Introduction
Diabetes mellitus (DM) is a type of endocrine
disease which is estimated to affect 284.6 million people
worldwide. According to previous reports, the number of people with
DM is predicted to increase to 438 million in 2030, which accounts
for 6.4% of the global population (1). Diabetic retinopathy (DR), the most
universal and severe complication of DM, is one of the primary
causes of blindness in adults (2).
The occurrence of DR is associated with the duration of DM.
Retinopathy rarely occurs in the first few years of diabetes,
however the likelihood of development increases to 50% following 10
years, and 90% following 25 years of suffering with DM (3). DR results in loss of vision and
blindness, which may reduce quality of life and result in an
economic burden to patients and the country. Therefore, the
prevention and treatment of DR is of primary concern for
researchers and clinicians.
The pathogenesis of DR has not been completely
elucidated, however it is believed to be correlated with
synergistic effects of a variety of factors. The pro-angiogenic
cytokine vascular endothelial growth factor (VEGF) is the primary
factor involved in neovascularization, which is the pathological
basis of DR (4–7). VEGF upregulation has been detected in
the vitreous humour and the fibrovascular tissues from eyes with DR
(8–13). VEGF activates two tyrosine kinase
receptors, VEGF receptor (R)-1 and VEGFR-2. These two receptors
regulate the physiological and pathological angiogenesis process.
It has been demonstrated that VEGFR-2 activation stimulates
endothelial cell proliferation, migration, and survival, in
addition to mediating angiogenesis and microvascular permeability
in DR (14). Furthermore, various
studies suggest that leukocyte aggregation resulting from
overexpression of intercellular adhesion molecule (ICAM)-1 is an
important factor in inducing the destruction of the blood-retinal
barrier (15). Previous studies
indicate that leukocyte adhesion is important in the pathogenesis
of DR. It is reported that the region of endothelial cell
destruction, capillary loss, and leukocyte extravasation is often
adjacent to static leukocytes (16). Therefore, evaluation of VEGF and
ICAM-1 expression levels is commonly used to assess retinal
vascular injury in DM.
Salvia miltiorrhiza Bunge (Danshen), which is an
important source of numerous active natural products, is divided
into aqueous and lipid soluble (diterpene) fractions (17). Tanshinone IIA (TSA) is the most
active diterpenoid quinine pigment in Danshen. The prominent
benefits of TSA on DM have been validated in numerous studies. It
is reported that TSA ameliorates glucose tolerance and decreases
the low-density to high-density lipoprotein ratio without altering
food intake in a high-fat diet induced obese animal model (18). Similar results have been reported
in db/db mice with DM, whereby TSA reduces the level of blood
glucose (19). Furthermore,
treatment with TSA reduces infarct area and ameliorates cardiac
dysfunction following ischemia/reperfusion injury in diabetic rats
(20). TSA has additionally been
demonstrated to inhibit vascular smooth muscle cell proliferation
and alleviate intimal hyperplasia (21). However, to the best of the author's
knowledge, no studies to date have focused on the effect of TSA on
HRECs under high glucose (HG) conditions mimicking DM.
The present study investigated the effects of TSA on
the proliferation, migration and vascularization of HRECs under HG
conditions. Following this, the effects of TSA on VEGF and ICAM-1
expression levels in HREC were analyzed.
Materials and methods
Reagents
TSA was obtained from Sigma-Aldrich (Merck KGaA,
Darmstadt, Germany). ICAM-1 and VEGF antibodies were purchased from
Abcam (Cambridge, UK). Antibodies for β-actin was purchased from
Cell Signaling Technology Inc., (Danvers, MA, USA).
Cell culture
The HREC cell line was purchased from Shanghai Cell
bank, Type Culture Collection Committee, Chinese Academy of
Sciences (Shanghai, China). The cells were cultured in Dulbecco's
modified Eagle's medium (DMEM) containing normal glucose (NG, 5.5
mM) or HG (25 mM), from Invitrogen; Thermo Fisher Scientific Inc.,
(Waltham, MA, USA), supplemented with 10% fetal bovine serum
(Transgen Biotech, Beijing, China, http://www.transgen.com.cn/) and grown in a humidified
incubator at 37°C, in an environment containing 5%
CO2.
Cell growth assay
Cell viability was measured with a Cell Counting
Kit-8 (CCK-8; Dojindo Molecular Technologies, Inc., Kumamoto,
Japan). The single cell suspension (5×104/ml, 100 µl) of
HRECs were dispensed in a 96-well plate and cultured for 0, 24, 48
and 72 h. At the designated time point, 10 µl of CCK-8 reagent was
added into each well and incubated for another 1.5 h. Then, the
absorbance was measured at a wavelength of 450 nm, using a scanning
microplate reader.
Wound healing assay
The migratory behavior of cells was assessed using a
wound healing assay as previously described (22). Briefly, a monolayer of HRECs were
wounded with a plastic pipette tip and rinsed twice with PBS to
remove the dead cells and incubated in serum-free medium. At the
designated time-point (0, 24 and 48 h), five randomly selected
fields were photographed under an Olympus IX-71 inverted
microscope.
