Introduction
Vascular smooth muscle cells (VSMCs) are an
essential component of arterial walls (1), and their dysfunction may result in
pathological processes and cause cardiovascular diseases. For
example, over-proliferation or reduced apoptosis of VSMCs
contributes to the development of atherosclerosis (2) and in-stent restenosis (ISR) (3). However, the underlying mechanisms are
not fully understood.
Over the past few years, an increasing number of
studies have indicated the important contribution of the nuclear
factor (NF)-κB signaling pathway in the proliferation of VSMCs
(4,5). NF-κB dimers exist in the cytoplasm as
cytoplasmic latent complexes consisting of three subunits: p50, p65
and nuclear factor of κ light polypeptide gene enhancer in B-cells
inhibitor-α (IκBα) (6). Following
exposure to proinflammatory stimuli, IκBα is rapidly phosphorylated
and can be degraded by the 26S proteasome. This action results in
the nuclear translocation of NF-κB dimers, which then bind to
specific DNA sites (κB sites) (7),
leading to the transcription of NF-κB regulated genes and
proliferation of VSMCs.
Paeoniflorin (PF) is one of the principal bioactive
ingredients of peonies, which have been used in traditional Chinese
medicine for >1,200 years (8).
Accumulating evidence has demonstrated that PF exerts anti-cancer
(9,10), anti-atherosclerosis (11) and immunoregulatory (12) pharmacological activities, with low
toxicity and few side effects. Recent findings by Lee et al
(13) have revealed that PF
significantly inhibits the proliferation and migration of
platelet-derived growth factor (PDGF)-BB-stimulated VSMCs. However,
its effects on the proliferation and apoptosis of VSMCs and the
underlying molecular mechanisms are still unknown.
The present study investigated the
anti-proliferative and apoptotic effects of PF on VSMCs and
explored the possible underlying mechanisms. To the best of our
knowledge, this study demonstrated for the first time that PF
inhibits proliferation and promotes apoptosis of VSMCs by
inhibiting the NF-κB signaling pathway and increasing caspase
expression, respectively. Therefore, PF may be a potential
therapeutic strategy for the treatment of atherosclerosis and
ISR.
Materials and methods
Culture of VSMCs
The mouse aorta smooth muscle cell line was
purchased from China Center for Type Culture Collection (Shanghai,
China). The cells were cultured in Dulbecco's modified Eagle's
medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 1% penicillin-streptomycin and 10% fetal bovine
serum (FBS; Gibco; Thermo Fisher Scientific, Inc.), and were
maintained at 37°C in a humidified atmosphere of 5% CO2.
Cell culture passage numbers 4–6 were used for experiments.
Cell counting kit (CCK)-8 assay and
bromodeoxyuridine (BrdU) incorporation assays of VSMCs
The viability of VSMCs was detected using a CCK-8
assay (Dojindo Molecular Technologies, Inc., Kumamoto, Japan).
Briefly, cells were transferred to a 96-well plate
(2×105 cells/ml) and incubated with fresh medium at 37°C
in 5% CO2 for 24 h. Following this, the cells were
pretreated with PF (Shanghai Winherb Medical S&T Development
Co., Ltd., Shanghai, China) for 12, 24 or 48 h. CCK-8 solution (10
µl) was then added to each well, followed by a 2-h incubation. The
optical density at 450 nm was read using a microplate reader and
the cell viability was subsequently expressed as a percentage of
the control groups.
The proliferation of PF-treated VSMCs was studied
using a BrdU incorporation assay (Roche Applied Science, Mannheim,
Germany). VSMCs were cultured in a 6-well plate (2×105
cells/ml) with 10% FBS for 48 h and then serum-starved by
incubating without FBS for 24 h. The VSMCs were subsequently
pretreated with PF (25, 50 or 100 µg/ml) for 12, 24 or 48 h and
then the proliferation was measured using BrdU incorporation
assays. The optical density was measured at 370 nm using an
enzyme-linked immunosorbent assay (ELISA) plate reader.
