone or MCSF with
RANKL alone, the formation of osteoclasts was not observed.
Consequently, it is necessary to use a two-step method in order to
construct osteoclasts. Due to the suspension characteristics of
THP-1 cells, PMA is required to promote cellular adherence to the
bottom of the culture plate.
At present, the cell types which have been used in
osteoclast models are murine BMCs, murine RAW 264.7 cells and human
PBMCs. BMCs from mice may imitate the formation of true
osteoclasts; however, it is difficult to obtain BMCs from humans.
In order to investigate osteoclasts in humans, using PMBCs is a
feasible method; however, there are ethical considerations and it
is difficult to separate and purify monocytes from peripheral
blood; using the classical method (10) typically yields few PMBCs, which
additionally lack proliferation ability and exhibit a limited
lifespan. In order to construct an osteoclast model, the THP-1 cell
line was used. THP-1 cells are characterized by a high rate of
proliferation and immortality. A large number of cells may be
obtained within a short time and ethical issues, as well as the
consideration of individual differences within human PMBCs, may be
avoided.
In the present study, high-dose PMA combined with
MCSF and RANKL was used to reliably induce osteoclast formation.
The two-step method described in the present study mimics the two
stages of osteoclast formation and provides a tool to investigate
the mechanisms underlying the functions of osteoclasts. The method
described in the present study is associated with the PKC and
RANKL-RANK signaling pathways, which serve a role in bone
resorption during inflammation. In order to construct an improved
osteoclast model, it is suggested that the number of cells seeded
be increased and that the culture time be prolonged.
Acknowledgements
The present study was supported in part by the
National Natural Science Foundation of China (grant no.
81371082).
References
|
1
|
Soares-Schanoski A, Gómez-Piña V, del
Fresno C, Rodríguez-Rojas A, García F, Glaría A, Sánchez M,
Vallejo-Cremades MT, Baos R, Fuentes-Prior P, et al:
6-Methylprednisolone down-regulates IRAK-M in human and murine
osteoclasts and boosts bone-resorbing activity: A putative
mechanism for corticoid-induced osteoporosis. J Leukoc Biol.
82:700–709. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Charles JF and Aliprantis AO: Osteoclasts:
More than ‘bone eaters’. Trends Mol Med. 20:449–459. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sloan AJ, Taylor SY, Smith EL, Roberts JL,
Chen L, Wei XQ and Waddington RJ: A novel ex vivo culture model for
inflammatory bone destruction. J Dent Res. 92:728–734. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Flecher X, Rolland C, Rixrath E, Argenson
JN, Robert P, Bongrand P, Wendling S and Vitte J: Local and
systemic activation of the mononuclear phagocyte system in aseptic
loosening of total hip arthroplasty. J Clin Immunol. 29:681–690.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Crump TB, Wimmer KL, Reinhardt AL, Schmid
MJ, Meyer CR, Robinson DH, Marx DB, Bhattacharyya I and Reinhardt
RA: Effects of locally-delivered human macrophage products and
estrogen on murine inflammatory bone resorption. J Periodontal Res.
37:101–109. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee HP, Lin YY, Duh CY, Huang SY, Wang HM,
Wu SF, Lin SC, Jean YH and Wen ZH: Lemnalol attenuates mast cell
activation and osteoclast activity in a gouty arthritis model. J
Pharm Pharmacol. 67:274–285. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kawamura H, Arai M and Togari A:
Inhibitory effect of chlorpromazine on RANKL-induced
osteoclastogenesis in mouse bone marrow cells. J Pharmacol Sci.
117:54–62. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ueki Y, Lin CY, Senoo M, Ebihara T, Agata
N, Onji M, Saheki Y, Kawai T, Mukherjee PM, Reichenberger E and
Olsen BR: Increased myeloid cell responses to M-CSF and RANKL cause
bone loss and inflammation in SH3BP2 ‘cherubism’ mice. Cell.
128:71–83. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jung YK, Han SW, Kim GW, Jeong JH, Kim HJ
and Choi JY: DICAM inhibits osteoclast differentiation through
attenuation of the integrin αVβ3 pathway. J Bone Miner Res.
27:2024–2034. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Petrova NL, Petrov PK, Edmonds ME and
Shanahan CM: Novel use of a Dektak 150 surface profiler unmasks
differences in resorption pit profiles between control and Charcot
patient osteoclasts. Calcif Tissue Int. 94:403–411. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Uchiyama S and Yamaguchi M: Inhibitory
effect of beta-cryptoxanthin on osteoclast-like cell formation in
mouse marrow cultures. Biochem Pharmacol. 67:1297–1305. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Park EK, Jung HS, Yang HI, Yoo MC, Kim C
and Kim KS: Optimized THP-1 differentiation is required for the
detection of responses to weak stimuli. Inflamm Res. 56:45–50.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tundup S, Srivastava L, Nagy T and Harn D:
CD14 influences host immune responses and alternative activation of
macrophages during Schistosoma mansoni infection. Infect Immun.
82:3240–3251. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang RF, Zhao GW, Liang ST, Chen HZ and
Liu DP: Lysine-specific demethylase 1 represses THP-1
monocyte-to-macrophage differentiation. Chin Med Sci J. 28:82–87.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pivetta E, Wassermann B, Bulian P, Steffan
A, Colombatti A, Polesel J and Spessotto P: Functional
osteoclastogenesis: The baseline variability in blood donor
precursors is not associated with age and gender. Oncotarget.
6:31889–31900. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ritchlin CT, Haas-Smith SA, Li P, Hicks DG
and Schwarz EM: Mechanisms of TNF-alpha- and RANKL-mediated
osteoclastogenesis and bone resorption in psoriatic arthritis. J
Clin Invest. 111:821–831. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang Y, Dong G, Jeon HH, Elazizi M, La LB,
Hameedaldeen A, Xiao E, Tian C, Alsadun S, Choi Y and Graves DT:
FOXO1 mediates RANKL-induced osteoclast formation and activity. J
Immunol. 194:2878–2887. 2015. View Article : Google Scholar : PubMed/NCBI
|