Angiogenesis in vitro
Matrigel (BD Biosciences, Franklin Lakes, NJ, USA)
was added into an eight-chamber slide and allowed to gel for 2 h at
37°C. Cells were serum deprived overnight in serum-free medium
prior to detaching. The cells (5×104) were suspended in
the NG or HG medium containing 0, 10, 20, 30 µg/ml TSA and were
added to each chamber. Cell migration and rearrangement were
recorded following 6 h. Randomly selected fields were photographed
using an Olympus IX-71 inverted microscope.
RNA extraction, cDNA synthesis and
reverse transcription-quantitative polymerase chain reaction
(RT-qPCR)
RT-qPCR was performed as previously described
(23). RNA was isolated from the
cells treated with NG medium or HG medium supplemented with 0, 10,
20 or 30 µg/ml TSA and the cDNA was constructed with the M-MLV
reverse transcription reagents (Roche Diagnostics, Basel,
Switzerland) according to the manufacturer's protocol. RT-qPCR was
carried out using an ABI 7300 real-time PCR instrument (Applied
Biosystems; Thermo Fisher Scientific, Inc.) using SYBR Green (Roche
Diagnostics, Basel, Switzerland). The thermocycling conditions
were: 5 min at 95°C for pre-denaturation, 30 sec at 95°C, 30 sec at
55°C, 30 sec at 72°C, the three steps (30 sec at 95°C, 30 sec at
55°C and 30 sec at 72°C) were repeated for 25 cycles and the final
step was 5 min at 72°C. The primers used to amplify VEGF and
β-actin were as follows: Forward, TGG TCC CAG GCT GCA CCC AT and
reverse, CGC ATC GCA TCA GGG GCA CA for VEGF; forward, CAT GTA CGT
TGC TAT CCA GGC and reverse, CGC TCG GTG AGG ATC TTC ATG for
β-actin. The products were 184 bp and 195 bp, respectively. For
each sample, the threshold cycle (Ct) was determined and normalized
to the average of the housekeeping gene (ΔCt = Ct unknow n - Ct
housekeeping gene). The gene transcript levels in each sample was
determined using the 2−ΔΔCq method (24).
Protein extraction and western
blotting
At the designated time points, the cells were
harvested and lysed with radioimmunoprecipitation assay buffer
(Beyotime Institute of Biotechnology, Haimen, China). The
concentrations of total protein were determined by a BCA Protein
Assay kit (Beyotime Institute of Biotechnology). A total of 50 µg
total protein was separated by 10% SDS-PAGE and then
electrophoretically transferred to a polyvinylidene fluoride
membrane (EMD Millipore, Billerica, MA, USA). The membranes were
incubated in 3% bovine serum albumin (Sigma-Aldrich; Merck KGaA) in
PBS for 2 h at room temperature, and then with primary antibodies
(anti-ICAM1 antibody, cat no. ab53013, 1:500; anti-VEGFA antibody,
cat no. ab183100, 1:1,000; β-actin, cat no. 3700, 1:4,000) at 4°C
overnight. On the following day, the membranes were incubated with
secondary antibodies (goat anti-mouse IgG-HRP, 1:5,000; cat no.
AP124P; goat anti-rabbit IgG-HRP, 1:5,000, cat no. AP132P) (both
from EMD Millipore) at room temperature for 1 h. Finally, the
membranes were detected using Pierce ECL Plus western blotting
substrate (Thermo Fisher Scientific, Inc.) and exposed to X-ray
films. Band densities were quantified by ImageJ software (version
2.1.4.7; Wayne Rasband; National Institutes of Health, Bethesda,
MD, USA). The relative amount of protein was determined by
normalizing the densitometry value of the protein of interest to
that of the loading control.
Immunofluorescence analysis
The cells grown on 13-mm diameter coverslips were
treated with TSA for 48 h and then fixed with 4% paraformaldehyde
for 30 min at room temperature and permeabilized in 0.2% Triton
X-100 for 10 min at room temperature. Unspecific binding was
blocked via a reaction with 10% normal goat serum (Beyotime
Institute of Biotechnology) for 30 min at room temperature.
Subsequently, the cells were incubated with rabbit anti-ICAM-1
antibody (Abcam; 1:100) overnight at 4°C. On the following day, the
cells were incubated with Alexa Fluor 488-conjugated goat
anti-rabbit IgG (Abcam, cat no. ab150077, 1:1,000) for 1 h at room
temperature. Finally, the nuclei were stained with
4,6-diamidino-2-phenylindole (DAPI; 1 µg/ml; Sigma-Aldrich; Merck
KGaA) for 10 min at room temperature. The cells were observed and
recorded using a fluorescence microscope (IX71; Olympus
Corporation, Tokyo, Japan). The fluorescence was quantified by
Image J Software (version 2.1.4.7; Wayne Rasband, National
Institutes of Health, MD).
Statistical analysis
The results are representative of experiments that
were repeated ≥ three times and quantitative data were expressed as
the mean ± standard error. Statistical analyses were performed
using SPSS software, version 13.0 (SPSS, Inc., Chicago, IL, USA).
Differences in multiple groups were determined using a one-way
analysis of variance followed by a Tukey's post-hoc test.
Comparisons between two groups were performed using a Student's
t-test. P<0.05 was considered to indicate a statistically
significant difference.