Annexin V-fluorescein isothiocyanate
(FITC)/propidium iodide (PI) assay of VSMCs
The effects of PF on the cell cycle of VSMCs were
analyzed using an Annexin V-FITC/PI flow cytometric assay (BD
Biosciences, Franklin Lakes, NJ, USA) according to the
manufacturer's protocol. The VSMCs were cultured in a 6-well plate
at a density of 1×105/well with PF (50 and 100 µg/ml)
for 24 h, washed once with phosphate-buffered saline (PBS), and
then centrifuged at 2,067 × g and at 4°C for 5 min, followed by
resuspension in 500 µl binding buffer. Next, the VSMCs were
incubated with Annexin-V-FITC for 20 min, followed by PI for 10 min
at room temperature, and then the apoptotic cells were evaluated
using a BD FACSCanto™ II flow cytometry system (BD
Biosciences).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) assay of VSMCs
The results of the previous experiments in this
study indicated that the optimal concentration of PF was 100 µg/ml;
therefore, this was used in subsequent experiment with VSMCs. Total
RNA was extracted using TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol after
the VSMCs were treated with PF (100 µg/ml) for 24 h. To synthesize
cDNA, 2 µl total RNA was reverse-transcribed using a cDNA synthesis
kit (Roche Applied Science). qPCR was performed using SYBR green
PCR master mix (Roche Applied Science) according to the
manufacturer's protocol. The Light Cycler 480 instrument with
designated software (version 1.5; Roche Diagnostics, Basel,
Switzerland) was used for PCR amplifications. The PCR thermal
cycling protocol was as follows: 1 cycle of initial denaturation at
94°C for 2 min, followed by 40 cycles of denaturation at 94°C for
40 sec, annealing at 58°C for 45 sec and extension at 72°C for 1
min. The following primer sequences were used: Cyclin D1 forward,
5′-CCCGAGGAGTTGCTGCAAATGGA-3′ and reverse,
5′-AGGGCCACAAAGGTCTGTGCA-3′; cyclin E forward,
5′-TGGTGTCCTCGCTGCTTCTGCT-3′ and reverse,
5′-TGCTTGGGCTTTGTCCAGCAAG-3′; cyclin-dependent kinase (CDK)4
forward, 5′-CAATGTTGTACGGCTGATGG-3′ and reverse,
5′-GGAGGTGCTTTGTCCAGGTA-3; CDK2 forward, 5′-GCTTTCTGCCATTCTCATCG-3′
and reverse, 5′-GTCCCCAGAGTCCGAAAGAT-3; and p21 forward,
5′-GCTTTCTGCCATTCTCATCG-3′ and reverse,
5′-TCGCCATGAGCGCATCGCAAT-3′; GAPDH forward,
5′-GACATGCCGCCTGGAGAAAC-3′ and reverse, 5′-AGCCCAGGATGCCCTTTAGT-3′.
GAPDH served as a reference gene. The 2−∆∆Cq method was
used to analyze data from qPCR experiments (14).
Terminal deoxynucleotidyl transferase
deoxyuridine 5′-triphosphate nick-end labeling (TUNEL)/4′,
6-diamidino-2-phenylindole (DAPI) assays of VSMCs
The TUNEL analysis (Vazyme, Piscataway, NJ, USA) was
used to detect the DNA fragments generated during apoptosis.
Briefly, VSMCs were cultured on chamber slides and the cells were
pretreated with PF (50, 100 µg/ml) for 24 h. Subsequently, cells
were fixed with 2% paraformaldehyde and incubated with the TUNEL
assay reagents for 1 h at 37°C. The nuclei were stained with DAPI
for 10 min, and then the cells were imaged using fluorescence
microscopy. The percentage of apoptotic cells was calculated as the
ratio of the TUNEL-positive (stained green) to the total of
DAPI-positive (stained blue) cells.
Western blot analysis of VSMCs
VSMCs were cultured in 100 mm petri dishes and
pretreated with PF (25, 50 and 100 µg/ml) for 3 and 24 h for
phosphorylated-protein and protein expression determination,
respectively. After incubation, the cells were lysed with
radioimmunoprecipitation assay buffer (Beyotime Institute of
Biotechnology, Haimen, China), and centrifuged at 2,067 × g at 4°C
for 10 min. Cell lysates (50 ug samples) were separated by 10%
SDS-PAGE, followed by electrotransfer to polyvinylidene fluoride
membranes (EMD Millipore, Billerica, MA, USA). Subequently, the
membranes were blocked with 5% non-fat milk at room temperature for
3 h and incubated with primary antibodies overnight at 4°C, p65
(1:1,000; cat. no. 8242; Cell Signaling Technology, Inc., Danvers,
MA, USA), p-P65 (1:1,000; cat. no. 3033; Cell Signaling Technology,
Inc.), IκBα (1:1,000; cat. no. 4814P; Cell Signaling Technology,
Inc.), p-IκBα (1:1,000; cat. no. 2859P; Cell Signaling Technology,
Inc.), GAPDH (1:1,000; cat. no. 2118; Cell Signaling Technology,
Inc.), rabbit monoclonal cleaved (c)-caspase-3 (1:1,000; cat. no.
9664; Cell Signaling Technology, Inc.), rabbit polyclonal
c-caspase-8 (1:1,000; cat. no. 9429; Cell Signaling Technology,
Inc.), rabbit polyclonal c-caspase-9 (1:1,000; cat. no. 9509P; Cell
Signaling Technology, Inc.). This was followed by incubation with
the secondary antibody, goat anti-rabbit immunoglobulin G (1:100;
cat. no. 926-32211; LI-COR Biosciences, Lincoln, NE, USA), at room
temperature for 60 min. The western blots were scanned using a
two-color infrared Odyssey imaging system (version 3.0, LI-COR
Biosciences, USA) to quantify the protein expression.
Statistical analysis
Statistical analysis was performed using SPSS
version 17.0 (SPSS, Inc., Chicago, IL, USA). Data are expressed as
the mean ± standard deviation. The different groups were compared
using one-way analysis of variance followed by Student-Newman-Keuls
tests. P<0.05 was considered to indicate a statistically
significant difference.