Results
TSA inhibits high glucose-induced HREC
proliferation
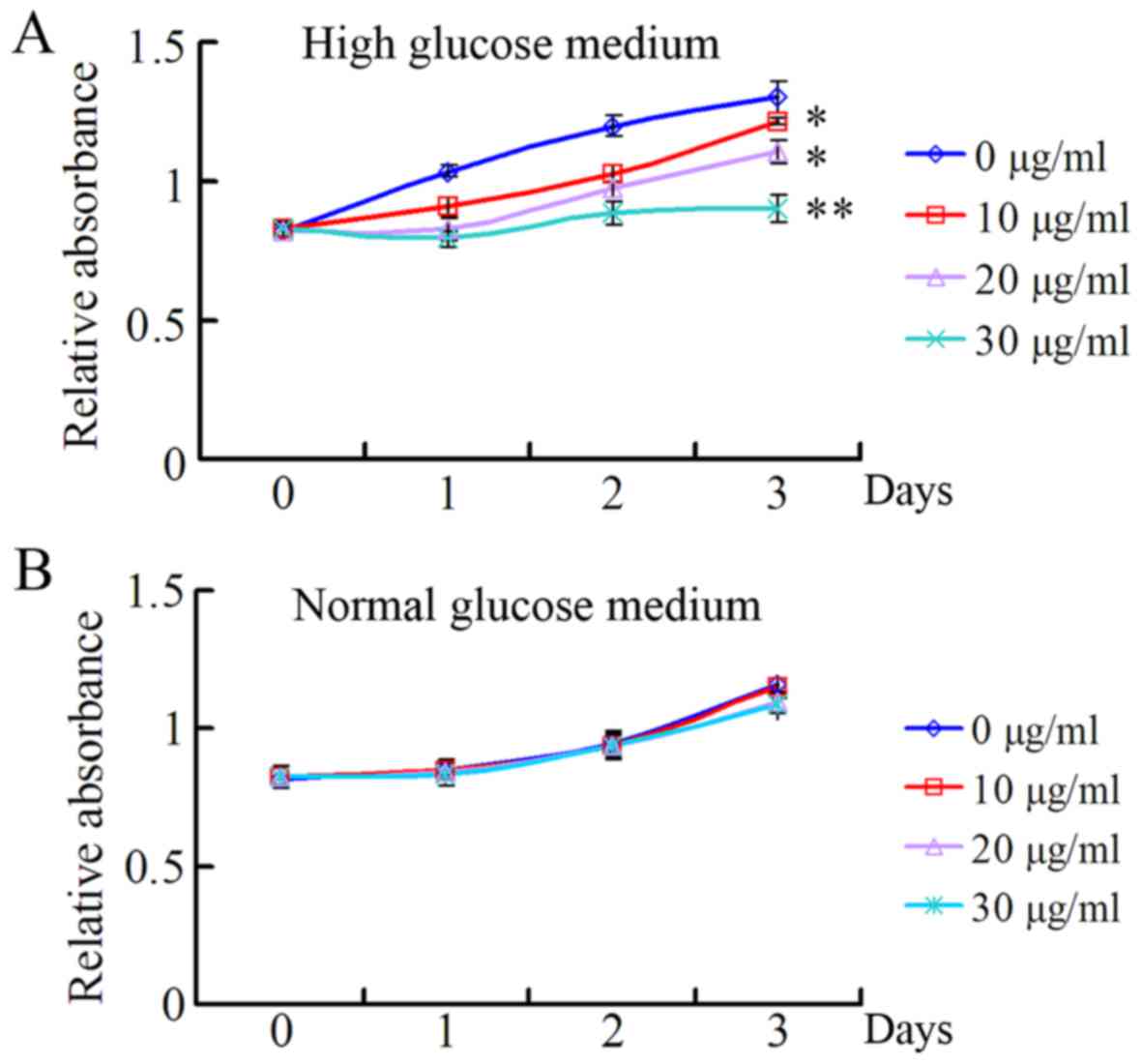
In order to investigate the role of TSA on HRECs
under high and normal concentrations of glucose, the present study
evaluated the effects of 10, 20 and 30 µg/ml TSA on the
proliferation of HRECs under the conditions of high and normal
glucose, using a CCK-8 assay. As presented in Fig. 1A, TSA exhibited a significant
inhibitory effect on the proliferation of HRECs in a dose-dependent
manner, under HG medium conditions. However, there was no
significant inhibitory effect on the proliferation of HRECs under
normal glucose conditions (Fig.
1B).
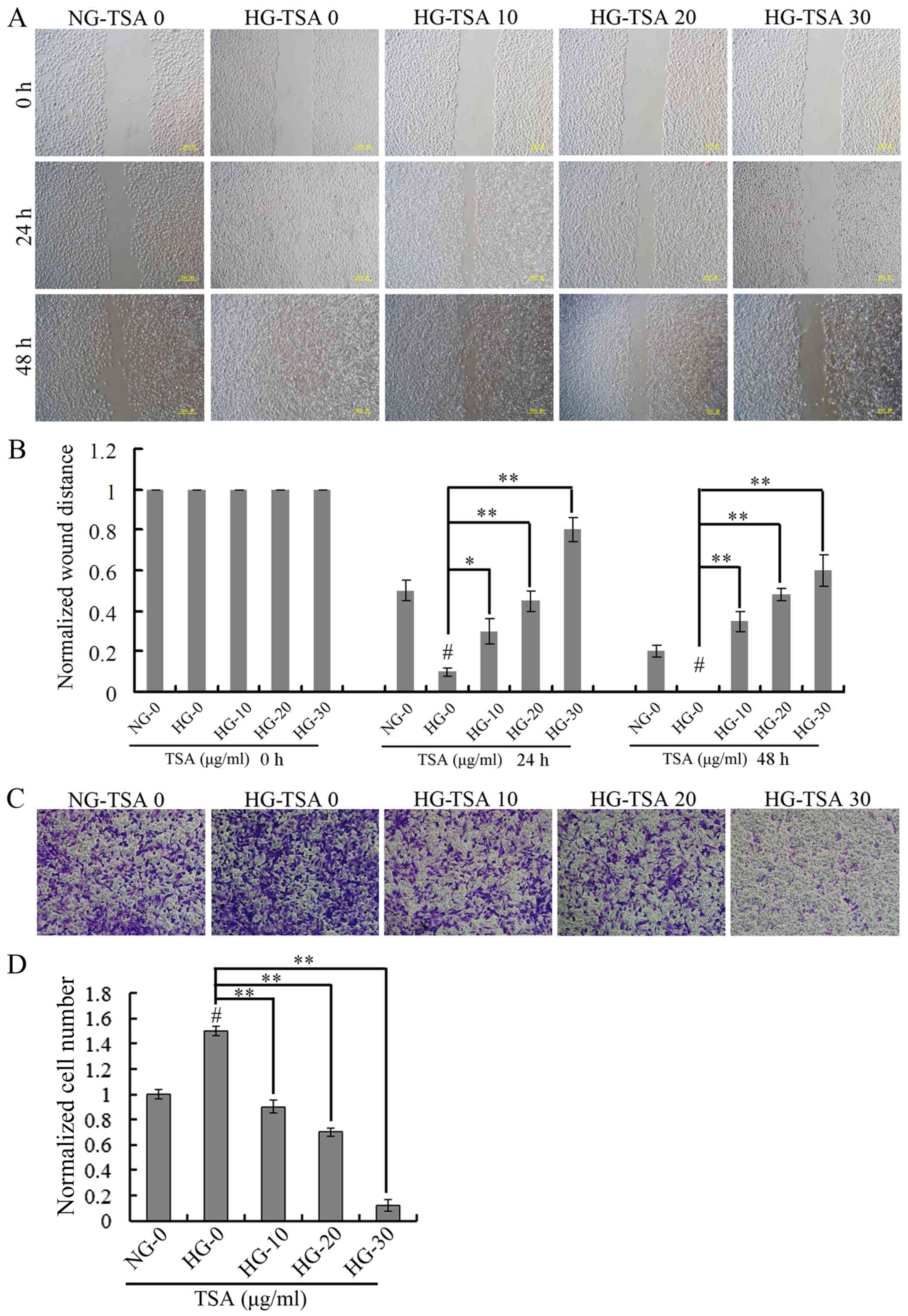
TSA suppresses high glucose-induced
HREC migration
The wound-healing assay was used for evaluating the
migration of HRECs under HG concentration. The wound healing was
analyzed at 24 and 48 h. It was demonstrated that high glucose
stimulated the migration of HRECs, however, TSA significantly
suppressed high glucose-induced HRECs migration in a dose-dependent
manner (Fig. 2).
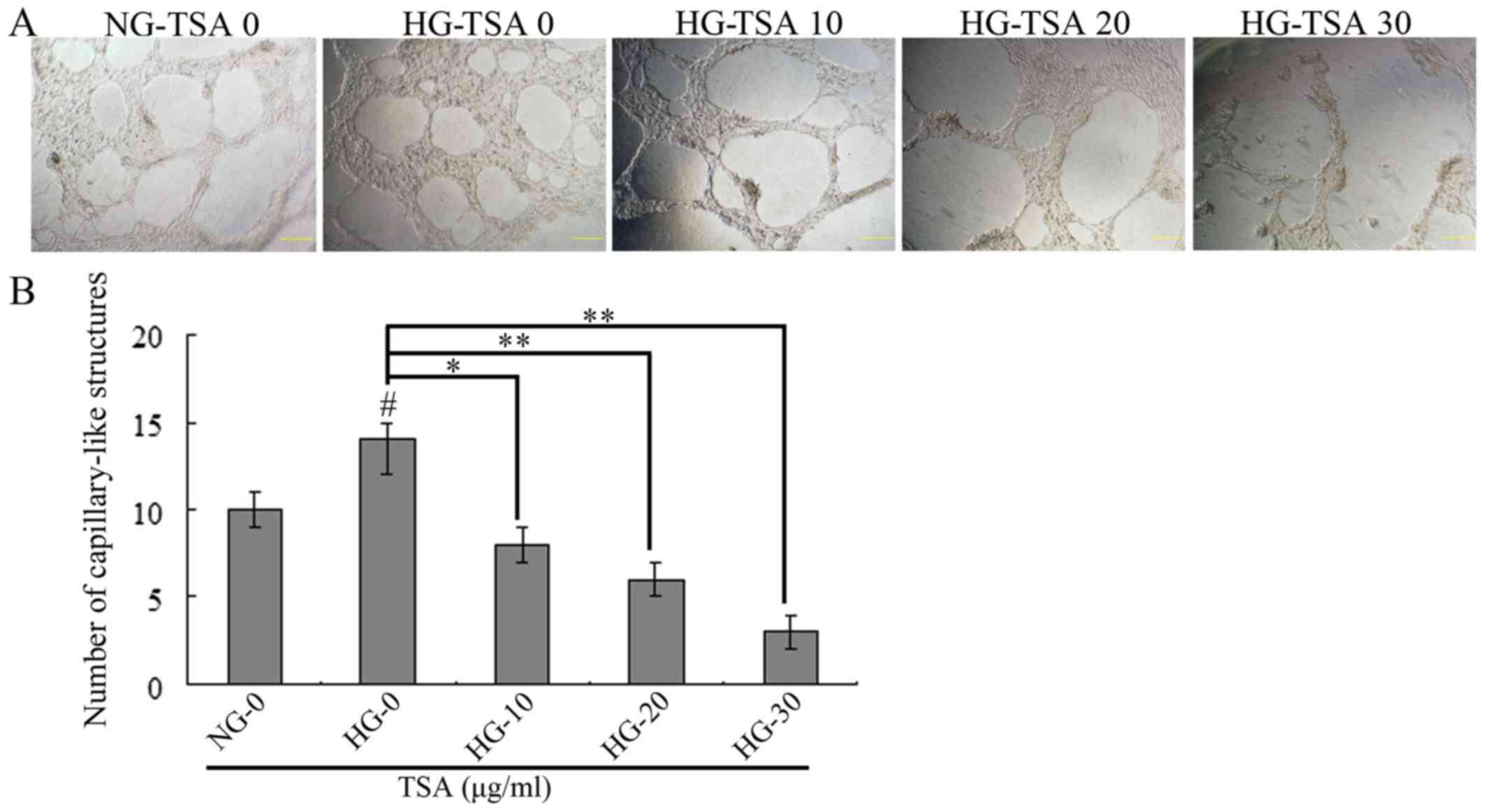
TSA inhibits high glucose-induced HREC
vascularization
The in vitro matrigel angiogenesis model was
performed to evaluate the vascularization of HRECs under HG
concentration. As presented in Fig.
3, HG stimulated the vascularization of HRECs, however, TSA
significantly inhibited HG-induced HRECs vascularization in a
dose-dependent manner.
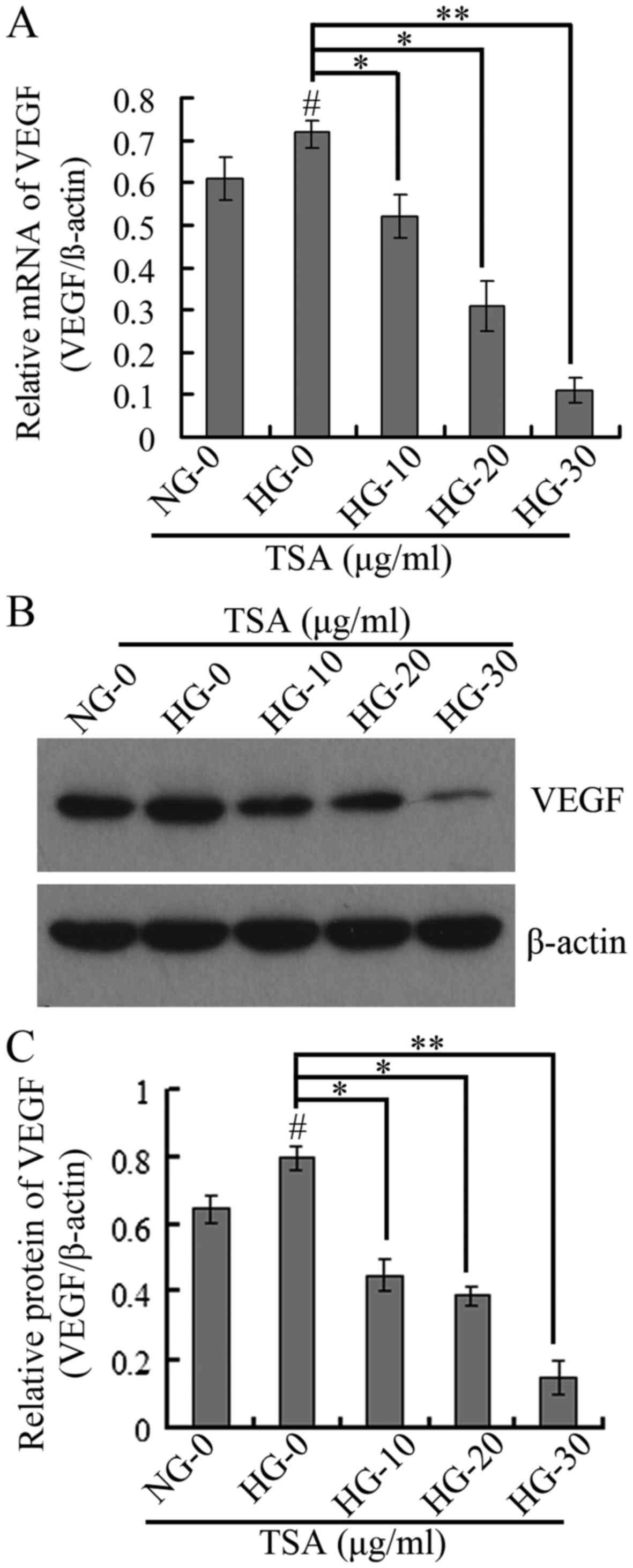
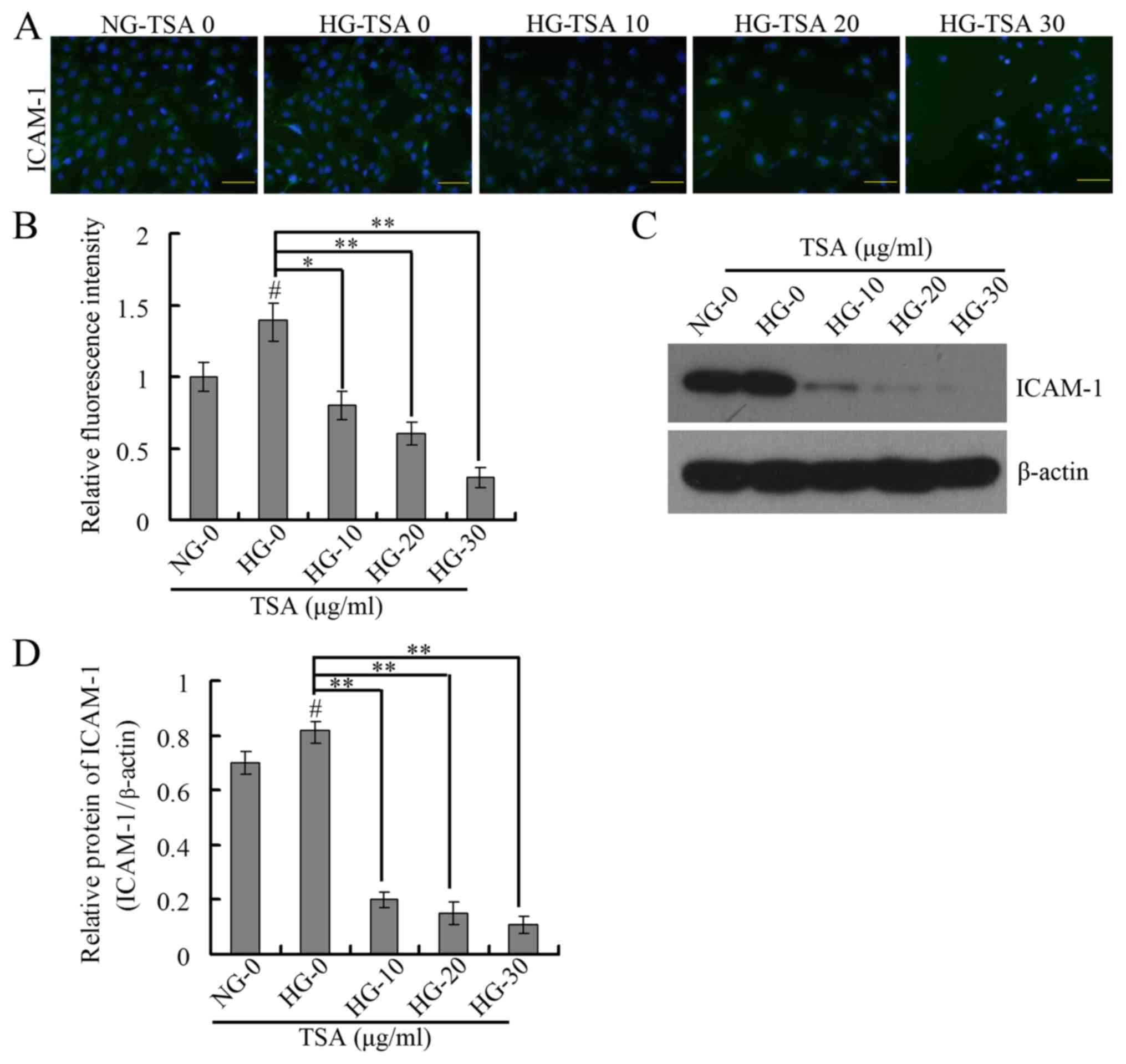