Results
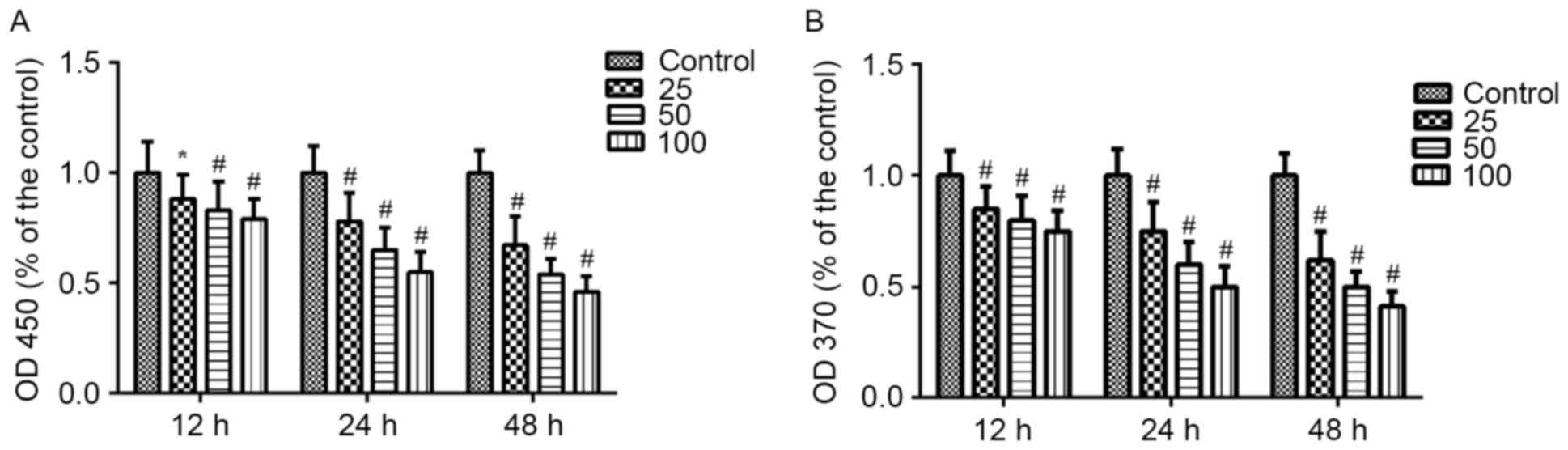
Effects of PF on VSMC viability and
proliferation
The effects of PF on the viability and proliferation
of VSMCs were measured using CCK-8 and BrdU assays. VSMCs cultured
with PF (25, 50 and 100 µg/ml) for 12, 24 or 48 h exhibited
decreased viability compared with the control group (P<0.05;
Fig. 1A). Similarly, PF reduced
BrdU incorporation in VSMCs (P<0.01; Fig. 1B). Both assays demonstrated that PF
inhibited cell viability and proliferation in a time- and
concentration-dependent manner.
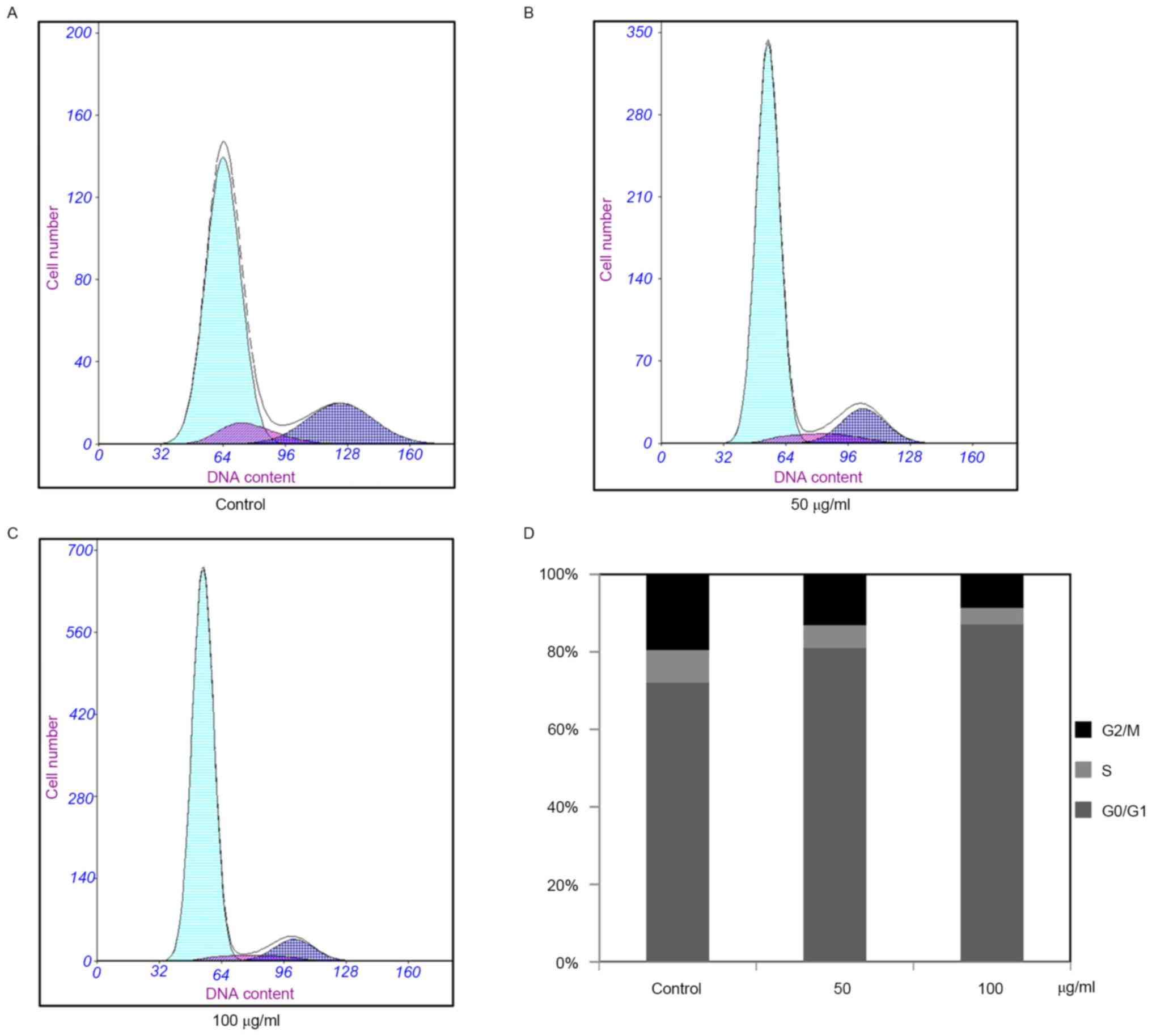
Effects of PF on cell cycle of
VSMCs
The potential altering effect of PF on the cell
cycle of VSMCs was analyzed. The results demonstrated that the
percentage of cells in the G1 phase increased from 72.1 to 80.4%
and 72.1 to 87.5% in the control and PF-treated groups,
respectively (P<0.05 vs. control group). Furthermore, the
control and PF-treated cells in the S phase decreased from 8.4 to