TSA downregulates VEGF and ICAM-1
expression levels in HRECs
It has been demonstrated that VEGF is important in
diabetic microvascular complications by promoting retinal
angiogenesis and increasing vascular permeability (25). At the same time, leukocyte
aggregation that results from overexpression of ICAM-1 is an
important factor in the destruction of the blood-retinal barrier,
loss of retinal vascular perfusion and initiation of angiogenesis
(15). The present study therefore
analyzed the expression levels of VEGF and ICAM-1 using RT-qPCR,
western blotting and immunofluorescence. The RT-qPCR and western
blotting results indicated that TSA significantly downregulated the
mRNA and protein expression levels of VEGF in a dose-dependent
manner under HG conditions (Fig.
4). The immunofluorescence and western blotting indicated that
TSA additionally significantly decreased the protein expression
level of ICAM-1 in a dose-dependent manner, under HG conditions
(Fig. 5).
Discussion
High glucose is considered an important risk factor
in the development of DR. It results in cellular stress and injury
of vascular pericytes and endothelial cells, and induces the
formation of aberrant capillaries (26,27).
Endothelial cells are critical in regulating vascular tension, and
damage of these cells is the earliest event that leads to
irreversible structural abnormalities (28). In the present study, HRECs were
used to simulate the pathogenesis of DR under conditions of high
glucose. Similar to previous reports, HRECs in the present study
were cultured in either normal (5.5 mM) or high glucose (25 mM)
media (28,29). In accordance with previous studies,
the results of the present study indicated that high glucose
promoted the proliferation, migration and vascularization of
HRECs.
Tanshinone IIA (TSA), which is a major lipophilic
component isolated from Danshen, demonstrates various therapeutic
and pharmacological effects including vasodilative, antithrombotic,
anti-inflammatory, anti-oxidant, anti-ischemic, anti-arrhythmia,
anti-hyperplasia, anti-atherosclerosis and lipid-lowering
properties (30). However, whether
TSA ameliorates DR is still unknown. The results of the present
study indicated that TSA significantly inhibited the high glucose
induced proliferation, migration and vascularization of HRECs.
Based on these findings, it was hypothesized that TSA may act as an
alternative inhibitor for intervention of DR.
The angiogenic cascade is a complicated and
multi-step process. The migration and proliferation of vascular
endothelial cells are the primary step in angiogenesis, and
following this, endothelial cells differentiate into a
capillary-like network (31). This
process is tightly controlled by a battery of pro- and
anti-angiogenic factors under physiological conditions. An
imbalance of these factors may result in severe pathological
consequences. (32). Notably, VEGF
is regarded as a primary factor of the aberrant angiogenesis in
response to high glucose. Therefore, anti-VEGF therapy is
considered a sufficient treatment strategy for DR (33,34).
In 2012, ranibizumab, a monoclonal antibody targeting VEGF designed
for use in the eye, became the first and only U.S. Food and Drug
Administration-approved reagent for DR (34,35).
In the present study, the results of mRNA and protein analysis
revealed that TSA decreased the increased VEGF expression in high
glucose treated HRECs. These results suggest that TSA may serve as
a potential anti-VEGF reagent in the treatment of DR.
It has been reported that ICAM-1 is a primary factor
of leukocyte aggregation (15). In
the present study, the immunofluorescence and western blotting
assays demonstrated that TSA decreased the increased ICAM-1
expression in high glucose treated HRECs. TSA may therefore
additionally serve as an anti-ICAM-1 reagent in the treatment of
DR.
In conclusion, the present study demonstrated that
TSA inhibited the proliferation, migration and vascularization of
HRECs by affecting the expression of VEGF and ICAM-1. Although the
findings provide evidence that TSA may act as a prospective drug to
restrain the development and progression of DR in the future,
further in vivo studies are still required to evaluate its
efficacy and safety.
Acknowledgements
The present study was supported by the National
Natural Science Foundation in China (NSFC, grant no. 30972712) and
Suzhou Municipal Natural Science Foundation (grant no.
SYS201448).
References
|
1
|
Ginter E and Simko V: Global prevalence
and future of diabetes mellitus. Adv Exp Med Biol. 771:35–41.
2012.PubMed/NCBI
|
|
2
|
Willard AL and Herman IM: Vascular
complications and diabetes: Current therapies and future
challenges. J Ophthalmol. 2012:2095382012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kowluru RA and Chan PS: Oxidative stress
and diabetic retinopathy. Exp Diabetes Res. 2007:436032007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Witmer AN, Vrensen GF, Van Noorden CJ and
Schlingemann RO: Vascular endothelial growth factors and
angiogenesis in eye disease. Prog Retin Eye Res. 22:1–29. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Abu E, l-Asrar AM, Nawaz MI, Kangave D,
Siddiquei M Mairaj and Geboes K: Angiogenic and vasculogenic
factors in the vitreous from patients with proliferative diabetic
retinopathy. J Diabetes Res. 2013:5396582013.PubMed/NCBI
|
|
6
|
Kalka C, Masuda H, Takahashi T, Gordon R,
Tepper O, Gravereaux E, Pieczek A, Iwaguro H, Hayashi SI, Isner JM
and Asahara T: Vascular endothelial growth factor(165) gene
transfer augments circulating endothelial progenitor cells in human
subjects. Circ Res. 86:1198–1202. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li B, Sharpe EE, Maupin AB, Teleron AA,
Pyle AL, Carmeliet P and Young PP: VEGF and PlGF promote adult
vasculogenesis by enhancing EPC recruitment and vessel formation at
the site of tumor neovascularization. FASEB J. 20:1495–1497. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Aiello LP, Avery RL, Arrigg PG, Keyt BA,
Jampel HD, Shah ST, Pasquale LR, Thieme H, Iwamoto MA, Park JE, et
al: Vascular endothelial growth factor in ocular fluid of patients
with diabetic retinopathy and other retinal disorders. N Engl J
Med. 331:1480–1487. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang X, Wang G and Wang Y: Intravitreous
vascular endothelial growth factor and hypoxia-inducible factor 1a
in patients with proliferative diabetic retinopathy. Am J
Ophthalmol. 148:883–889. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Matsuoka M, Ogata N, Minamino K and
Matsumura M: Expression of pigment epithelium-derived factor and
vascular endothelial growth factor in fibrovascular membranes from
patients with proliferative diabetic retinopathy. Jpn J Ophthalmol.