7.4% and 8.4 to 4.3%, respectively (P<0.05 vs. control group),
while the G2 phase cells decreased from 19.5 to 12.6% and 19.5 to
8.2%, respectively (P<0.05 vs. control group, Fig. 2). These results indicated that PF
induced G1 cell cycle arrest in the VSMCs, and the percentage of
the S and G2/M phase cells decreased significantly.
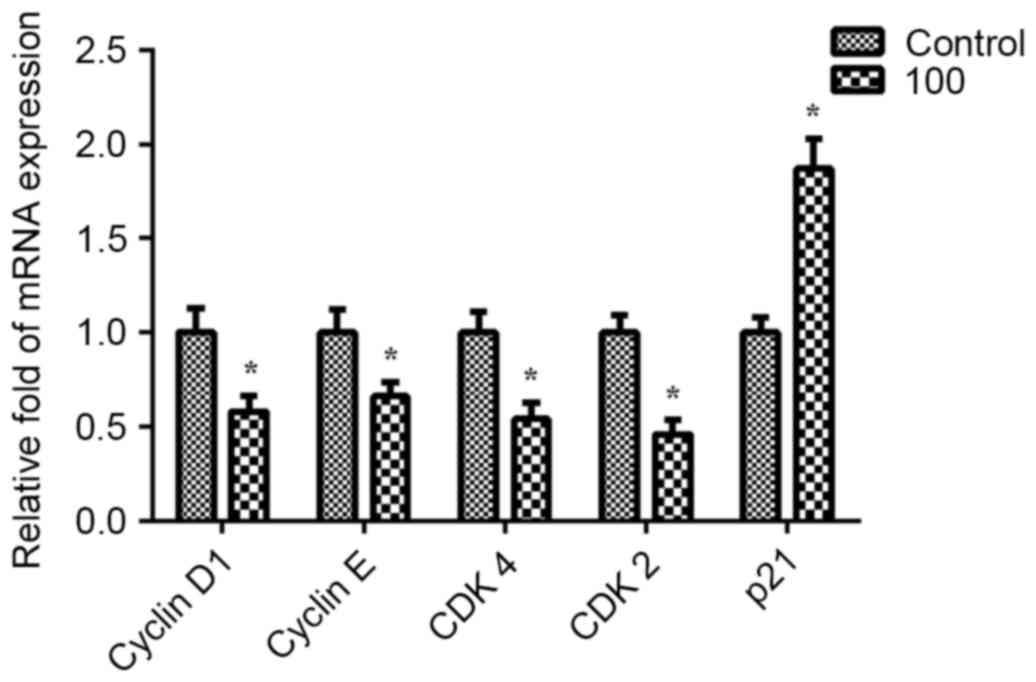
Effects of PF on the expression and
activation of cell cycle-associated molecules
To investigate the specific underlying mechanism of
PF, its effects on cell cycle regulatory proteins were determined
in VSMCs at the predetermined optimal concentration (100 µg/ml for
24 h) by using RT-qPCR. Pre-treatment with PF significantly
decreased the expression levels of cyclin D1, cyclin E, CDK4 and
CDK2 compared with the levels of the untreated control cells
(P<0.05). However, PF enhanced the expression of p21 (Fig. 3). These findings demonstrated that
the inhibitory effect of PF on VSMCs may be associated with cell
cycle inhibition.