50:116–120. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Abu E, l-Asrar AM, Missotten L and Geboes
K: Expression of hypoxia-inducible factor-1alpha and the protein
products of its target genes in diabetic fibrovascular epiretinal
membranes. Br J Ophthalmol. 91:822–826. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lim JI, Spee C and Hinton DR: A comparison
of hypoxia-inducible factor-α in surgically excised neovascular
membranes of patients with diabetes compared with idiopathic
epiretinal membranes in nondiabetic patients. Retina. 30:1472–1478.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chung EJ, Kang SJ, Koo JS, Choi YJ,
Grossniklaus HE and Koh HJ: Effect of intravitreal bevacizumab on
vascular endothelial growth factor expression in patients with
proliferative diabetic retinopathy. Yonsei Med J. 52:151–157. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shibuya M: Differential roles of vascular
endothelial growth factor receptor-1 and receptor-2 in
angiogenesis. J Biochem Mol Biol. 39:469–478. 2006.PubMed/NCBI
|
|
15
|
Hubbard AK and Rothlein R: Intercellular
adhesion molecule-1 (ICAM-1) expression and cell signaling
cascades. Free Radic Biol Med. 28:1379–1386. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Barouch FC, Miyamoto K, Allport JR, Fujita
K, Bursell SE, Aiello LP, Luscinskas FW and Adamis AP:
Integrin-mediated neutrophil adhesion and retinal leukostasis in
diabetes. Invest Ophthalmol Vis Sci. 41:1153–1158. 2000.PubMed/NCBI
|
|
17
|
Zhou L, Zuo Z and Chow MS: Danshen: An
overview of its chemistry, pharmacology, pharmacokinetics, and
clinical use. J Clin Pharmacol. 45:1345–1359. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gong Z, Huang C, Sheng X, Zhang Y, Li Q,
Wang MW, Peng L and Zang YQ: The role of tanshinone IIA in the
treatment of obesity through peroxisome proliferator-activated
receptor gamma antagonism. Endocrinology. 150:104–113. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hwang SL, Yang JH, Jeong YT, Kim YD, Li X,
Lu Y, Chang YC, Son KH and Chang HW: Tanshinone IIA improves
endoplasmic reticulum stress-induced insulin resistance through
AMP-activated protein kinase. Biochem Biophys Res Commun.
430:1246–1252. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang Y, Wei L, Sun D, Cao F, Gao H, Zhao
L, Du J, Li Y and Wang H: Tanshinone IIA pretreatment protects
myocardium against ischaemia/reperfusion injury through the
phosphatidylinositol 3-kinase/Akt-dependent pathway in diabetic
rats. Diabetes Obes Metab. 12:316–322. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li X, Du JR, Yu Y, Bai B and Zheng XY:
Tanshinone IIA inhibits smooth muscle proliferation and intimal
hyperplasia in the rat carotid balloon-injured model through
inhibition of MAPK signaling pathway. J Ethnopharmacol.
129:273–279. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen Z, Liu G, Xiao Y and Lu P:
Adrenomedullin22-52 suppresses high-glucose-induced migration,
proliferation, and tube formation of human retinal endothelial
cells. Mol Vis. 20:259–269. 2014.PubMed/NCBI
|
|
23
|
Dai C, Liu G, Li L, Xiao Y, Zhang X and Lu
P: ADP-ribosylation factor as a novel target for corneal
neovascularization regression. Mol Vis. 18:2947–2953.
2012.PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rajah TT and Grammas P: VEGF and VEGF
receptor levels in retinal and brain-derived endothelial cells.
Biochem Biophys Res Commun. 293:710–713. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Stitt AW, McGoldrick C, Rice-McCaldin A,
McCance DR, Glenn JV, Hsu DK, Liu FT, Thorpe SR and Gardiner TA:
Impaired retinal angiogenesis in diabetes: Role of advanced
glycation end products and galectin-3. Diabetes. 54:785–794. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dagher Z, Park YS, Asnaghi V, Hoehn T,
Gerhardinger C and Lorenzi M: Studies of rat and human retinas
predict a role for the polyol pathway in human diabetic
retinopathy. Diabetes. 53:2404–2411. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yuan L, Hu J, Luo Y, Liu Q, Li T, Parish
CR, Freeman C, Zhu X, Ma W, Hu X, et al: Upregulation of heparanase
in high-glucose-treated endothelial cells promotes endothelial cell
migration and proliferation and correlates with Akt and
extracellular-signal-regulated kinase phosphorylation. Mol Vis.
18:1684–1695. 2012.PubMed/NCBI
|
|
29
|
Premanand C, Rema M, Sameer MZ, Sujatha M
and Balasubramanyam M: Effect of curcumin on proliferation of human
retinal endothelial cells under in vitro conditions. Invest
Ophthalmol Vis Sci. 47:2179–2184. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shang Q, Xu H and Huang L: Tanshinone IIA:
A promising natural cardioprotective agent. Evid Based Complement
Alternat Med. 2012:7164592012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jeganathan VS: Anti-angiogenesis drugs in
diabetic retinopathy. Curr Pharm Biotechnol. 12:369–372. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kumar B, Gupta SK, Saxena R and Srivastava
S: Current trends in the pharmacotherapy of diabetic retinopathy. J
Postgrad Med. 58:132–139. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zechmeister-Koss I and Huic M: Vascular
endothelial growth factor inhibitors (anti-VEGF) in the management
of diabetic macular oedema: A systematic review. Br J Ophthalmol.
96:167–178. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Titchenell PM and Antonetti DA: Using the
past to inform the future: Anti-VEGF therapy as a road map to
develop novel therapies for diabetic retinopathy. Diabetes.
62:1808–1815. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Stewart MW: Critical appraisal of
ranibizumab in the treatment of diabetic macular edema. Clin
Ophthalmol. 7:1257–1267. 2013. View Article : Google Scholar : PubMed/NCBI
|