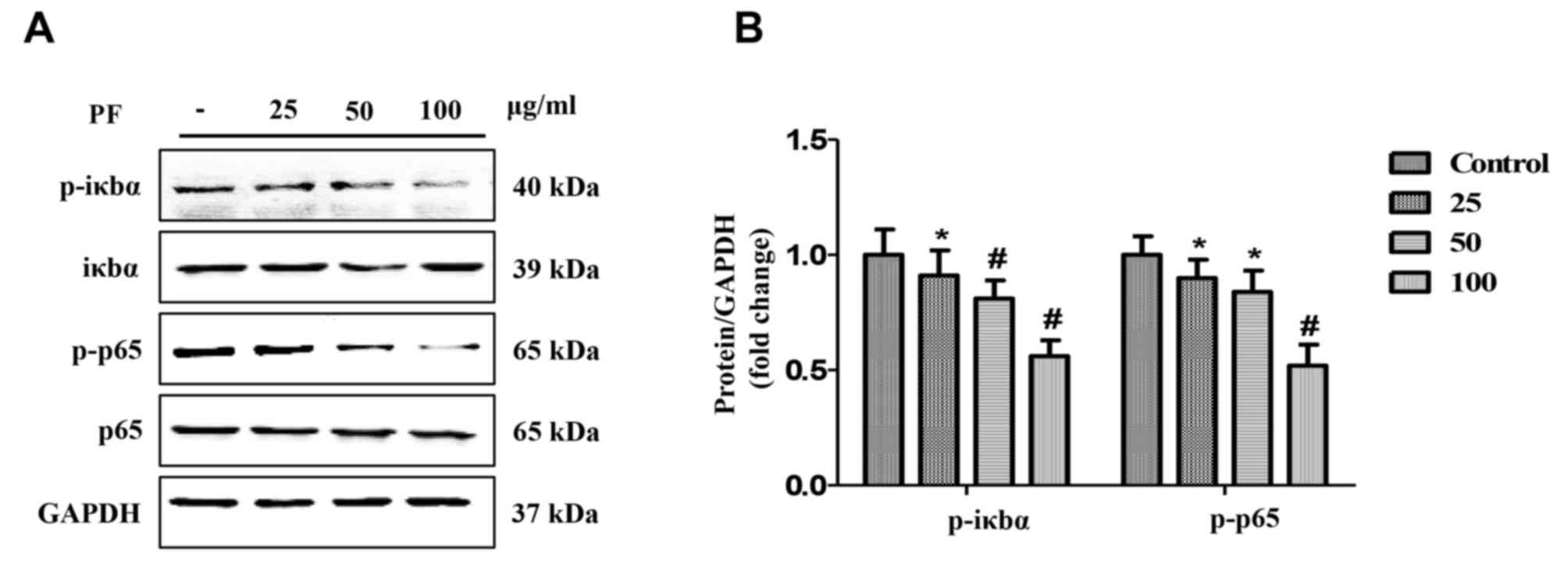
Effects of PF on expression of NF-κB
signaling pathway molecules
The levels of NF-κB signaling pathway proteins in
VSMCs cultured with PF (25, 50 and 100 µg/ml) for 3 h were analyzed
using western blotting. The results demonstrated that PF
significantly decreased the phosphorylation of p65 and IκBα in a
concentration-dependent manner (P<0.05; Fig. 4). However, PF had no effect on the
expression of un-phosphorylated p65 and IκBα.
Effects of PF on apoptosis of
VSMCs
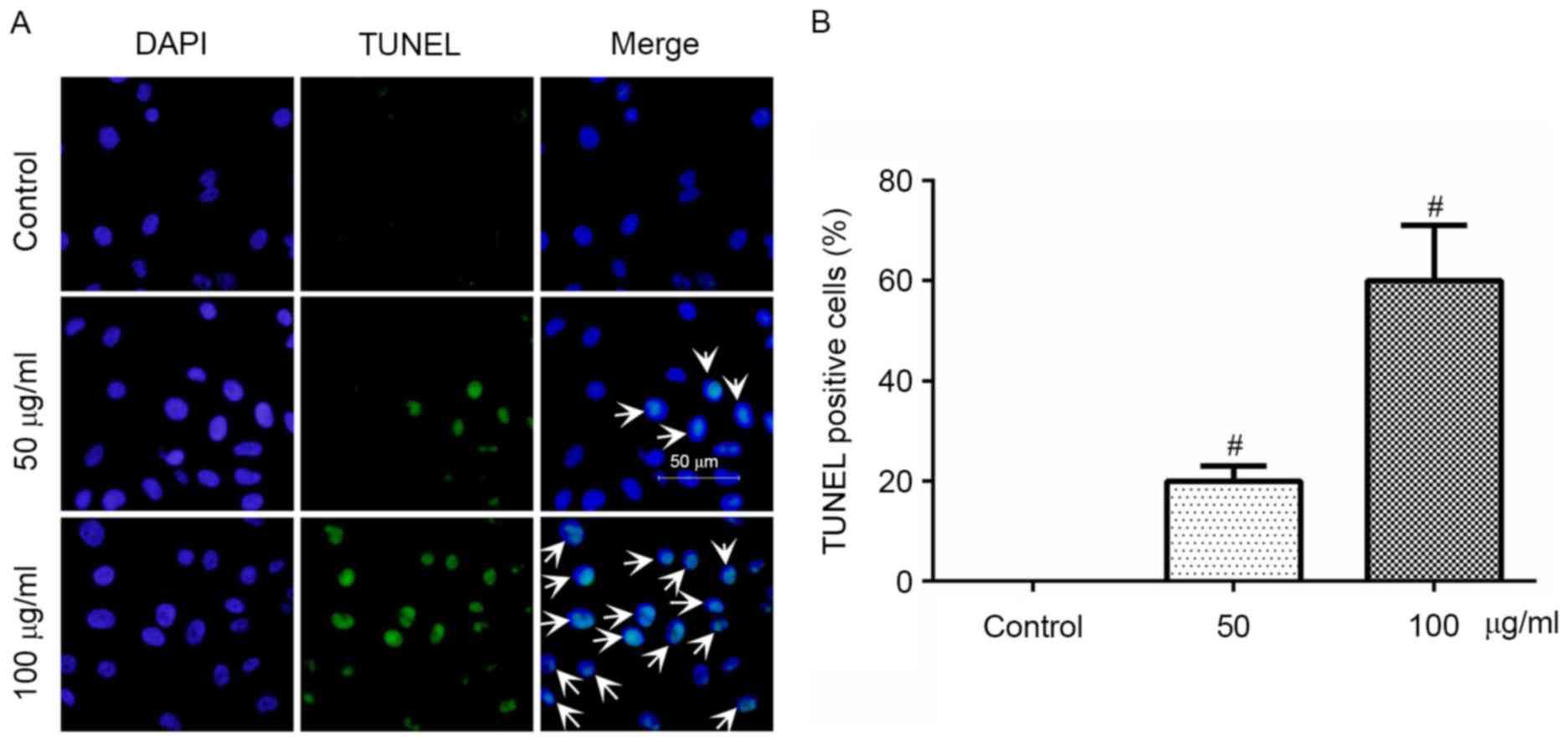
The apoptotic effects of PF on VSMCs were determined
using the TUNEL assay, which revealed that treatment with PF (50
and 100 µg/ml) for 24 h significantly increased the cells with
fragmented DNA (Fig. 5A). As
presented in Fig. 5B, the
percentage of TUNEL-positive cells in the control, and the 50 and
100 µg/ml groups, was 0±0, 19.8±1.2 and 61.3±1.5, respectively.
Therefore, these results suggested that PF induced the apoptosis of
VSMCs.
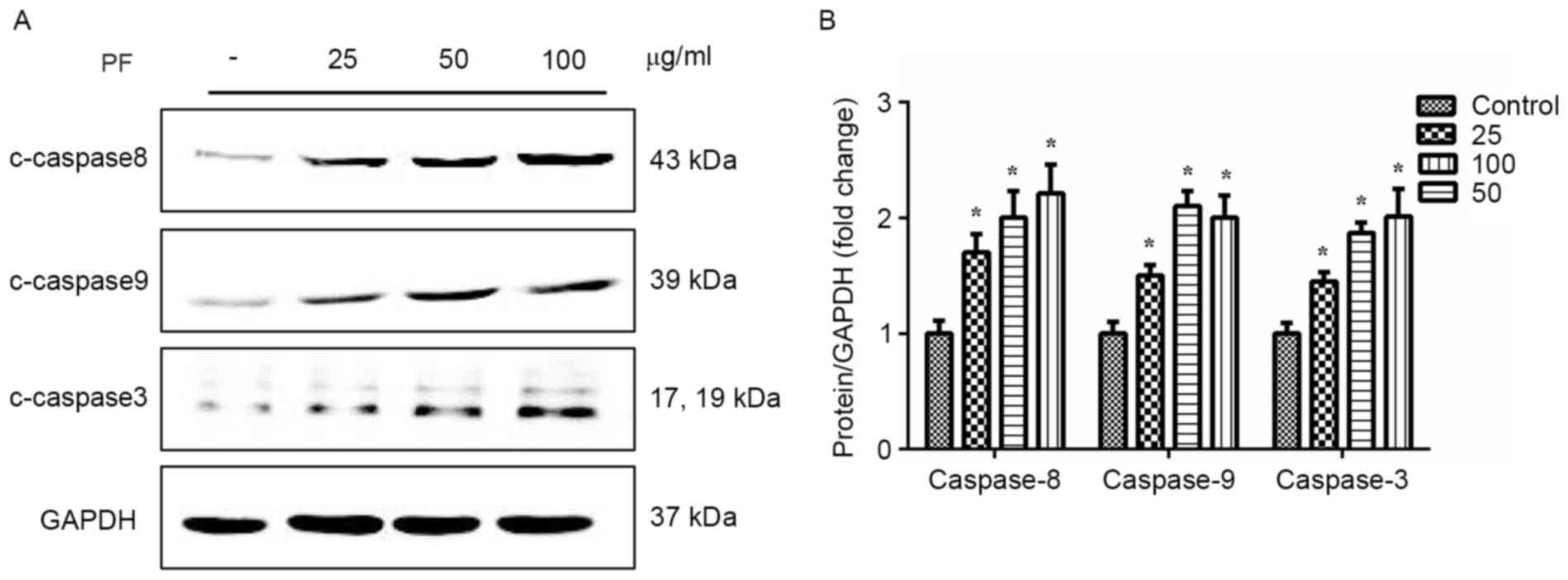
Effects of PF on caspase
expression
A significant increase in caspase-3 expression was
observed when VSMCs were cultured with PF (25, 50, and 100 µg/ml)
for 24 h (Fig. 6). Similarly, PF
significantly enhanced the expression of caspase-8 and −9 in a
concentration-dependent manner (P<0.01 vs. control group).
Discussion
The present study had two major findings. Firstly,
PF exhibited an inhibitory effect on VSMC proliferation as
demonstrated by the results of the CCK-8, BrdU and Annexin-V
FITC/PI assays. Notably, the results of western blot analysis
demonstrated that modulation of the NF-κB signaling pathway might
be an important contributing signal by inhibiting of the
phosphorylation of p65 and IκBα. Secondly, PF enhanced VSMC
apoptosis, as was demonstrated by the TUNEL assay. This enhancement
of apoptosis may be associated with increased expression of
caspases. Previous findings by Guo et al (15) demonstrated that PF reversed
ischemia-induced activation of the NF-κB signaling pathway and has
potential neuroprotective effects. These results indicated that PF
may be a good candidate for the prevention of atherosclerosis and
vascular restenosis. Several previous studies have reported the
effect of PF on the proliferation of human lung cancer (9), gastric carcinoma (16) and breast cancer cells (17). In addition, Wu et al
(18) demonstrated that PF
suppresses NF-κB activation by modulating IκBα and enhancing
5-fluorouracil-induced apoptosis of human gastric carcinoma cells.
The abnormal proliferation of VSMCs in arterial walls is known to
be an important pathogenic factor for atherosclerosis and
restenosis following angioplasty. Furthermore, Jeong et al
(19) demonstrated that the
inhibition of NF-κB activity prevents high glucose-induced VSMC
proliferation.
The association between the NF-κB signaling pathway
and the anti-proliferative effect of PF on VSMCs has not been well
reported. Therefore, the present study investigated the effects of
PF at different concentrations (25, 50 and 100 µg/ml) in VSMC
models. The data indicated that treatment with PF significantly
inhibited the proliferation of VSMCs in a time- and
concentration-dependent manner, which was confirmed using CCK-8,
BrdU and flow cytometry analyses. This experiment confirmed that
the optimal concentration of PF was 100 µg/ml, which was used in
the subsequent experiments with VSMCs. The results demonstrated
that PF treatment inhibited the proliferation of VSMCs by
downregulating the expression of four proteins involved in the
G0/G1 and G1/S transition (cyclin D1, cyclin E, CDK4 and CDK2).
Furthermore, PF upregulated the expression of p21, which is known
to form heterotrimetric complexes with cyclin-CDK complexes,
thereby inhibiting their activity. Finally, the western blot
analysis data, which provided insights into the mechanism of this
phenomenon, revealed that PF (25, 50 and 100 µg/ml) decreased the
phosphorylation of p65 and IκBα in a concentration-dependent
manner. These results indicated that PF exerted an inhibitory
effect on VSMCs proliferation by inhibiting activation of the NF-κB
signaling pathway.
Numerous studies have demonstrated that VSMC
apoptosis occurs during physiological vessel remodelling, including
after flow reduction (20),
atherosclerosis (21) and after
injury (22). However, the
apoptotic effect of PF on VSMCs is not widely reported. Thus, the
TUNEL assay results demonstrated that PF significantly increased
the apoptosis of VSMCs. Furthermore, the effect on VSMCs apoptosis
was confirmed by upregulation of caspase-3, −8 and −9
expression.
In conclusion, the results of the present study
suggested that PF has anti-proliferative effects in VSMCs, which
likely involve the NF-κB signaling pathway. In addition, PF
promoted the apoptosis of VSMCs by upregulating the expression of
caspases. Therefore, PF may have the potential for further
development as a candidate chemo-preventive or therapeutic agent
for the treatment of arteriosclerosis and restenosis.
Glossary
Abbreviations
Abbreviations:
|
CDK
|
cyclin-dependent kinase
|
|
VSMCs
|
vascular smooth muscle cells
|
|
PF
|
paeoniflorin
|
|
IκBα
|
nuclear factor of κ light polypeptide
gene enhancer in B-cells inhibitor-α
|
|
NF-κB
|
nuclear factor-κB
|
|
CCK
|
cell counting kit
|
|
FITC
|
fluorescein isothiocyanate
|
|
PI
|
propidium iodide
|
|
RT-qPCR
|
reverse transcription-quantitative
polymerase chain reaction
|
|
DAPI
|
4′,6-diamidino-2-phenylindole
|
|
TUNEL
|
terminal deoxynucleotidyl transferase
dUTP nick-end labeling
|
|
PDGF
|
platelet-derived growth factor
|
References
|
1
|
Ross R: Cell biology of atherosclerosis.
Annu Rev Physiol. 57:791–804. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lusis AJ: Atherosclerosis. Nature.
407:233–241. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Davies MG and Hagen PO: Pathobiology of
intimal hyperplasia. Br J Surg. 81:1254–1269. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Su G, Sun G, Liu H, Shu L, Zhang J, Guo L,
Huang C and Xu J: Niacin suppresses progression of atherosclerosis
by inhibiting vascular inflammation and apoptosis of vascular
smooth muscle cells. Med Sci Monit. 21:4081–4089. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yang J, Chen L, Ding J, Fan Z, Li S, Wu H,
Zhang J, Yang C, Wang H, Zeng P and Yang J: MicroRNA-24 inhibits
high glucose-induced vascular smooth muscle cell proliferation and
migration by targeting HMGB1. Gene. 586:268–273. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bonizzi G and Karin M: The two NF-kappaB
activation pathways and their role in innate and adaptive immunity.
Trends Immunol. 25:280–288. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li ZW, Chu W, Hu Y, Delhase M, Deerinck T,
Ellisman M, Johnson R and Karin M: The IKKbeta subunit of IkappaB
kinase (IKK) is essential for nuclear factor kappaB activation and
prevention of apoptosis. J Exp Med. 189:1839–1845. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wu H, Zhu Z, Zhang G, Zhao L, Zhang H, Zhu
D and Chai Y: Comparative pharmacokinetic study of paeoniflorin
after oral administration of pure paeoniflorin, extract of Cortex
Moutan and Shuang-Dan prescription to rats. J Ethnopharmacol.
125:444–449. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li CR, Zhou Z, Zhu D, Sun YN, Dai JM and
Wang SQ: Protective effect of paeoniflorin on irradiation-induced
cell damage involved in modulation of reactive oxygen species and
the mitogen-activated protein kinases. Int J Biochem Cell Biol.
39:426–438. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hung JY, Yang CJ, Tsai YM, Huang HW and
Huang MS: Antiproliferative activity of paeoniflorin is through
cell cycle arrest and the Fas/Fas ligand-mediated apoptotic pathway
in human non-small cell lung cancer A549 cells. Clin Exp Pharmacol
Physiol. 35:141–147. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hsu FL, Lai CW and Cheng JT:
Antihyperglycemic effects of paeoniflorin and
8-debenzoylpaeoniflorin, glucosides from the root of Paeonia
lactiflora. Planta Med. 63:323–325. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zheng YQ, Wei W, Zhu L and Liu JX: Effects
and mechanisms of Paeoniflorin, a bioactive glucoside from paeony
root, on adjuvant arthritis in rats. Inflamm Res. 56:182–188. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee KP, Kim JE, Kim H, Chang HR, Lee DW
and Park WH: Bo-Gan-Whan regulates proliferation and migration of
vascular smooth muscle cells. BMC Complement Altern Med.
16:3062016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Guo RB, Wang GF, Zhao AP, Gu J, Sun XL and
Hu G: Paeoniflorin protects against ischemia-induced brain damages
in rats via inhibiting MAPKs/NF-κB-mediated inflammatory responses.
PLoS One. 7:e497012012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zheng YB, Xiao GC, Tong SL, Ding Y, Wang
QS, Li SB and Hao ZN: Paeoniflorin inhibits human gastric carcinoma
cell proliferation through up-regulation of microRNA-124 and
suppression of PI3K/Akt and STAT3 signaling. World J Gastroenterol.
21:7197–7207. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang Q, Yuan Y, Cui J, Xiao T and Jiang
D: Paeoniflorin inhibits proliferation and invasion of breast
cancer cells through suppressing Notch-1 signaling pathway. Biomed
Pharmacother. 78:197–203. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wu H, Li W, Wang T, Shu Y and Liu P:
Paeoniflorin suppress NF-kappaB activation through modulation of I
kappaB alpha and enhances 5-fluorouracil-induced apoptosis in human
gastric carcinoma cells. Biomed Pharmacother. 62:659–666. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jeong IK, Oh DH, Park SJ, Kang JH, Kim S,
Lee MS, Kim MJ, Hwang YC, Ahn KJ, Chung HY, et al: Inhibition of
NF-κB prevents high glucose-induced proliferation and plasminogen
activator inhibitor-1 expression in vascular smooth muscle cells.
Exp Mol Med. 43:684–692. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cho A, Mitchell L, Koopmans D and Langille
BL: Effects of changes in blood flow rate on cell death and cell
proliferation in carotid arteries of immature rabbits. Circ Res.
81:328–337. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lutgens E, de Muinck ED, Kitslaar PJ,
Tordoir JH, Wellens HJ and Daemen MJ: Biphasic pattern of cell
turnover characterizes the progression from fatty streaks to
ruptured human atherosclerotic plaques. Cardiovasc Res. 41:473–479.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ashino T, Yamamoto M and Numazawa S:
Nrf2/Keap1 system regulates vascular smooth muscle cell apoptosis
for vascular homeostasis: Role in neointimal formation after
vascular injury. Sci Rep. 6:262912016. View Article : Google Scholar : PubMed/NCBI
|